Abstract
Since their recent discovery, T helper 17 (Th17) cells have been frequently detected in the tumor microenvironment of many malignancies, but their clinical implications remain largely unknown. Interleukin-17 (IL-17) detection is commonly related with poor outcomes in colorectal cancers, yet its presence is associated with antitumor responses in ovarian carcinomas. Numerous experimental models illustrate the divergent roles of Th17 cells in tumor immunity, which appears to be mainly dependent on the tumor context (type, location, and stage of cancer). It is recognized that IL-17 is produced by a variety of cell types and that Th17 cells are endowed with a unique functional plasticity. Therefore, when trying to elucidate potential immune biomarkers and immunotargets, it is extremely important to make a clear dissociation between strategies targeting Th17 versus its hallmark cytokine, IL-17. In this review, we will summarize the data regarding the detection of IL-17 and Th17 in human cancers, consider the experimental evidence on their respective roles in antitumor activity, and discuss the potential of IL-17 as an immune target for therapeutic interventions.
Immunotherapy in cancer
“Evading immune destruction” has recently been introduced by Hanahan and Weinberg in the revised definition of “hallmarks of cancer,” illustrating the remarkable progress made in the last decade to describe the role of inflammation in promoting cancer development.Citation1 It is well established that, during its progression, cancer 1) triggers an immune response, then 2) co-opts the immune cells and mediators for tumor growth and survival, and 3) can evade immune-mediated killing. The concept of cancer vaccine, a T-cell-based immunotherapy approach meant to eradicate cancer cells while establishing long lasting antitumor memory, has its roots in the demonstration that nascent cancer cells and their cortege of associated neoantigens trigger endogenous immune responses. Tumor-infiltrating cluster of differentiation (CD)8+ cytotoxic T lymphocytes (CTL) that recognize and eliminate cancer cells in an antigen specific manner are usually associated with antitumor immune response.Citation2,Citation3 However, even though numerous Phase I/II clinical trials demonstrated the proof of principle that antitumor immune responses can be efficiently induced, the paucity of objective clinical responses and data showing a benefit to patients forced the reconsideration of immunotherapeutic approaches.Citation4 One major obstacle for successful T-cell-based immunotherapies is the ability of cancer to escape the immune response by either suppressing the expression of tumor-associated antigens (loss of antigen, downregulation of major histocompatibility complex [MHC]), or subverting the tumor-associated inflammation via the recruitment of suppressive cells, like regulatory T cells (Treg) or immature myeloid cells, leading to inhibition of immune effectors such as CTL and natural killer (NK) cells.Citation5,Citation6 Molecular identification of these protumoral mediators (including interleukin [IL]-6, IL-23, and vascular endothelial growth factor [VEGF]) and immunosuppressive pathways (including signal transducer and activator of transcription [STAT]3, immune checkpoints such as programmed cell death protein 1[PD-1] or cytotoxic T-lymphocyte antigen 4 [CTLA-4]) have led to the development of immune targets for cancer therapy as recently successfully demonstrated in clinical trials.Citation7,Citation8 Ipilimumab, a monoclonal antibody (mAb) which blocks the immunomodulatory receptor CTLA-4 was approved by the Food and Drug Administration for the treatment of metastatic melanoma in 2011.Citation9 Similarly, successful clinical studies using the blockade of the PD-1/programmed cell death 1 ligand 1 (PD-L1) immunomodulatory pathway predict the rapid approval of these drugs for the treatment of advanced cancers such as refractory melanoma, renal carcinoma, and lung cancer.Citation10 This recent breakthrough in cancer immunotherapy justifies taking a closer look at biomarkers that can predict clinical outcome and response to therapies. For instance, the expression of PD-L1, an immunosuppressor ligand long considered to be a negative prognostic marker because it blocks the action of activated PD1+ CTL, was upregulated on melanoma cells in response to interferon gamma (IFN-γ) production.Citation11 In this study, PD-L1 became a biomarker of endogenous antitumor immune activity, predicting a positive clinical response to the PD-1 blockade.
These findings and others proved that local immune reactions influence the clinical outcome in human tumors and their response to treatment.Citation12 Whereas the density of CD8+ CTL and CD45RO+ memory T cells in tumor is correlated with better prognosis in a variety of cancers, the prognostic value of CD4+ cells infiltration, including Treg and IL-17A (IL-17) producing T helper 17 (Th17) cells, remains largely unclear. In recent times, IL-17 and Th17 detection have gained a lot of attention in the field and have triggered a lot of controversy in terms of their functional impact and significance as prognostic biomarkers. The nature of cytokines produced in the tumor niche and the respective tissue surroundings would determine the beneficial versus the detrimental effects of IL-17-mediated inflammation in cancer.Citation11,Citation13 However, much remains to be understood before we can consider Th17 a target for immune interventions in cancer therapy.
Physiological roles of IL-17 and Th17 cells
The Th17 group of cells was introduced in 2005 as a new committed lineage of CD4 cells, distinct from the IFN-γ-producing Th1, IL-4-producing Th2, and forkhead box P3+ (Foxp3+) Treg.Citation14,Citation15 Upon stimulation, naïve T cells commit to one of these Th subsets endowed with specific functions. The context in which a naïve T cell is stimulated, including the array of cytokines present and transcription factors activated, determines CD4 Th polarization. Although these different CD4 Th lineages were previously thought to be mutually exclusive, recent studies suggest Th subsets are functionally flexible.Citation16 Th17 cells are particularly well appreciated for their plasticity.Citation17 They can express Foxp3, produce IL-10, and become suppressive.Citation18–Citation20 Alternatively, Th17 cells can upregulate T-box transcription factor (TBET), produce IFN-γ, and mediate pathogenic or antitumor functions.Citation21,Citation22 Nevertheless, the Th17 lineage is known for producing the iconic IL-17, as well as IL-17F, IL-22, and IL-21, which critically impact inflammation and tumor-associated immunity.Citation23–Citation25 The extremely dynamic nature of CD4 cell polarization is a feature of equally dynamic regulation driven by local environmental changes and epigenetics.Citation17 Adding to Th17 instability, a variety of cell subsets have been identified as a source of IL-17 to date, highlighting the essential distinction that should be made between targeting Th17 or IL-17 as a strategy for cancer therapeutics.Citation26
Sources of IL-17
IL-17 is a member of the IL-17 family which also includes IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F. IL-17A and IL-17F share the most homology and are produced as homodimers (A/A and F/F) or heterodimers (A/F).Citation27 Murine Th17 cells, which are commonly derived in vitro by activation of naïve uncommitted CD4+ T cells in the presence of transforming growth factor beta (TGF-β), IL-6, and IL-23/IL-1β, remain rare in vivo in peripheral compartments such as blood and secondary lymphoid structures. However, they naturally concentrate in mucosal areas where they play an active role in keeping the microbial flora and pathogenic microorganisms at bay. Th17 cells are especially abundant in gut associated lymphoid tissues, where they have the crucial function, along with Treg, of allowing colonization by commensal flora without provoking tissues damage.Citation28 Under pathologic circumstances, Th17 cells accumulate in inflamed tissues during different types of events such as infections, autoimmune diseases, or neoplasia. It is now well appreciated that IL-17 produced by Th17 cells represents only a fraction of the total IL-17. Other sources of IL-17 are innate cells, including γδ-T cell receptor (TCR)+ T cells, which are particularly potent producers of IL-17 when stimulated by IL-1β or IL-23, independently of TGF-β and TCR ligation.Citation29 To date, the other cells described as sources of IL-17 include NK cells, lymphoid tissue inducer cells, and innate lymphoid cells.Citation26 Though controversial, myeloid-derived cells including polymorphonuclear cells, macrophages, and mast cells producing IL-17 have also been reported as sources.Citation30–Citation32 These innate immune cells could represent an early source of IL-17, intervening before the differentiation of Th17 cells and potentially influencing the ensuing Th17 response.Citation29 It is likely that the prognostic value of IL-17 detection in cancer differs depending on its source.
Th17 differentiation in tumor microenvironment
Plasticity of Th17 cells
Programming uncommitted Th cells (Th0) to become Th17 cells requires the combined action of soluble mediators (IL-6, TGF-β, and IL-23) and activation of STAT3 and retinoic acid receptor related orphan receptor gamma (RORγt) transcription factors.Citation33 Alternatively, the combination of IL-1β, IL-6, and IL-23 has also been shown to sustain differentiation of Th17 in a TGF-β-independent manner.Citation21 Indeed, conditional deletion of TGF-β signaling in murine CD4 cells did not compromise Th17 differentiation in the intestinal lamina propria.Citation21 IL-23 is involved in the maintenance of Th17 and the production of IL-17 by memory T cells, whereas TGF-β is thought to facilitate Th17 differentiation by repressing Th1 and Th2 polarizations.Citation22,Citation34 TGF-β-dependent and independent Th17 cells were distinguished by distinct functions and transcription profile ().Citation21 IL-6 and TGF-β induce poorly pathogenic Th17, characterized by the production of IL-10.Citation20 IL-23 in vivo holds a decisive role in stabilizing the Th17 phenotype, but also in inducing pathogenic properties of Th17 cells with production of IFN-γ and granulocyte-macrophage colony-stimulating factor (GM-CSF).Citation35 However, the dispensability of TGF-β has faced some controversy recently, since TGF-β is widely expressed by tissue and immune cells. Thus, it is not clear whether a TGF-β-independent Th17 commitment is physiologically relevant.Citation36 RORγt must cooperate with other transcription factors such as RORα, STAT3, and interferon regulatory factor 4 (IRF4) to drive the differentiation of the Th17 lineage.Citation37,Citation38 Interestingly, other immune and metabolic conditions, including the amount of available IL-2, tryptophan metabolites (indoleamine 2,3-dioxygenase [IDO] activity), and hypoxia present in the milieu of a neoplasm, may drive the differentiation of Th toward the Th17 lineage.Citation39–Citation41 IL-2 promotes Foxp3 expression in Th17 and inhibits its differentiation via the activation of STAT5 signaling, which was shown to compete with STAT3 for binding to the promoter of Il17a gene.Citation39 Treg were demonstrated to regulate the IL-2- dependent STAT5 signaling by absorbing the IL-2 in the microenvironment, paradoxically promoting Th17 differentiation.Citation42 Yet, Foxp3+ Treg can also regulate Th17 function through an IL-10/STAT3 pathway.Citation43,Citation44 Dang et al recently showed that hypoxia directs the differentiation of CD4 cells toward Th17, rather than Treg via hypoxia-inducible factor 1-alpha (HIF 1-α) which activates the transcription of RORγt and targets Foxp3 to proteasomal degradation.Citation40 IDO is a tryptophan-catabolizing enzyme with potent immunosuppressive functions in the tumor microenvironment (TME), especially via its impact on Treg.Citation45 It was recently shown that inhibition of IDO might reprogram Treg into Th17, resulting in increased CD8+ T cell infiltration and antitumor activity.Citation41 A similar functional plasticity demonstrated by the Treg allows skewing of Foxp3+ Tregs toward Th17 in the presence of IL-1β or IL-23.Citation46 Conversely, Th17 cells isolated from tumors were able to upregulate Foxp3 upon in vitro TCR-mediated stimulation and to convert to Treg with immunosuppressive functions independent of IL-10 and TGF-β.Citation18 Clearly, there is a finely regulated balance between Treg and Th17 differentiation, tuned by inflammatory mediators and the metabolism of the TME. The plasticity of Th17 cells is further demonstrated by their ability to upregulate IFN-γ and TBET, which orchestrate Th1 differentiation and mediate potent antitumor responses. Th17 or IL-17-producing CD8+ T cells (Tc17) injected into tumor-bearing mice can convert into Th1 or CTL, respectively, to promote antitumor responses.Citation47,Citation48 Given the aforementioned descriptions, the ontogenic and functional adaptive ability of Th17 accounts, at least in part, for the complexity of the clinical significance attributed to their detection in the TME (pro- versus anti-tumoral).
Figure 1 Differentiation and functional flexibility of Th17 in TME.
Abbreviations: APC, antigen-presenting cells; CCL, chemokine (C-C motif) ligand; CD, cluster of differentiation; DC, dendritic cells; Foxp3, forkhead box P3; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon gamma; IL, interleukin; RORγt, retinoic acid receptor related orphan receptor gamma; STAT3, signal transducer and activator of transcription 3; TAF, tumor associated fibroblast; TAM, tumor associated macrophage; TBET, T-box transcription factor; Th17, T helper 17 cells; TGF-β, transforming growth factor beta; TME, tumor microenvironment; Treg, regulatory T cells; Tum, tumor; Th0, Th cells.
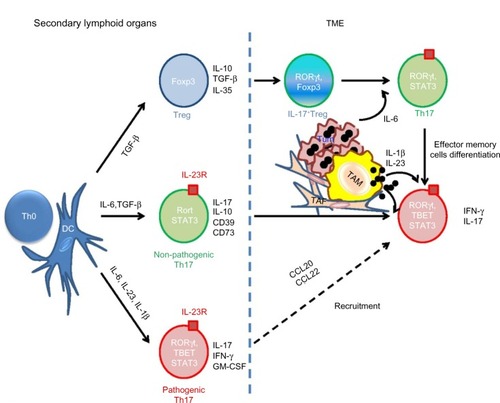
The role of antigen-presenting cells in Th17 polarization
It is not yet fully understood what factors in the TME turn CD4+ Th into “bad” or “good” Th17 cells. The nature of antigen-presenting cells (APC) and their cytokine profiles (IL-1β, IL-23, or TGF-β), which induce Th17 in regional lymph nodes or in tumor tissues, is thought to dictate the functional properties of Th17 in these tissues. APC and especially dendritic cells (DC), which are mainly involved in the education of naïve T cells in the lymph nodes, are also important sources of TGF-β in the TME. Integrin αvβ8 on DC was shown to play an important role in TGF-β activation and Th17 differentiation since mice lacking αvβ8 were fully protected from Th17-dependent experimental autoimmune encephalomyelitis.Citation49 Th17 can also be generated in the TME from effector memory cells. Studies have shown that tumor-associated macrophages (TAM) and resident DC are efficient in inducing antitumor Th17 response in ovarian cancer.Citation50,Citation51 Tumor-associated macrophages were especially potent inducers of Th17 polarization through the production of IL-1β, in the TME, whereas blocking IL-6 or TGF-β did not affect Th17 polarization and IL-23 was barely detectable in ascites produced by ovarian carcinomas.Citation50 IL-1β produced by APC in tumor lesions can contribute to the differentiation of antitumor skewed Th17 cells, which produce IFN-γ and induce the recruitment of effector cells.Citation51 Divergently, blocking IL-23 was shown to dramatically mitigate IL-17 production, impeding carcinogenesis in a murine model of colitis-associated cancer.Citation52 It is possible that the differential amount of TGF-β, IL-23, and/or IL-1β in the TME of these types of cancers may lead to functionally distinct Th17 subsets. Taken together, these studies suggest that influencing the recruitment and nature of APC (eg, producers of TGF-β, IL-23, and IL-1β) in the TME may become an effective strategy to improve antitumoral immunity.Citation53
Th17 and IL-17 functions in cancer
IL-17 is a pleiotropic proinflammatory cytokine whose impact in tumor progression is highly context-dependent. However, discrepancies observed regarding its role in the TME seem to be largely dependent on the experimental model utilized: transplantable tumors versus de novo carcinogenesis;Citation54,Citation55 lymphopenic versus immunocompetent systems;Citation47,Citation56–Citation58 over-expressed IL-17 versus endogenous IL-17 ().Citation55,Citation57
Table 1 List of experimental murine models analyzing the roles of IL-17/IL-23 in tumor immunity
IL-17 receptor signaling
Th17 are largely devoid of the effector molecules granzyme and perforin, suggesting that they do not exert effector functions by themselves. Instead, Th17 cells are proinflammatory as a result of their signature cytokines IL-17 and IL-17F, which mediate the recruitment of effector lymphocytes and phagocytes that dispose compromised cells and pathogens. They also play an active role in tissue repair. Although more work needs to be done to delineate the differential inflammatory properties of IL-17 and IL-17F, recent experimental models of inflammation using selective genetic ablation of either Il17a or Il17f genes showed that both cytokines mediate distinct proinflammatory functions. IL-17F, but not IL-17, knockout (KO) mice exhibit defective airway neutrophilia in response to allergen.Citation59 The molecular mechanisms of their functional specificity are not yet understood since they signal through the same heterodimeric IL-17R subunit A/subunit C (IL-17RA/C) receptor and are biologically active as homodimers as well as heterodimers. IL-17R signaling leads to the activation of the nuclear factor-kappaβ (NF-κB) pathway via the interaction of IL-17RA with the adaptor molecule tumor necrosis factor (TNF) receptor associated factor 3 (TRAF3) interacting protein 2 (TRAF3P2 or ACT1) and the TRAF6.Citation27 In contrast to other cytokines, IL-17/IL-17R signaling does not directly use the Janus kinase (JAK) and STAT transduction routes, but rather induces an indirect activation of the protumoral STAT3 pathway through an IL-6/STAT3 feedforward loop.Citation54,Citation60 IL-17R is widely expressed in epithelial cells, fibroblasts, endothelial cells, and hematopoietic cells, mediating IL-17 pleiotropic properties widely in the TME.Citation27
Recruitment of immune effector cells
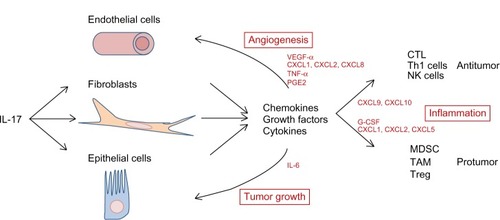
IL-17 signals epithelial cells and fibroblasts to produce growth factors and a large array of chemokines, which are critical for the immune polarization mediating protumoral versus antitumoral effects. Indeed, in some cancers, such as in the case of non-small-cell lung carcinomas (NSCLC), cervical cancers, or colorectal cancers (CRC), IL-17 induces the secretion of chemokine (C-X-C motif) ligand 1 (CXCL1), CXCL2, CXCL5, and CXCL8, mediating the recruitment of neutrophils, macrophages, and immature myeloid cells, well known for their protumoral function.Citation58,Citation61 Conversely, other groups have shown that genetic ablation of IL-17 signaling impairs tumor growth through decreased myeloid-derived suppressor cells (MDSC) accumulation and increased cytotoxic CD8+ T cell infiltration.Citation54,Citation62–Citation64 Also, other tumors such as ovarian carcinomas produce CXCL9 and CXCL10 in the presence of IL-17, which attract effector cells like CD8+ CTL and NK cells to mediate antitumor immune response.Citation50 IL-17 contributes also to the recruitment of APC such as DC via the induction of chemokine (C-C motif) ligand 20 (CCL20) production in the tumor bed.Citation53,Citation65 Adoptive transfer of tumor-specific Th17 in a model of pulmonary melanoma showed a dramatic reduction of lung metastasis associated with a CCL20-dependent recruitment of DC and activation of CD8+ CTL.Citation53 These observations unravel that according to the type of cancer cells, activation by IL-17 can lead to opposite effects via the induction of a distinct array of chemokines by target cells.
Angiogenesis
In a lymphopenic system missing adaptive immunity, IL-17 demonstrated protumoral properties by sustaining angiogenesis and tumor growth.Citation58 Indeed, IL-17 is a pro-angiogenic factor that induces neovascularization and tumor growth via the direct induction of VEGF and angiogenin-2 production by stromal (myeloid cells and fibroblasts) and epithelial cells.Citation57,Citation60,Citation63,Citation66,Citation67 IL-17 can similarly facilitate tumor angiogenesis independently of VEGF via its action on the tumor-associated stromal cells.Citation63,Citation67 Chung et al’s group showed that IL-17 can activate tumor-associated fibroblasts (TAF) to secrete granulocyte colony-stimulating factor (G-CSF), which in turn attract myeloid cells that produce angiogenic mediators such as prokineticin 2/Bv8, matrix metalloproteinase 9 (MMP9), as well as the proinflammatory S100A8/9 molecules.Citation63 Importantly, IL-17 also mediates protumoral effects indirectly through the IL-6/STAT3 signaling, promoting the expression of genes involved in cell proliferation and survival (eg, Cyclin D1, Bcl-xl, or survivin).Citation60
Th17-induced tumorigenesis
Two recent experimental models of colitis demonstrated the contribution of the IL-23/Th17 axis in colon tumorigenesis.Citation52,Citation68 Wu et al were able to reduce enterotoxigenic Bacteroides-fragilis-driven colon tumorigenesis in mice with heterozygous null mutation of adenomatous polyposis coli (Apc) gene (MinApc−/+ mice) via neutralization of IL-23R and IL-17.Citation68 Grivennikov et al showed that genetic deletion of IL-17R or IL-23 deficiency in myeloid cells reduced the tumor burden in MinApc−/+ mice.Citation52 The authors proposed that barrier breach provoked by colonic tumors triggered IL-23 production by myeloid cells and Th17 polarization, although the molecular mechanisms sustaining the tumorigenic activities of IL-17 remain largely unknown. Nevertheless, these experimental data concur with the observations made in human CRC, where the IL-17/Th17 signature is associated with diminished survival of patients.Citation13
In summary, despite the fact that Th17 cells make up a small part of circulating CD4+ T cells (in mice and humans), they accumulate in the TME of most solid tumors when compared to normal counterpart tissues. Different subsets of Th17 (IFN-γ+ versus IL-10+) are endowed with distinct functions in regards to their ability to promote tumors or, conversely, eliminate tumors. Th17 exercise their opposing functions depending on the features of the TME (nature of the APC, presence of TGF-β, IL-1β or IL-23). These cells can mediate antitumoral effects by their ability to recruit immune effector cells (CTL, NK, DC) into the tumor bed and they can promote tumor growth by means of angiogenesis (production of VEGF, angiogenic chemokines) and/or through immunosuppression (Treg conversion, MDSC recruitment) (). Th17 cells are functionally unstable and capable of converting into Treg or Th1-type cells causing antagonistic consequences in tumor immunity. Moreover, discrepancies have been found when comparing the development of Th17 in humans as opposed to murine models, emphasizing the need to exercise caution when extrapolating between species.Citation69 The preceding described aspects of the Th17 biology account for the complexity of IL-17/Th17 significance in human cancer and the uncertainty of using this biomarker for therapeutic purposes.
Figure 2 Role of IL-17 in tumor progression.
Abbreviations: CCL, chemokine (C-C motif) ligand; CTL, cytotoxic T lymphocytes; CXCL, chemokine (C-X-C motif) ligand; DC, dendritic cells; G-CSF, granulocyte colony-stimulating factor; IL, interleukin; MDSC, myeloid-derived suppressor cells; NK, natural killer; PGE2, prostaglandin E2; STAT3, signal transducer and activator of transcription 3; TAF, tumor associated fibroblasts; TAM, tumor associated macrophages; Th1, T helper 1; TME, tumor microenvironment; TNF, tumor necrosis factor; Treg, regulatory T cells; VEGF, vascular endothelial growth factor.
Clinical implications
Th17 cells are key players in inflammation and autoimmune disorders; however, the clinical significance of their detection in the TME remains unclear. The phenotypic and functional heterogeneity of the Th17 lineage along with its functional plasticity underscore the context-dependency of their prognostic and therapeutic implications as a cancer-associated biomarker (cancer type and stage, infiltration density, nature, and location). Importantly, in light of recent studies seeking to demonstrate a link between IL-17 detection and cancer promotion (), a distinction should be made between IL-17 and Th17. A variety of cell types characterized by distinct cytokine profiles and effector functions are able to produce IL-17. Many reports state that IL-17 protein and/or messenger RNA (mRNA) are detected in tumor beds, but the cellular sources of production were often not characterized.Citation70
Table 2 List of studies in humans analyzing the links between cancer type and IL-17/Th17 detection
The prognostic value of IL-17 and/or Th17 in cancer
Blood versus tumor tissues
A growing list of cancers has recently been investigated for the presence of Th17 cells in order to gain insight about the clinical significance of their existence in tumor lesions. However, while most of the studies have been performed with peripheral blood from patients, Th17 cells accumulate predominantly in the neoplastic sites. Yamada et al recently showed the preferential accumulation of Th17 cells in gastric cancer lesions compared to peripheral blood and normal gastric tissue specimens.Citation71 They also found a strong negative correlation between high level of IL-17 in the sera of gastric cancer patients and their 5-year survival.Citation71 Unfortunately, they did not address the prognostic value of having IL-17 and/or Th17 detected in stomach samples. Meanwhile, an earlier study performed by Chen et al found that gastric cancer patients expressing higher levels of intratumoral IL-17 had a better survival.Citation72 Despite relative ease of accessibility, the blood compartment is probably not representative of the intratumoral Th17 response, and investigations should concentrate on determining the clinical impact of tumor infiltrating IL-17-producing cells. Kryczek et al published one of the first comprehensive analyses characterizing tumor infiltrating Th17 cells and demonstrated that IL-17 is a positive prognostic marker of antitumor immune response in ovarian carcinomas.Citation50 They showed that although no significant difference could be found between normal and cancer patient blood samples, the prevalence of Th17 among CD4+ T cells was much higher in tumor tissues. Th17 are recruited, induced, or expanded in the TME via the production of mediators by tumor and/or stromal cells. Interestingly, the detection of IL-17 (and not Th17 per se) in ovarian cancer ascites has been correlated with an increased survival of patients. The authors of this study made an elegant charinacterization of the infiltrating Th17 showing a lack of CD25, human leukocyte antigen (HLA)-DR, and granzyme, therefore distinguishing them from conventional effector cells. Of note, these Th17 cells produced IFN-γ, IL-2, and TNF-α, but no IL-10.Citation50
Intratumoral IL-17 and chemokines
Kryczek et al found in their study that ovarian tumor cells produced CXCL9 and CXCL10, which recruited immune effectors into the tumor bed.Citation50 No correlation was observed between Th17 and neutrophils, eosinophils, macrophages, or DC. They instead established a correlation with CD8+ T cells and NK infiltration, well known for their active participation in antitumor immune responses.Citation50 Similar results have been described in esophageal squamous cell carcinoma (ESCC) and lung cancer.Citation65,Citation73,Citation74 Though Lu et al did not characterize Th17 per se, they reported that the number of IL-17-producing cells correlated with a higher number of CD8+ T and NK cells invading the tumor area.Citation65 Interestingly, they were able to positively link the number of IL-17-producing cells with the recruitment of CD1a+ DC, a mechanism that seems to be associated with the capacity of ESCC cells to produce CCL20 upon IL-17 stimulation.Citation65 Patients showing a high density of CD1a+ DC were found to have a better survival. These results were in line with those obtained by Martin-Orozco et al, who discovered that the adoptive transfer of Th17 cells in a murine model of melanoma lung metastasis was related to the recruitment of DC in a CCL20/chemokine receptor 6 (CCR6)-dependent manner.Citation53 In lung cancer, a high Th17 frequency detected in malignant pleural effusions was positively correlated with patients’ survival,Citation74 and CCL20 was shown to be a key chemotactic mediator produced by tumor cells in the recruitment of CCR6+ Th17.Citation74 The positive association between IL-17 or Th17 and cancer patient survival demonstrated in these different types of cancers (ovarian, lung, and esophageal) is due to the role of IL-17 in shaping the chemokine milieu via its interaction with tumor cells or tumor-associated stromal cells (fibroblast or myeloid cells). On the one hand, IL-17 can promote the production of angiostatic CXCL9 and CXCL10 (and not VEGF) by ovarian cancer cells, but, on the other hand, IL-17 can induce the production of angiogenic VEGF, CXCL1, or CXCL8 (and repressed CXCL9 or CXCL10) in CRC cells.Citation75,Citation76 IL-17 in NSCLC patients was associated with detection of CXCL1, CXCL5, or CXCL8.Citation61 Furthermore, the administration of recombinant CXCL10 improved the survival of NSCLC-bearing severe combined immunodeficiency (SCID) mice and injection of anti-CXCR2 (CXCL1, CXCL5, and CXCL8 receptor) mAb blocked the protumoral effect of IL-17.Citation61,Citation77 In concordance with these results, the density of pro-angiogenesis IL-17+ cells in CRC and NSCLC correlated with poor prognosis for these patients.Citation13 Recently, a large intergene correlation study performed in CRC patients looking for genes highly associated with tumor-infiltrating memory T cells and effector T cells (antitumor immune signature) identified CXCL9 and CXCL10 genes at the top of the list.Citation78 This finding concurred with the observation that IL-17, which does not induce the production of these angiostatic chemokines by CRC cells, is associated with poor prognosis in this type of malignancy.Citation13 It is therefore tempting to postulate that knowing the sensitivity of the different tumor types to IL-17 may allow clinicians to predict whether infiltrating Th17 would be beneficial or deleterious for patient survival. Unfortunately, the pleiotropic IL-17 targets not only epithelial/tumoral cells but also stromal cells, including fibroblast (TAF), endothelial cells, and myeloid-derived cells, which can also polarize tumor immune responses toward tumor promotion or tumor eradication (). TAF have been shown to promote tumor-associated angiogenesis when stimulated by IL-17 through the G-CSF-dependent recruitment of MDSC.Citation63 IL-17 has also been found to be capable of directly activating the immunosuppressive function of MDSC and monocytes.Citation62,Citation79
Table 3 Drugs affecting IL-17 and Th17 functions in preclinal and clinical development
Heterogeneity of intratumoral Th17
Th17 cells are functionally heterogeneous and different Th17 subsets may have a distinct impact on tumor progression. Zhao et al recently demonstrated that CCR4+CCR6+ Th17, but not CCR4−CCR6+ Th17, were able to inhibit the proliferation and IFN-γ production by CD8+ T cells in hepatocellular carcinoma (HCC) patients.Citation80 This study was performed in the peripheral blood compartment and it would be informative to characterize these Th17 subsets in the tumor tissue and link their detection with antitumor immune responses. On this note, Zhang et al previously showed that, despite CCR6 expression by virtually all Th17 in blood and tumors of HCC patients, a higher proportion of them expressed CCR4 in the tumor tissue.Citation81 Though the cohort included a relatively limited number of patients, they showed that CCR4 expression was significantly increased according to the stage of the disease. Here again, the expression of CCR4 and CCR6 on Th17 was associated with higher levels of CCL20 and CCL22 in tumor tissue compared to normal counterparts. This suggests that the density of Th17 cells and the differential representation of each Th17 subset in tumor tissue may be driven by a CCL20/CCL22-dependent recruitment mechanism. Therefore, additional functional markers (eg, CCR4 and CCR6) could be used to characterize intratumoral Th17 and further stratify the clinical value of their detection in tumors.Citation12,Citation78
IL17 and immune contexture
The nature and anatomic localization of IL-17+ cells in the tissue have also been recently appreciated as an important factor in determining clinical prognosis.Citation12,Citation30 There are numerous prospective clinical studies looking at the penetration of this subset of T helper cells in cancer tissues raising the concern of utilizing IL-17 detection versus Th17 cells as prognostic markers. Since IL-17 is produced by a variety of adaptive and innate immune cells, it seems that the characterization of IL-17+ cells, and not only Th17, should be performed (). IL-17 mediates different types of effects depending on whether it is being produced by Th17, γδ-T cells, macrophages, or mast cells dictated by the nature of the cytokines and growth factors co-produced by these cells. Th17 cells are capable of producing other proinflammatory cytokines such as IL-21 or IFN-γ, which can attract immune effector cells (CTL and NK) to promote an antitumor immune response. In turn, macrophages can provide the TME with a variety of angiogenic (VEGF, TNF-α) and protumoral mediators (IL-6).Citation30,Citation31,Citation82 Wang et al described that IL-17-producing mast cells in ESCC were mainly concentrated in the muscularis propria and their density predicted a better outcome of the disease by means of recruiting more effector CTL and macrophages to the area.Citation30 This distribution of immune effector cells is different in CRC, where the IL-17+ cells accumulate in the lamina propria and indicate a poor prognosis.Citation13 These reports show that not only the functional properties of IL-17 are important, but also that the context in which this proinflammatory cytokine is being produced is critical. Galon et al recently introduced the concept of immune contexture in cancer, which defines the density, functional polarization, localization (core versus invasive margin) of the immune infiltration.Citation83 They demonstrated the profound impact of immune contexture on clinical outcome independently of the pathological stage in a large cohort of CRC specimens.Citation12,Citation83 Essentially, they found that the density of memory CD4+ T cells and memory CD8+ T cells in the core of CRC lesions was negatively correlated with tumor recurrence. In a following study, they established that the combined analysis of the expression of cytotoxic (IFNG, TAP1, and GZMB) and Th17 (IL17a and RORC)-related clusters of genes allows a better prediction of relapse.Citation13 The detection of a Th17 cluster was associated with the expression of CCL24, but not CXCL9 or CXCL10.
In conclusion, IL-17 detection in the tumor site has a complex clinical significance. For that reason, its geographic localization in the neoplasm plus the concomitant characterization of functional markers including chemokines (CXCL9, CXCL10 versus CXCL1, CXCL5), receptors (eg, CCR4), cytokines (eg, IFN-γ), and lineage markers (eg, γδ-TCR or myeloid markers) might dramatically clarify and improve the prognostic value of IL-17.
Therapeutic perspectives of Th17 and IL-17 in cancer
It is only recently that we started to appreciate the functional diversity and plasticity of Th17 cells. The association of IL-17 and Th17 with a variety of chronic inflammatory disorders has led to the development of an increasing arsenal of inhibitors being tested in clinical trials to develop immunotherapies targeting the pathogenic IL-23/IL-17 axis.Citation84,Citation85 These trials have posted some success, especially in conditions such as psoriasis, ankylosing spondylitis, rheumatoid arthritis, and inflammatory bowel disease (IBD).Citation86 However, no clinical trials specifically targeting IL-17 have yet been attempted as a treatment for cancer. As a result, therapeutic tools in the form of drugable blocking mAbs (humanized or fully human) or small-molecule inhibitors have flourished and are being interrogated for their clinical activity and toxicity profile (Phase I and II clinical trials; ). Recent results of clinical trials in inflammatory disorders should be used as leverage to test these tools in cancer immunotherapy. Obviously, two radically opposed strategies should be applied according to the type of cancer and the clinical impact of IL-17 in tumor progression: 1) blocking deleterious IL-17-driven inflammation in cases where IL-17 is associated with poor prognosis; or 2) promoting antitumor Th17 response in cases where IL-17 is associated with good prognosis.
Blocking IL-17/Th17
Different approaches could be exploited to block protumoral effects of IL-17 adopting results extrapolated from the inflammatory disease immunotherapy field. The first option relies on the use of mAb blocking IL-17 or IL-17R and IL-23; the second choice consists of using small-molecule inhibitors that target key molecules in the IL-17 pathway (eg, STAT3 or ROR).
Pharmacological blocking of IL-17 signaling (anti-IL17 and anti-IL17R mAbs)
IL-17 and related cytokines have been successfully targeted for the treatment of autoimmunity.Citation84 A variety of clinical trials focused on hindering the proinflammatory properties of IL-17 in autoimmune disorders including psoriasis, IBD, sclerosis, and even in allergies. Whereas blockade of IL-17 or IL-17R is effective in the therapy of psoriasis, so far little benefit has been observed in Crohn’s disease. These discrepancies between inflammatory disorders emphasize how complex the pathogenic role of IL-17 and Th17 is. Of note, blockade of IL-17 and IL-23 signaling pathways was shown to significantly decrease the incidence of colon tumors in experimental models of colitis-associated cancer related to chronic inflammatory disorders, and where IL-17 has been proven to be responsible in the promotion of tumorigenesis.Citation52,Citation68 On these grounds, it is reasonable to postulate that even though blockade of IL-17 (with or without blockade of IL-23) did not improve colitis in humans, it may decrease the long-term pathogenic effects of IL-17 on the epithelium, therefore reducing the CRC incidence in this patient population. Furthermore, considering that the IL-17/Th17 pathway has been linked to poor prognosis in CRC patients,Citation13 it could be anticipated that the detection of an IL-17 signature in cancer lesions could render patients eligible for adjuvant anti-IL-17 and/or IL-23 immunotherapies.
Blocking Th17 differentiation
Several protocols that could affect the Th17 polarization are already in place for the treatment of inflammatory disorders, like mAbs against IL-23 and IL-6R, or IL-1R antagonists and small-molecule inhibitors that target STAT3 (). Given that IL-23 promotes the expansion and survival of Th17, blocking the IL-23 pathway is one of the possibilities to directly impact the IL-17 response and tumor development. However, just like IL-17 and Th17, the role of IL-23 should be considered according to the context of the cancer of origin. While IL-23 deficient mice have been shown to be resistant to carcinogen-induced skin cancer, IL-23-transduced DC-based vaccine can lead to strong antitumor responses with increased CD8+ cytotoxic T cell recruitment.Citation87,Citation88 A Phase II clinical trial of therapies targeting IL-12/p40, common to IL-12 and IL-23, showed encouraging results in the treatment of psoriasis, but rendered individuals with an increased susceptibility to infections.Citation84 A Phase III randomized controlled trial that used the fully human IL-12/IL-23 mAb (briakinumab) in moderate-to-severe psoriasis reported that briakinumab was paradoxically associated with an increased risk of cancer, including non-melanoma skin cancer, although this increase was not statistically significant and not clearly linked to a direct effect of IL-12/IL-23 or the use of adjuvant phototherapy.Citation89 A potential explanation could be that the blockade of IL-12 decreased antitumor immunosurveillance (IFN-γ, TH1, and CTL), leading to an increased risk of developing skin tumors. Accordingly, the use of a more specific mAb such as MK-3222 (IL-23 and IL-19 inhibitor), which should spare the IL-12/IFN-γ axis classically associated with antitumor response, appears more appropriate for the cancer immunotherapeutic approach. Anti-IL-6R mAb (tocilizumab) and IL-1Ra antagonist (anakinra) are also available in the clinical setting. However, despite being involved in Th17 polarization, IL-1 and IL-6 have been shown to be dispensable cytokines for effector Th17 cells.
Because the clinical impact of IL-17 detection in tumor is largely unknown, it is hard to predict whether blockade of IL-17 or inhibition of Th17 differentiation would bring any therapeutic benefit to patients, or if it would aggravate the disease. The use of these regimes would seem more applicable in reducing the incidence of cancer in patients already suffering from chronic inflammatory disorders (eg, IBD and CRC; dermatoses and skin cancers; pancreatitis and HCC). To our knowledge, the only listed cancer-related clinical trials targeting the IL-17 pathway are agents using the STAT3 inhibition route. Even though most of the trials listed in clinicaltrials. gov are not specifically targeting IL-17, these small-molecule inhibitors have been tested in numerous different types of hematopoietic-derived cancers and solid tumors. Admitting that most of these studies are incomplete, very limited benefits have been obtained. In certain clinical trials, like in the case of renal cell carcinoma (NCT00550277; a Phase II trial testing LBH589 for the treatment of patients with refractory clear cell renal carcinomaCitation90), no promising results were observed and the recruitment of the study was stopped. It is, however, fair to note that the effects of such treatment on IL-17 or Th17 infiltration were not documented. Nevertheless, one clinical trial using histone deacetylase (HDAC) inhibitors in patients with cutaneous T cell lymphomas (CTCL) and benign dermatoses is monitoring Th17 in cancer lesions (NCT01663571Citation91). HDAC inhibitors regulate STAT3 transcriptional activity and hence can potentially be active in CTCL through modulation of this proinflammatory pathway. The investigators will investigate whether HDAC inhibitors have a direct impact on the number of Th17 cells, the cytokine production by these cells, and phosphorylated STAT3 protein in CTCL with subsequent treatment cycles.
Small-molecule inhibitors to oppose IL-17 production
Inhibitors of Th17 lineage transcription factors RORγt and RORα have beneficial anti-inflammatory effects in experimental models of inflammatory disease.Citation92 These agents hold promise to dim the protumoral effect of IL-17 in certain types of cancers in which the detection of IL-17 has been associated with bad outcomes. SR1001, a high-affinity synthetic ligand that is specific to both RORα and RORγt and which inhibits Th17 cell differentiation and function, has been shown to inhibit sclerosis in animal models.Citation93 Yet, attention should be paid to this drug due to its critical regulation in the hepatic metabolism. Vidofludimus (4SC-101) (NCT00820365Citation94: Crohn’s disease or ulcerative colitis [UC]; NCT01010581Citation95: rheumatoid arthritis) is a potent inhibitor of human dihydroorotate dehydrogenase and of IL-17 secretion in vitro and in vivo. It was examined in several relevant animal models, showing beneficial effects in the case of acute colitis in mice with a coincidental decrement in IL-17A/A and A/F production.Citation96 In fact, a Phase II trial in Crohn’s disease using vidofludimus is being pursued as the next step. Tofacitinib (CP-690,550) inhibits JAK1, JAK3, STAT3, and IL-17 and it is being utilized in the treatment of rheumatoid arthritis, UC, and psoriasis. Despite the fact that both inhibitors have demonstrated a relatively good safety profile in clinical studies, the concern is that vidofludimus and tofacitinib also reduce IFN-γ secretion, an effect which could be detrimental in the treatment of cancer. Much further optimization of these small-molecule inhibitors should therefore be performed in preclinical and clinical studies to avoid undesirable side effects.
Interventions promoting IL-17/TH17
Adoptive transfer of TH17 or Tc17
In vitro polarized CD4 and CD8 T cells that produce IL-17 have been shown to have antitumor activity when adoptively transferred into tumor-bearing mice.Citation47,Citation48,Citation53,Citation97 However, whereas infusion of in vitro expanded CD8+ T cells in cancer patients has been intensively studied in clinical trials, there has been very little effort to use polarized CD4+ cells (including Th17) in the clinic because of their functional instability rendering their therapeutic efficiency relatively uncertain.Citation98,Citation99
Inhibition of IDO and arginase
Amino acid metabolism can profoundly affect immune cell function; two key amino acids in this respect are arginine and tryptophan. IDO metabolizes the essential amino acid L-tryptophan and arginase metabolizes arginine. High levels of either enzyme are associated with functional inhibition of T cells and other cell populations, such as DC. IDO overexpression is observed in a variety of human cancers and can be an independent adverse prognostic factor.Citation100 IDO can also be induced in DC and macrophages in the TME by Treg cells. A specific small-molecule inhibitor of IDO, 1-methyl-tryptophan and a similar inhibitor for arginase, N-Methylarginine, are being studied preclinically and clinically. Sharma et al demonstrated that inhibition of IDO reprograms Treg toward Th17 in situ and increased CD8+ CTL.Citation41 With this rationale, a combination of IDO-inhibitor drugs plus chemotherapy and perhaps immunotherapy could help block the reestablishment of suppressive activity by IDO in the tumor bed. Indoximod (1-methyl-D-tryptophan inhibitor) is currently being tested in refractory solid tumors including breast, lung, melanoma, and pancreatic malignancies (Phase I and II clinical trials). Alternatively, a study employing an IDO peptide vaccination in NSCLC patients was completed in a Phase I trial and results remain to be published (NCT01219348Citation101). Once again, these approaches are expected to be beneficial only in cancers in which Th17 detection is associated with a proper antitumor response and increased survival.
Conclusion
The therapeutic strategies targeting IL-17 in cancer remain in their infancy, but a plethora of drugs, mainly developed to block IL-17/Th17 in inflammatory disorders, are already in place for clinical development.Citation84,Citation92 However, mixed results of clinical trials aiming at inhibiting the Th17/IL-17 pathway in inflammatory diseases, predict that cancer patients should be carefully selected for IL-17-targeted therapy. The complexity of targeting Th17 for therapeutic purpose is indeed illustrated by the lack of improvement of Crohn’s disease when blocking IL-17, which even led to deterioration of the disease in some clinical trials. It appears that epigenetic and transcriptional modifications dictated by the TME and specific to each cancer may account for the functional plasticity of infiltrating Th17, making it difficult to predict the role of this cell subset in tumor progression (protumor versus antitumor). Therefore, before targeting the IL-17 pathway to treat cancer, a better understanding of the mechanisms involved in IL-17 bioactivities during tumor development is required for each type of cancer.
Accordingly, the prognostic value of Th17 in cancer is highly heterogeneous and must be integrated to the immune “contexture.”Citation12 A stratified selection of the patients based on the definition of multiple biomarkers and bioassays would help to improve clinical prognosis and personalize therapeutic decisions. Meanwhile, several recent studies reported that a polymorphism in Il17, but not Il17f, genes may influence the susceptibility in cancer, including cervical,Citation102 breast,Citation103 and gastric.Citation104,Citation105 Though yet limited, such genetic link between IL-17 and cancers may allow future routine testing to better evaluate the risk of cancer and the therapeutic response.
Disclosure
The authors report no conflicts of interest in this work.
References
- HanahanDWeinbergRAHallmarks of cancer: The next generationCell2011144564667421376230
- PardollDMCancer vaccinesNat Med19984Suppl 55255319585204
- DranoffGImmunotherapy at large: Balancing tumor immunity and inflammatory pathologyNat Med20131991100110124013749
- RosenbergSAYangJCRestifoNPCancer immunotherapy: Moving beyond current vaccinesNat Med200410990991515340416
- van den BoornJGHartmannGTurning tumors into vaccines: co-opting the innate immune systemImmunity2013391273723890061
- MotzGTCoukosGDeciphering and reversing tumor immune suppressionImmunity2013391617323890064
- PardollDMSpinning molecular immunology into successful immunotherapyNat Rev Immunol20022422723812001994
- PardollDMThe blockade of immune checkpoints in cancer immunotherapyNat Rev Cancer201212425226422437870
- LipsonEJDrakeCGIpilimumab: An anti-CTLA-4 antibody for metastatic melanomaClin Cancer Res201117226958696221900389
- PardollDMImmunology beats cancer: A blueprint for successful translationNat Immunol201213121129113223160205
- TopalianSLHodiFSBrahmerJRSafety, activity, and immune correlates of anti-PD-1 antibody in cancerN Engl J Med2012366262443245422658127
- FridmanWHPagèsFSautès-FridmanCGalonJThe immune contexture in human tumours: Impact on clinical outcomeNat Rev Cancer201212429830622419253
- TosoliniMKirilovskyAMlecnikBClinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancerCancer Res20117141263127121303976
- HarringtonLEHattonRDManganPRInterleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineagesNat Immunol20056111123113216200070
- ParkHLiZYangXOA distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17Nat Immunol20056111133114116200068
- O’SheaJJPaulWEMechanisms underlying lineage commitment and plasticity of helper CD4+ T cellsScience201032759691098110220185720
- MuranskiPRestifoNPEssentials of Th17 cell commitment and plasticityBlood2013121132402241423325835
- YeJSuXHsuehECHuman tumor-infiltrating Th17 cells have the capacity to differentiate into IFN-gamma+ and FOXP3+ T cells with potent suppressive functionEur J Immunol201141493695121381020
- BeriouGCostantinoCMAshleyCWIL-17-producing human peripheral regulatory T cells retain suppressive functionBlood2009113184240424919171879
- McGeachyMJBak-JensenKSChenYTGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathologyNat Immunol20078121390139717994024
- GhoreschiKLaurenceAYangXPGeneration of pathogenic T(H)17 cells in the absence of TGF-beta signallingNature2010467731896797120962846
- LeeYKTurnerHMaynardCLLate developmental plasticity in the T helper 17 lineageImmunity20093019210719119024
- KirchbergerSRoystonDJBoulardOInnate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse modelJ Exp Med2013210591793123589566
- PetrellaTMTozerRBelangerKInterleukin-21 has activity in patients with metastatic melanoma: A phase II studyJ Clin Oncol201230273396340122915661
- TongZYangXOYanHA protective role by interleukin-17F in colon tumorigenesisPLoS One201274e3495922509371
- CuaDJTatoCMInnate IL-17-producing cells: The sentinels of the immune systemNat Rev Immunol201010747948920559326
- ChangSHDongCSignaling of interleukin-17 family cytokines in immunity and inflammationCell Signal20112371069107521130872
- MaloyKJPowrieFIntestinal homeostasis and its breakdown in inflammatory bowel diseaseNature2011474735129830621677746
- SuttonCELalorSJSweeneyCMBreretonCFLavelleECMillsKHInterleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunityImmunity200931233134119682929
- WangBLiLLiaoYMast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinomaCancer Immunol Immunother201362101575158523912243
- ZhuXMulcahyLAMohammedRAIL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell linesBreast Cancer Res2008106R9519014637
- LinAMRubinCJKhandpurRMast cells and neutrophils release IL-17 through extracellular trap formation in psoriasisJ Immunol2011187149050021606249
- MiossecPKornTKuchrooVKInterleukin-17 and type 17 helper T cellsN Engl J Med2009361988889819710487
- McGeachyMJChenYTatoCMThe interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivoNat Immunol200910331432419182808
- El-BehiMCiricBDaiHThe encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSFNat Immunol201112656857521516111
- BasuRHattonRDWeaverCTThe Th17 family: Flexibility follows functionImmunol Rev201325218910323405897
- QuintanaFJBassoASIglesiasAHControl of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptorNature20084537191657118362915
- HuberMBrüstleAReinhardKIRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotypeProc Natl Acad Sci U S A200810552208462085119088203
- YangXPGhoreschiKSteward-TharpSMOpposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5Nat Immunol201112324725421278738
- DangEVBarbiJYangHYControl of T(H)17/T(reg) balance by hypoxia-inducible factor 1Cell2011146577278421871655
- SharmaMDHouDYLiuYIndoleamine 2,3-dioxygenase controls conversion of Foxp3+ tregs to TH17-like cells in tumor-draining lymph nodesBlood2009113246102611119366986
- PandiyanPContiHRZhengLCD4(+)CD25(+)Foxp3(+) regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse candida albicans Th17 cell infection modelImmunity201134342243421435589
- HuberSGaglianiNEspluguesETh17 cells express interleukin-10 receptor and are controlled by Foxp3 and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent mannerImmunity201134455456521511184
- ChaudhryARudraDTreutingPCD4+ regulatory T cells control TH17 responses in a Stat3-dependent mannerScience2009326595598699119797626
- Godin-EthierJHanafiLAPiccirilloCALapointeRIndoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectivesClin Cancer Res201117226985699122068654
- KoenenHJSmeetsRLVinkPMvan RijssenEBootsAMJoostenIHuman CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cellsBlood200811262340235218617638
- MuranskiPBoniAAntonyPATumor-specific Th17-polarized cells eradicate large established melanomaBlood2008112236237318354038
- YenHRHarrisTJWadaSTc17 CD8 T cells: Functional plasticity and subset diversityJ Immunol2009183117161716819917680
- MeltonACBailey-BucktroutSLTravisMAFifeBTBluestoneJASheppardDExpression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in miceJ Clin Invest2010120124436444421099117
- KryczekIBanerjeeMChengPPhenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environmentsBlood200911461141114919470694
- ZielinskiCEMeleFAschenbrennerDPathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1βNature2012484739551451822466287
- GrivennikovSIWangKMucidaDAdenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growthNature2012491742325425823034650
- Martin-OrozcoNMuranskiPChungYT helper 17 cells promote cytotoxic T cell activation in tumor immunityImmunity200931578779819879162
- WangLYiTZhangWPardollDMYuHIL-17 enhances tumor development in carcinogen-induced skin cancerCancer Res20107024101121012021159633
- KryczekIWeiSSzeligaWVatanLZouWEndogenous IL-17 contributes to reduced tumor growth and metastasisBlood2009114235735919289853
- BenchetritFCireeAVivesVInterleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanismBlood20029962114212111877287
- NumasakiMFukushiJOnoMInterleukin-17 promotes angiogenesis and tumor growthBlood200310172620262712411307
- TartourEFossiezFJoyeuxIInterleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude miceCancer Res199959153698370410446984
- YangXOChangSHParkHRegulation of inflammatory responses by IL-17FJ Exp Med200820551063107518411338
- WangLYiTKortylewskiMPardollDMZengDYuHIL-17 can promote tumor growth through an IL-6-Stat3 signaling pathwayJ Exp Med200920671457146419564351
- NumasakiMWatanabeMSuzukiTIL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesisJ Immunol200517596177618916237115
- HeDLiHYusufNIL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cellsJ Immunol201018452281228820118280
- ChungASWuXZhuangGAn interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapyNat Med20131991114112323913124
- CharlesKAKulbeHSoperRThe tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humansJ Clin Invest2009119103011302319741298
- LuLPanKZhengHXIL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survivalJ Immunother201336845145823994890
- WakitaDSumidaKIwakuraYTumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesisEur J Immunol20104071927193720397212
- BergerHVégranFChikhMSOCS3 transactivation by PPARγ prevents IL-17-driven cancer growthCancer Res201373123578359023619236
- WuSRheeKJAlbesianoEA human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responsesNat Med20091591016102219701202
- Acosta-RodriguezEVNapolitaniGLanzavecchiaASallustoFInterleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cellsNat Immunol20078994294917676045
- KatoTFurumotoHOguraTExpression of IL-17 mRNA in ovarian cancerBiochem Biophys Res Commun2001282373573811401524
- YamadaYSaitoHIkeguchiMPrevalence and clinical relevance of Th17 cells in patients with gastric cancerJ Surg Res2012178268569122940035
- ChenJGXiaJCLiangXTIntratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patientsInt J Biol Sci201171536021234303
- LvLPanKLiXDThe accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinomaPLoS One201163e1821921483813
- YeZJZhouQGuYYGeneration and differentiation of IL-17-producing CD4+ T cells in malignant pleural effusionJ Immunol2010185106348635420952674
- LiuJDuanYChengXIL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinomaBiochem Biophys Res Commun2011407234835421396350
- LeeJWWangPKattahMGDifferential regulation of chemokines by IL-17 in colonic epithelial cellsJ Immunol200818196536654518941244
- ArenbergDAWhiteESBurdickMDStromSRStrieterRMImproved survival in tumor-bearing SCID mice treated with interferon-gamma-inducible protein 10 (IP-10/CXCL10)Cancer Immunol Immunother2001501053353811776375
- MlecnikBTosoliniMCharoentongPBiomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancerGastroenterology201013841429144019909745
- ZhaoQXiaoXWuYInterleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patientsEur J Immunol20114182314232221674477
- ZhaoFHoechstBGamrekelashviliJHuman CCR4+ CCR6+ Th17 cells suppress autologous CD8+ T cell responsesJ Immunol2012188126055606222615204
- ZhangJPYanJXuJIncreased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patientsJ Hepatol200950598098919329213
- VykhovanetsEVMaclennanGTVykhovanetsOVGuptaSIL-17 expression by macrophages is associated with proliferative inflammatory atrophy lesions in prostate cancer patientsInt J Clin Exp Pathol20114655256521904631
- GalonJCostesASanchez-CaboFType, density, and location of immune cells within human colorectal tumors predict clinical outcomeScience200631357951960196417008531
- MiossecPKollsJKTargeting IL-17 and TH17 cells in chronic inflammationNat Rev Drug Discov2012111076377623023676
- NeurathMFNew targets for mucosal healing and therapy in inflammatory bowel diseasesMucosal Immunol Epub1022013
- JonesSASuttonCECuaDMillsKHTherapeutic potential of targeting IL-17Nat Immunol201213111022102523080193
- LangowskiJLZhangXWuLIL-23 promotes tumour incidence and growthNature2006442710146146516688182
- OverwijkWWde VisserKETirionFHImmunological and antitumor effects of IL-23 as a cancer vaccine adjuvantJ Immunol200617695213522216621986
- LangleyRGPappKGottliebABSafety results from a pooled analysis of randomized, controlled phase II and III clinical trials and interim data from an open-label extension trial of the interleukin-12/23 monoclonal antibody, briakinumab, in moderate to severe psoriasisJ Eur Acad Dermatol Venereol201227101252126123157612
- SCRI Development Innovations, LLCLBH589 Treatment for Refractory Clear Cell Renal Carcinoma Available from: http://clinicaltrials.gov/show/NCT00550277. NLM identifier: NCT00550277Accessed January 7, 2014
- New York University School of MedicineSTAT3 in T Cells: At The Crossroads of Inflammation and Cancer Available from: http://clinicaltrials.gov/show/NCT01663571. NLM identifier: NCT01663571 Accessed January 7, 2014
- FitzpatrickLRInhibition of IL-17 as a pharmacological approach for IBDInt Rev Immunol2013325–654455523886112
- SoltLAKumarNNuhantPSuppression of TH17 differentiation and autoimmunity by a synthetic ROR ligandNature2011472734449149421499262
- 4SC AGSC12267 (4SC-101) for Treatment of Patients With Inflammatory Bowel Disease (ENTRANCE) Available from: http://clinicaltrials.gov/show/NCT00820365. NLM identifier: NCT00820365Accessed January 7, 2014
- 4SC AGSC12267 (4SC-101) in Combination With Methotrexate in Patients With Rheumatoid Arthritis (COMPONENT) Available from: http://clinicaltrials.gov/show/NCT01010581. NLM identifier: NCT01010581Accessed January 7, 2014
- FitzpatrickLRDemlLHofmannC4SC-101, a novel immunosuppressive drug, inhibits IL-17 and attenuates colitis in two murine models of inflammatory bowel diseaseInflamm Bowel Dis201016101763177720310011
- HinrichsCSKaiserAPaulosCMType 17 CD8+ T cells display enhanced antitumor immunityBlood2009114359659919471017
- ZouWRestifoNPT(H)17 cells in tumour immunity and immunotherapyNat Rev Immunol201010424825620336152
- HinrichsCSGattinoniLRestifoNPProgramming CD8+ T cells for effective immunotherapyCurr Opin Immunol200618336337016616471
- UyttenhoveCPilotteLThéateIEvidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenaseNat Med20039101269127414502282
- Inge Marie SvaneIDO Peptid Vaccination for Stage III-IV Non Small-cell Lung Cancer Patients. (IDOvaccine) Available from: http://clinicaltrials.gov/show/NCT01219348. NLM identifier: NCT01219348Accessed January 7, 2014
- QuanYZhouBWangYAssociation between IL17 polymorphisms and risk of cervical cancer in chinese womenClin Dev Immunol2012201225829323049595
- WangLJiangYZhangYAssociation analysis of IL-17A and IL-17F polymorphisms in chinese han women with breast cancerPLoS One201273e3440022461912
- RafieiAHosseiniVJanbabaiGPolymorphism in the interleukin-17A promoter contributes to gastric cancerWorld J Gastroenterol201319345693569924039363
- ArisawaTTaharaTTsutsumiMShibataTInfluence of IL17A polymorphisms on the aberrant methylation of DAPK and CDH1 in non-cancerous gastric mucosaBMC Med Genet2012135922827846
- DroeserRAGüthUEppenberger-CastoriSHigh IL-17-positive tumor immune cell infiltration is indicative for chemosensitivity of ovarian carcinomaJ Cancer Res Clin Oncol201313981295130223624523
- ChenWCLaiYHChenHYGuoHRSuIJChenHHInterleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factorHistopathology201363222523323738752
- BenevidesLCardosoCRTiezziDGMaranaHRAndradeJMSilvaJSEnrichment of regulatory T cells in invasive breast tumor correlates with the upregulation of IL-17A expression and invasiveness of the tumorEur J Immunol20134361518152823529839
- ChenXWanJLiuJIncreased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patientsLung Cancer201069334835420022135
- LiQHanYFeiGGuoZRenTLiuZIL-17 promoted metastasis of non-small-cell lung cancer cellsImmunol Lett2012148214415023089548
- ZhangYLLiJMoHYDifferent subsets of tumor infiltrating lymphocytes correlate with NPC progression in different waysMol Cancer20109420064222
- LiaoRSunJWuHHigh expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinomaJ Exp Clin Cancer Res201332323305119
