Abstract
Developed countries have experienced a steady increase in atopic disease and disorders of immune dysregulation since the 1980s. This increase parallels a decrease in infectious diseases within the same time period, while developing countries seem to exhibit the opposite effect, with less immune dysregulation and a higher prevalence of infectious disease. The “hygiene hypothesis”, proposed by Strachan in 1989, aimed to explain this peculiar generational rise in immune dysregulation. However, research over the past 10 years provides evidence connecting the commensal and symbiotic microbes (intestinal microbiota) and parasitic helminths with immune development, expanding the hygiene hypothesis into the “microflora” and “old friends” hypotheses, respectively. There is evidence that parasitic helminths and commensal microbial organisms co-evolved with the human immune system and that these organisms are vital in promoting normal immune development. Current research supports the potential for manipulation of the bacterial intestinal microbiota to treat and even prevent immune dysregulation in the form of atopic disease and other immune-mediated disorders (namely inflammatory bowel disease and type 1 diabetes). Both human and animal model research are crucial in understanding the mechanistic links between these intestinal microbes and helminth parasites, and the human immune system. Pro-, pre-, and synbiotic, as well as treatment with live helminth and excretory/secretory helminth product therapies, are all potential therapeutic options for the treatment and prevention of these diseases. In the future, therapeutics aimed at decreasing the prevalence of inflammatory bowel disease, type 1 diabetes, and atopic disorders will likely involve personalized microbiota and/or helminth treatments used early in life.
Introduction
The Millennial generation (born 1980–1999) displays a marked increase in prevalence of atopic diseases (asthma, anaphylaxis, allergic rhinitis, food allergy, and atopic dermatitis [AD]) and immune-mediated disorders (including type 1 diabetes [T1D], and inflammatory bowel disease [IBD]), which have been steadily increasing in developed countries since the 1980s.Citation1–Citation3 These disorders comprise a unique sector within immune dysregulation characterized by an irrational immune cell response to a foreign (or in the case of autoimmunity, a self) antigen which would, under normal circumstances, not occur. The short developmental timeframe of these diseases (from the 1980s onward, roughly within one generation) decreases the likelihood that a changing genetic component is significantly involved. Hence, researchers are assessing the potential effects of environmental factors, such as diet and antibiotic exposure.Citation4 Furthermore, the increase in immune disorders and atopic diseases parallels a decrease in prevalence of infectious diseases over the same time period, which can be attributed to increased vaccine and antibiotic treatments, and improved sanitation standards.Citation4 An in-depth look at the effects of these “hygienic” environmental factors suggests that lack of exposure to infectious agents may be the culprit for the increase in immune-mediated and atopic disease prevalence, a concept most commonly referred to today as the “hygiene hypothesis”. This review aims to provide readers with the historical and current perspectives of the hygiene hypothesis and to elaborate on the modern scientific and medical applications of this theory. We also discuss the increasing evidence connecting the hygiene hypothesis to the development of atopic disease and immune-mediated disorders, in addition to discussing future therapies capitalizing on this knowledge.
A history of “hygiene” in immune modulation
One of the first observations relating infectious agents and immune dysregulation occurred in Western Nigeria, where Greenwood noted the low incidence of rheumatoid arthritis and deduced that this low incidence may be attributed to immunological disturbance resulting from frequent exposure to malaria ().Citation5 Greenwood et al also observed suppressed spontaneous autoimmune disease, characterized by delayed Coombs test positivity and reticulocytosis in mice infected with Plasmodium berghei (a causative agent of rodent malaria).Citation6 In the late 1970s, a discrepancy between urbanized and rural environments emerged when Gerrard et al observed a lower prevalence of allergy in indigenous populations in Northern Canada compared to urban Caucasian populations.Citation7
Figure 1 Timeline displaying key findings leading up to the proposal of the “hygiene hypothesis”, proposal of the “old friends” and “microflora” hypotheses, and key microbiological and immunological findings in support of these theories.
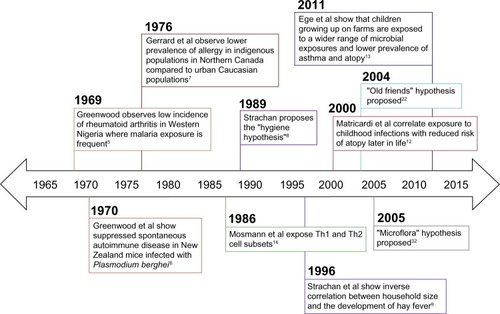
In 1989, Strachan proposed the hygiene hypothesis of allergic disease after observing that hay fever was less common among children with older siblings.Citation8 He reasoned that children growing up in larger families may experience increased exposure to microbes in early childhood due to inevitable unhygienic contact with older siblings or prenatal exposure from the mother infected by similar unhygienic contact.Citation8 Strachan proposed that this increased microbial exposure in early life could protect children from developing immune hypersensitivities later in life.Citation8 Strachan et al supported this theory by assessing family history, medical records, and allergy skin prick test results in a cohort of 11,765 children and found that household size was inversely correlated with the development of hay fever.Citation9 Additional epidemiological studies supporting the hygiene hypothesis associate a reduction in allergen sensitization with pet exposure, daycare attendance, and an increased number of siblings.Citation10,Citation11 Early childhood infections have also been associated with decreased atopy in children. A retrospective case-control study showed that atopic patients exhibited a lower prevalence of Toxoplasma gondii, Helicobacter pylori, and hepatitis A when compared to non-atopic controls.Citation12 More recently, single-strand polymorphism analysis and culture techniques were used to identify microbial exposures among two cohort studies of European children.Citation13 In both cohort studies, researchers found that children growing up on farms in Central Europe encountered a wider range of microbial exposures and had a lower prevalence of asthma and atopy than the reference group.Citation13 A closer look at the immunological mechanisms behind Strachan’s hygiene hypothesis of allergic disease will enhance the connection between early life infectious exposures and the development of immune tolerance.
Immunological support for the hygiene hypothesis
In 1986, just prior to Strachan’s proposal of the hygiene hypothesis, Mosmann et al described the T-helper (Th)1 and Th2 cell subtypes, providing an immunological basis for this otherwise observational theory.Citation14 They discovered that fully differentiated murine CD4+ T-cells secreted two separate cytokine profiles (Th1: IFN-γ; Th2: IL-4) and that the different cytokines produced two different inflammatory responses.Citation14 Th2 cells play a primary role in the allergen sensitization process.Citation15 Infection with viruses and intracellular bacteria generally stimulates Th1 immune responses, which suppress Th2 cytokine activity through the induction of IFN-γ.Citation16,Citation17 Consequently, the concept of a Th1 versus Th2 balance arose whereby a Th1-dominated immune phenotype (brought on by early life microbial exposures) was thought to inhibit atopic immunopathology. Research related to helminth parasites stimulated the need for further explanation beyond this binary view, as these organisms paradoxically induce Th2 responses while suppressing allergic reactivity.Citation18 T-cell plasticity and additional T-cell phenotypes (eg, Th17, Th9, and T regulatory [Treg] cells) have more recently been implicated in the control of hypersensitivity disorders.Citation19,Citation20 Additionally, many innate cytokines (eg, IL-25, IL-33, and thymic stromal lymphopoietin) and cell types (eg, eosinophils, basophils, mast cells, and epithelial cells) also play significant roles in hypersensitivity disease.Citation21 It is now understood that the process of allergen presentation and consequent initiation of the allergic response involves both the innate and adaptive branches of the immune system. Thus, the immunological foundation of the hygiene hypothesis has been modified to consider the balance between many adaptive and innate immune cell populations. Further, extending the hygiene hypothesis to account for the role of various parasites (ie, intestinal helminths) and microbiota compositional shifts provides insight into how early life environmental exposures shape the human immune system. These extensions are known as the “old friends” and “microflora” hypotheses, respectively.Citation22,Citation23
The old friends hypothesis: parasitic helminths
The old friends hypothesis, proposed by Rook et al, notes the co-evolution of microorganisms and macroorganisms, such as parasitic helminths, with the development of the human immune system.Citation22 Similar to the hygiene hypothesis, it suggests that these organisms are required for normal immune system development.Citation22,Citation24 For example, a study in Gabon found that school children diagnosed with schistosomiasis, caused by infection with helminth parasites from the Schistosoma genus, exhibited lower levels of allergen reactivity than their uninfected classmates.Citation25 Since then, additional studies have highlighted this seemingly protective effect of helminths in many mouse models of allergic diseases.Citation26–Citation28 A live Heligmosomoides polygyrus (H. polygyrus; a murine helminth parasite) infection reduces lung cellular influx, eosinophilia, allergen recall responses, bronchial hyperreactivity, and histopathology in ovalbumin (OVA)- and house dust mite (HDM)-driven mouse models of asthma.Citation26,Citation27 Additionally, Schistosoma mansoni infection has been shown to be protective in an experimental mouse model of fatal anaphylaxis, probably due to the induction of a regulatory IL-10-producing B cell population.Citation28 There is also experimental animal model evidence suggesting the ability of helminths to ameliorate symptoms of T1D and colitis (). Non-obese diabetic (NOD) mice spontaneously develop T1D, which is significantly inhibited when they are infected with H. polygyrus or the filarial nematode Litomosoides sigmodontis.Citation29–Citation32 Helminth infection has also been shown to reduce inflammation in murine models of colitis.Citation33 Studies such as these support live helminth infection as a potential therapy to combat hypersensitivity and other immune disorders; however, referring back to Strachan’s original hygiene hypothesis, the question of whether live helminth infection in early life is an effective treatment to protect against the development of these disorders is still unclear. Future therapeutics to treat immune dysregulation may involve the excretory/secretory (ES) products of these parasites and/or the intestinal microbiota ( and ).
Table 1 Helminth-based therapeutic studies
Table 2 Microbiota-based therapeutic studies
The microflora hypothesis
The microflora hypothesis is another modern extension of the hygiene hypothesis, which suggests that early life perturbations (driven by factors such as antibiotic use, infection, or diet) to the bacteria residing in the human intestine (the intestinal microbiota) disrupt the normal microbiota-mediated mechanisms promoting immunological tolerance and consequently bias the immune system toward a state that promotes hypersensitivity disorders.Citation23 Current research focuses on the mechanisms by which the intestinal microbiota influences immune system development and homeostasis, and potentially confers protection against immune dysregulation.Citation35–Citation50
A mutualistic bond
The human intestine is a densely populated zone in the body harboring a diverse microbial community of 500–1,000 different bacterial species among other microbes such as archaea, eukarya, and viruses.Citation34 The most striking illustration of the importance of the intestinal microbiota for mammalian immune development comes from studies conducted in germ-free (GF) mice, in which the lack of a microbiota results in reduced Peyer’s patches, smaller germinal centers and fewer plasma cells, and increased susceptibility to pathogen invasion when compared to conventionally raised mice.Citation35–Citation38 Although GF murine models are valuable in mechanistic studies, they do have many caveats.Citation39 To fully elucidate the underlying mechanisms driving the relationship of the gut microbiota with atopic disease development, many different murine models, including GF, gnotobiotic, and antibiotic-treated models, along with models supplemented with specific bacterial species, should be used. In addition, murine systems with a reconstituted human immune system would be even more valuable.
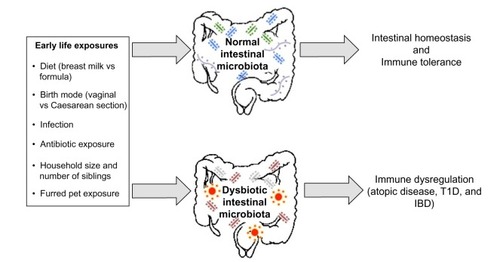
Specific bacterial species within the microbiota have been shown to induce expression of antimicrobial peptides (eg, Bacteroides thetaiotaomicron induction of regenerating islet-derived 3γ expression by Paneth cells) and mucin production, which ultimately confers protection against pathogen invasion, and combined with regular stimulation of pattern recognition receptors, contributes to intestinal homeostasis.Citation40–Citation42 The presence of the microbiota can stimulate CD4+ T-cell proliferation, Th17 cell differentiation through the induction of IL-1β, and accumulation of colonic Tregs.Citation43–Citation45 The intestinal microbiota also metabolizes food components that are indigestible by mammalian enzymes, such as human milk oligosaccharides (HMOs) and dietary fiber.Citation46,Citation47 This produces short-chain fatty acids (SCFAs), which are essential energy sources for many host tissues and prominent immune modulators.Citation48–Citation50 There are many factors that likely contribute to the development of immune dysregulation: perturbations to the composition of the intestinal microbiota, caused by environmental factors such as antibiotic exposure, birth mode, or diet, are one potential explanation linking early life hygiene with the development of atopic and immune-mediated disorders ().
Figure 2 A depiction of the early life environmental exposures differentially associated with promoting a healthy intestinal microbiota, which results in intestinal homeostasis and immune tolerance, and a dysbiotic (unhealthy) intestinal microbiota, which may induce the development of immune dysregulation.
The intestinal microbiota in atopic disease: human studies
A longitudinal study comparing the early life intestinal microbiota compositions of school-age asthmatic and non-asthmatic children showed that significant decreases in overall gut microbial diversity at 1 week and 1 month of age were correlated with asthma development at school age.Citation51 Additionally, a recent characterization of the gut microbiota of 166 Canadian infants revealed an increased Enterobacteriaceae/Bacteroidaceae ratio in children sensitized to food allergens at 3 months and 1 year of age compared to non-sensitized children.Citation52 Also, lower gut microbial richness was observed at 3 months of age only.Citation52 Studies such as these suggest that therapeutic microbial intervention early in human life may be favorable, and highlight the need for animal studies in which experimentation to confirm causality is possible.
Many human studies lend support for the hygiene and microflora hypotheses by assessing the impact of early life environmental factors known to disturb the intestinal microbiota on atopic disease development later in life. For example, antibiotic usage in the first 2 years of life has been associated with the development of asthma at 7.5 years of age in a dose-dependent manner.Citation53 Additionally, antibiotic usage was reported to precede the manifestation of wheeze in the first 2 years of life in a questionnaire-based analysis of the KOALA (acronym in Dutch for “Child, parents and health: lifestyle and genetic constitution”) Birth Cohort Study in the Netherlands.Citation54 Birth by Caesarean section was associated with lower total microbial diversity, delayed colonization with Bacteroidetes, and decreased Th1 responses in the first 2 years of life.Citation55 Breastfeeding promotes colonization with commensal microbes such as Bifidobacteria spp. and provides the intestinal microbiota with necessary nutrients in the form of HMOs.Citation46,Citation56 Specific HMOs, short-chain galactooligosaccharides (GOSs) and long-chain fructooligosaccharides (FOSs), administered in the first 6 months of life have been shown to reduce the cumulative incidences of AD, recurrent wheezing, and allergic urticaria.Citation57 In line with Strachan’s original proposal, one study found that an increased number of older siblings was associated with decreased colonization with Clostridium difficile and Clostridium cluster 1, and a decreased risk of developing AD.Citation58 Correlative human studies such as these shed light on the environmental factors that may be associated with atopic disease through manipulation of the intestinal microbiota; however, research regarding factors such as antibiotic exposure, breastfeeding, and birth mode remains controversial, and there are studies that suggest these factors have little or no effect on atopic disease development.Citation59–Citation64 Additional longitudinal human studies are necessary to determine which early life factors are most influential in promoting the intestinal dysbiosis associated with the development of immune hypersensitivities, and animal model research is a crucial complementary approach to elucidate the mechanisms behind these associations.
The intestinal microbiota in atopic disease: mouse models
Murine model studies mechanistically support a link between the intestinal microbiota and atopic disorders through the experimental manipulation of microbiota compositions. In an OVA-driven model of asthma, Forsythe et al show that oral supplementation with live Lactobacillus reuteri reduced airway hyperresponsiveness as well as levels of TNFalpha, monocyte chemotactic protein 1, IL-5, and IL-13 in the bronchoalveolar lavage fluid (BALF), while treatment with Lactobacillus salivarius had no effect.Citation65 Intranasal supplementation of mice, polysensitized to birch and grass pollen allergens, with Bifidobacterium longum and Lactobacillus paracasei at the time of sensitization resulted in reduced IgE-dependent basophil degranulation in response to allergen challenge.Citation66 Only B. longum displayed protective effects when mice were supplemented prior to allergen sensitization.Citation66 Additionally, oral supplementation of mice with B. longum protected against airway inflammation, increased Peyer’s patch and splenic Tregs, and blocked serum IgE induction in OVA-sensitized animals.Citation67
More recent research focuses on the earliest time point at which gut microbial intervention must occur to prevent the onset of hypersensitivity disease. In an OVA-driven model of allergic inflammation, neonatal (but not adult) exposure of previously GF mice to a conventional microbiota reduced the severity of allergic inflammation characterized by decreased accumulation of invariant natural killer (NK) T-cells to the lung and reduced serum IgE levels and eosinophil frequencies in the BALF.Citation68 Arnold et al show in OVA- and HDM-driven mouse models of allergic inflammation that oral infection of neonatal mice with H. pylori prior to OVA or HDM challenge resulted in the significant reduction of eosinophils in the BALF, and a decrease in IL-5 and IL-13 cytokine levels when compared to uninfected mice and infected adult mice.Citation69 Russell et al found that perinatal vancomycin treatment of OVA-challenged mice alters gut microbial composition and exacerbates asthma-related immune responses, which may be driven by increased serum IgE levels and reduced Treg populations.Citation70 Interestingly, perinatal treatment with streptomycin did not result in exacerbated disease after OVA challenge, but perinatally streptomycin-treated mice showed exaggerated lung inflammation when compared to untreated or vancomycin-treated mice in a Th1/Th17-driven model of hypersensitivity pneumonitis.Citation70,Citation71 This highlights the ability of altered microbiota compositions to differentially control disease severity depending on the immunological basis of the disease.Citation71 Additional studies including human subjects and supporting mechanistic animal models are necessary to provide a holistic view of the role of the intestinal microbiota in atopic disease. Currently, there is also increasing evidence supporting a role of the intestinal microbiota and early life environmental exposures in other immune-mediated disorders.Citation72 For the purpose of this review, we focus on IBD and T1D.
The hygiene and microflora hypotheses and immune-mediated disorders
IBD
IBD is an inflammatory disorder of the gastrointestinal (GI) tract encompassing Crohn’s disease (CD) and ulcerative colitis (UC), both of which are highest in prevalence in North America and Europe.Citation73 The presence of intestinal bacteria appears to be required for the development of experimental colitis, while the composition influences the severity of IBD. GF IL-10-deficient mice show no evidence of experimental colitis, while IL-10-deficient mice housed under specific pathogen-free (SPF) conditions spontaneously develop the disease.Citation74 Additionally, antibiotics have been shown to attenuate the symptoms of experimental colitis.Citation75–Citation77 Exposure of SPF IL-10-deficient mice to antibiotics displays differential and localized roles of specific bacteria in mediating experimental colitis.Citation77 For example, treatment of SPF IL-10-deficient mice with vancomycin–imipenem and metronidazole eliminated anaerobic bacteria and reduced colonic injury, while ciprofloxacin and vancomycin–imipenem decreased cecal inflammation and reduced the prevalence of Escherichia coli and Enterococcus faecalis.Citation77
Some human studies suggest that early life antibiotic exposure is associated with IBD.Citation78–Citation80 This discrepancy is likely because antibiotics in murine IBD experiments are typically given as treatment after disease onset, whereas human studies are often retrospective and assess the effects of antibiotic exposure prior to disease onset. In a nested case-control study, children diagnosed with IBD at approximately 8 years of age were 2.9 times more likely to have received antibiotics in the first year of life.Citation80 Additionally, antibiotic exposure in the first 3 months of life was associated with childhood CD.Citation79 Conversely, antibiotic combination therapy has been shown to be effective in treating UC in humans.Citation81 Thus, effects after antibiotic exposure in humans are likely disease specific and/or dependent on when antibiotics are administered (ie, before or after disease onset).
Diet may also play an important role in IBD. Maternal secretory IgA (a component of breast milk) has been shown to alter the intestinal microbiota composition and the expression of genes associated with intestinal inflammation.Citation82 Additionally, a systematic review negatively correlated breast milk exposure with the development of early onset IBD in humans, suggesting a protective effect of breastfeeding on IBD development.Citation83
Altogether, these results suggest that IBD is driven by the composition of the intestinal microbiota, which is strongly influenced by early life environmental factors. Early life diet (breastfeeding) is likely protective against IBD development, while effects of antibiotic exposure are more complicated. If antibiotics are given in early life, they may result in an intestinal microbiota that promotes IBD development.Citation79,Citation80 However, after disease onset, antibiotics alleviate disease severity by shifting the prevalence of specific microbes that may be promoting the disease.Citation75–Citation77,Citation79,Citation81 Regardless, factors related to early life hygiene are involved in IBD development, and there is also evidence that the hygiene and microflora hypotheses are applicable to immune-mediated disorders not associated with the GI tract, such as T1D.Citation84–Citation96
T1D
The prevalence of childhood T1D, an autoimmune disorder resulting from T-cell mediated destruction of beta cells in the pancreas, is steadily increasing worldwide, and developed countries such as Canada and the UK exhibit the highest incidences of the disease.Citation84,Citation85 Epidemiological evidence supports a link between environmental factors associated with the hygiene hypothesis and the onset of T1D. Having older siblings is negatively correlated with childhood onset T1D, suggesting a protective effect.Citation86 Furred pet exposure seems to also play a role, as one study found in a birth cohort of 3,000 children: children exposed to an indoor dog were less likely to develop T1D than unexposed children.Citation87 Breastfeeding has been associated with protection from T1D, and children born by Caesarean section exhibit a higher risk of T1D than children born vaginally.Citation88–Citation90
Lending support for the microflora hypothesis, a recent study compared the gut microbial compositions of children with T1D and healthy children and concluded that children with T1D showed a significant increase in Bacteroides spp., which was later reduced to that of controls after insulin treatment for 2 years.Citation91 Oral administration of Lactobacillus johnsonii isolated from bio-breeding (BB) diabetes-resistant rats was shown to delay the onset of T1D in BB-diabetes prone rats.Citation92 Additionally, MyD88-deficient NOD mice are protected from disease onset in SPF environments, and segmented filamentous bacteria have been reported to protect female NOD mice from disease development.Citation93,Citation94 Additionally, antibiotic therapy in mice has been shown to protect against virus-induced T1D through the alteration of intestinal microbiota composition.Citation95 However, in humans the contribution of antibiotics to T1D development is currently unclear, as a population-based human cohort study found no association between T1D and antibiotic exposure in the first 8 years of life.Citation96 Thus, similar to atopic disease and IBD, early life factors common to industrialized countries such as birth mode, diet, and antibiotic exposure seem to play a role in T1D development. However, additional mechanistic research is needed before significant conclusions regarding the gut microbial composition and immunological consequences can be made. The use of appropriate animal models will be critical in continuing to determine whether the relationship between microbiota composition and immune dysregulation is causal, or an effect of a dysregulated immune environment. Regardless, research related to the hygiene, old friends, and microflora hypotheses supports early life intervention as the primary therapeutic component for averting immune dysregulation in the form of atopic and immune-mediated disorders.
Future therapeutics
Future therapeutic options to prevent the development of immune dysregulation will likely involve the millions of micro- and macroorganisms living commensally or symbiotically (microbiota), or even parasitically (helminths) in the human body. In this section, we discuss potential helminth-based () and microbiota-based therapies () in the prevention of these disorders.
Helminth-based therapies
Clinical trials to date have focused on the use of live helminth infection as an ameliorative, rather than preventative, strategy due to the potential for diminished vaccine responsiveness in mice and humans infected with helminths early in life.Citation97–Citation101 The majority of early phase clinical trials to determine the safety and efficacy of live helminth infection have been conducted in CD and UC patients.Citation102 Initial clinical trials using ova from the porcine whipworm, Trichuris suis, or larvae from the human hookworm, Necator americanus, have not yet found any cause for major safety concerns in IBD or asthma patients.Citation103–Citation107 T. suis ova administration seemed to reduce intestinal inflammation in a small number of CD and UC patients, and administration of N. americanus larvae to CD patients resulted in a nonsignificant improvement in intestinal inflammation scores.Citation104–Citation108 These initial clinical trials were promising, although follow-up studies with the inclusion of placebo control groups show mixed results.Citation102,Citation107,Citation108
Live helminth parasites release a suite of ES immunomodulatory products that likely mediate many of their suppressive effects in models of allergic disease and experimental colitis.Citation109 In mice exposed to both OVA- and Alternaria alternata-driven asthma models, administration of ES material from the murine intestinal nematode, H. polygyrus (HES), was sufficient to suppress lung eosinophilia and histopathology in response to antigen challenge.Citation110,Citation111 HES appears to suppress lung inflammation when given at the point of antigen sensitization and antigen challenge, making it a promising therapeutic candidate.Citation110 Soluble products from several different helminth parasites have also been shown to reduce measures of disease severity in murine models of trinitrobenzene sulfonic acid-induced and dextran sulfate sodium-induced colitis and T1D.Citation112–Citation116 Administration of helminth ES products rather than live helminths has not yet begun in human patients, but evidence from murine models suggests that this is a promising approach for future clinical trials.
Researchers are beginning to elucidate the mechanisms that mediate the potent immunoregulatory effects of these helminth products. ES products from N. americanus mediate the rapid proteolysis of eotaxin, an eosinophil chemoattractant, and HES can stimulate the induction of Tregs through a TGF-β-dependent pathway.Citation117,Citation118 Whether the administration of helminth products modifies the composition of the intestinal microbiota is not yet reported. However, infection of mice with live helminth parasites results in a marked disruption of intestinal microbiota composition, suggesting that the immunosuppressive effects following helminth infection could be due to an indirect modulation of the microbiota.Citation119–Citation121 The relative contribution of the microbiota or helminth-secreted products in ameliorating immune dysregulation remains to be determined. If microbiota compositional shifts following helminth infection are shown to have a direct role in disease modulation, future probiotic administration to drive the microbiota composition toward that seen during helminth infection may be a novel therapeutic approach.
Microbiota-based therapies
Probiotics are live bacteria which, when administered, are beneficial to host health. Animal model research using probiotics shows their ability to ameliorate symptoms in atopic disease, IBD, and T1D.Citation66,Citation92,Citation119,Citation122–Citation125 Additionally, probiotic administration in humans has been shown to protect against allergic rhinitis, peanut allergy, AD, and UC.Citation124,Citation126–Citation129 However, research thus far reveals many gaps in probiotic therapy, likely due to individualized disease phenotypes that may or may not be linked to the specific microbial species tested.Citation130–Citation132 Consequently, prebiotic and synbiotic therapeutics are also being explored.
Prebiotics are chemicals or food components (eg, inulin, pectin, GOSs, and FOSs), which are indigestible by pancreatic and intestinal enzymes, but are important in the growth and proliferation of intestinal microbiota.Citation133 Prebiotic substances can induce the production of SCFAs by intestinal microbes, which have been shown to promote effector (Th1 and Th17) and anti-inflammatory IL-10-producing FoxP3+ and non-FoxP3+ T-cell differentiation.Citation48,Citation49 As such, they continue to be a promising microbe-based therapeutic option to modulate intestinal immune responses. Supplementation of mice with a mixture of short-chain GOS, long-chain GOS, and pectin-derived acidic oligosaccharides prior to OVA challenge suppressed airway inflammation and hyperresponsiveness compared to controls.Citation134 Additionally, Trompette et al show that a high-fiber diet (diet supplemented with 30% pectin) metabolized by the gut microbiota increases the concentrations of circulating SCFAs and decreases allergic inflammation in the lungs of an HDM-driven model of allergic inflammation.Citation47 In humans, prebiotic oligosaccharide formula supplementation in the first 6 months of life has been associated with decreased incidences of allergic manifestations until 2 years of age, supporting early life intervention in humans.Citation57 Additionally, prebiotics have been implicated in protection from IBD development. Human leukocyte antigen-B27 transgenic (HLA-B27, TG) rats supplemented with FOS and inulin prior to disease onset showed decreased intestinal inflammation compared to untreated rats; however, FOS-treated rats compared to inulin-treated rats showed less intestinal inflammation, suggesting FOS as a more effective prebiotic treatment for spontaneous colitis.Citation135 Conversely, FOS was not an effective treatment for CD, as patients receiving the treatment after 4 weeks exhibited higher GI symptoms compared to the placebo group, despite the reduced IL-6 and increased IL-10 production from lamina propria dendritic cells.Citation136
Synbiotic therapies involve supplementation with both pre- and probiotics. In a murine model for cow’s milk allergy, mice fed the synbiotic mixture (GOS, FOS, and Bifidobacterium breve M-16V) showed increased galectin-9 expression by intestinal epithelial cells, which correlated with reduced acute skin reaction and mast cell degranulation.Citation137 Similar results were measured in humans fed the synbiotic mixture, suggesting a mechanism by which this therapy may be effective in protecting against AD in humans.Citation137 Conversely, a clinical trial using a similar synbiotic mixture, Immunofortis®, found no difference in AD severity in the synbiotic group versus the placebo group.Citation138 However, this research group did later find in infants with AD that supplementation with this mixture for 12 weeks correlated with decreased prevalence of wheezing and asthma medication usage after 1 year.Citation139 Synbiotics are also potential therapeutics for IBD. In a controlled pilot trial involving 18 patients with active UC, short-term synbiotic therapy combining B. longum and inulin-oligofructose significantly reduced chronic inflammatory biomarkers of the disease, including decreased TNFα and IL-1α levels.Citation140
The effects of early life factors such as diet and antibiotic exposure discussed throughout this review suggest that the application of live helmiths/helminth ES products, and pro-, pre-, and synbiotics prior to disease onset may be key in averting disease development, because interventions occurring later in life or after disease onset may be ineffective after the neonatal immune developmental window has closed. The timing of this developmental window could be driven by epigenetic alterations to specific, microbially regulated factors, such as the CXCL16 gene described by Olszak et al.Citation68 In previously GF mice colonized neonatally with a conventional microbiota, the presence of a conventional microbiota decreased hypermethylation of CXCL16, which consequently decreased accumulation of invariant NK T-cells in the colon (this did not occur in previously GF mice colonized until they reached adulthood). This suggests that microbe-based therapeutics aimed at protecting against hyperinflammatory diseases are age-sensitive.Citation67 Additionally, the incongruity of current research highlights the need for future microbiota-based treatments that are constructed as individualized therapeutics specific to the disease phenotype and microbiota of the affected patient.
Conclusion
The progression of research since Strachan’s 1989 proposal of the hygiene hypothesis exemplifies the scientific method in health research, progressing from observational theory to experimental therapy. The hygiene hypothesis has been expanded today to include commensal and symbiotic intestinal microbes, which are profoundly involved in human immune development, and parasitic helminths, which are also strong therapeutic candidates to protect against immune dysregulation. More research addressing the early life “critical window” for microbiota intervention, currently being assessed in mice for hypersensitivity diseases, is needed if researchers hope to use these therapeutics to prevent immune dysregulation in humans.Citation68–Citation71 Children undergo large shifts in their intestinal microbiota compositions throughout the first few months of life; thus, it may be possible in the near future to shift the gut microbial composition using pro-, pre-, and synbiotics toward a microbiota that promotes immune tolerance.Citation72
Acknowledgments
LT Stiemsma is supported by the University of British Columbia Four-Year Fellowship. SET holds the Aubrey J Tingle Professorship in Pediatric Immunology and is a clinical scholar of the Michael Smith Foundation for Health Research. Work in the Finlay and Turvey labs is supported by a Canadian Institutes of Health Research (CIHR) Emerging Team Grant in partnership with Genome BC and AllerGen NCE, the Allergy, Genes and Environment Network.
Disclosure
The authors report no conflicts of interest in this work.
References
- No authors listedWorldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering CommitteeLancet19983519111122512329643741
- AnandanCNurmatovUvan SchayckOCSheikhAIs the prevalence of asthma declining? Systematic review of epidemiological studiesAllergy201065215216719912154
- OkadaHKuhnCFeilletHBachJFThe ‘hygiene hypothesis’ for autoimmune and allergic diseases: an updateClin Exp Immunol201016011920415844
- BrooksCPearceNDouwesJThe hygiene hypothesis in allergy and asthma: an updateCurr Opin Allergy Clin Immunol2013131707723103806
- GreenwoodBMPolyarthritis in Western Nigeria. I. Rheumatoid arthritisAnn Rheum Dis19692854884964186678
- GreenwoodBMHerrickEMVollerASuppression of autoimmune disease in NZB and (NZB x NZW) F1 hybrid mice by infection with malariaNature197022652422662675437516
- GerrardJWGeddesCARegginPLGerrardCDHorneSSerum IgE levels in white and metis communities in SaskatchewanAnn Allergy197637291100987744
- StrachanDPHay fever, hygiene, and household sizeBMJ19892996710125912602513902
- StrachanDPTaylorEMCarpenterRGFamily structure, neonatal infection, and hay fever in adolescenceArch Dis Child19967454224268669958
- OwnbyDRJohnsonCCPetersonELExposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of ageJAMA2002288896397212190366
- BennCSMelbyeMWohlfahrtJBjörksténBAabyPCohort study of sibling effect, infectious diseases, and risk of atopic dermatitis during first 18 months of lifeBMJ20043287450122315121716
- MatricardiPMRosminiFRiondinoSExposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological studyBMJ2000320723241241710669445
- EgeMJMayerMNormandACGABRIELA Transregio 22 Study GroupExposure to environmental microorganisms and childhood asthmaN Engl J Med2011364870170921345099
- MosmannTRCherwinskiHBondMWGiedlinMACoffmanRLTwo types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteinsJ Immunol19861367234823572419430
- BosnjakBStelzmuellerBErbKJEpsteinMMTreatment of allergic asthma: modulation of Th2 cells and their responsesRespir Res20111211421867534
- HuangLKriegAMEllerNScottDEInduction and regulation of Th1-inducing cytokines by bacterial DNA, lipopolysaccharide, and heat-inactivated bacteriaInfect Immun199967126257626310569735
- OrissTBMcCarthySAMorelBFCampanaMAMorelPACrossregulation between T helper cell (Th)1 and Th2: inhibition of Th2 proliferation by IFN-gamma involves interference with IL-1J Immunol19971588366636729103429
- MaizelsRMMcSorleyHJSmythDJHelminths in the hygiene hypothesis: sooner or later?Clin Exp Immunol20141771384624749722
- O’SheaJJPaulWEMechanisms underlying lineage commitment and plasticity of helper CD4+ T cellsScience201032759691098110220185720
- LloydCMSaglaniST cells in asthma: influences of genetics, environment, and T-cell plasticityJ Allergy Clin Immunol2013131512671274 quiz 127523541326
- HolgateSTInnate and adaptive immune responses in asthmaNat Med201218567368322561831
- RookGAAdamsVHuntJPalmerRMartinelliRBrunetLRMycobacteria and other environmental organisms as immunomodulators for immunoregulatory disordersSpringer Semin Immunopathol2004253–423725515007629
- NoverrMCHuffnagleGBThe ‘microflora hypothesis’ of allergic diseasesClin Exp Allergy200535121511152016393316
- RookGABrunetLRMicrobes, immunoregulation, and the gutGut200554331732015710972
- van den BiggelaarAHvan ReeRRodriguesLCDecreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10Lancet200035692431723172711095260
- WilsonMSTaylorMDBalicAFinneyCAMLambJRMaizelsRMSuppression of allergic airway inflammation by helminth-induced regulatory T cellsJ Exp Med200520291199121216275759
- KitagakiKBusingaTRRacilaDElliottDEWeinstockJVKlineJNIntestinal helminths protect in a murine model of asthmaJ Immunol200617731628163516849471
- ManganNEFallonRESmithPvan RooijenNMcKenzieANFallonPGHelminth infection protects mice from anaphylaxis via IL-10-producing B cellsJ Immunol2004173106346635615528374
- LiuQSundarKMishraPKHelminth infection can reduce insulitis and type 1 diabetes through CD25- and IL-10-independent mechanismsInfect Immun200977125347535819752032
- MishraPKPatelNWuWBleichDGauseWCPrevention of type 1 diabetes through infection with an intestinal nematode parasite requires IL-10 in the absence of a Th2-type responseMucosal Immunol20136229730822806101
- HübnerMPStockerJTMitreEInhibition of type 1 diabetes in filaria-infected non-obese diabetic mice is associated with a T helper type 2 shift and induction of FoxP3+ regulatory T cellsImmunology2009127451252219016910
- HübnerMPShiYTorreroMNHelminth protection against autoimmune diabetes in nonobese diabetic mice is independent of a type 2 immune shift and requires TGF-betaJ Immunol2012188255956822174447
- McSorleyHJMaizelsRMHelminth infections and host immune regulationClin Microbiol Rev201225458560823034321
- O’haraAMShanahanFThe gut flora as a forgotten organEmbo Reports20067768869316819463
- PollardMSharonNResponses of the Peyer’s patches in germ-free mice to antigenic stimulationInfect Immun1970219610016557807
- RoundJLMazmanianSKThe gut microbiota shapes intestinal immune responses during health and diseaseNat Rev Immunol20099531332319343057
- FagundesCTAmaralFAVieiraATTransient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree miceJ Immunol201218831411142022210917
- InagakiHSuzukiTNomotoKYoshikaiYIncreased susceptibility to primary infection with Listeria monocytogenes in germfree mice may be due to lack of accumulation of L-selectin+ CD44+ T cells in sites of inflammationInfect Immun1996648328032878757865
- YiPLiLThe germfree murine animal: an important animal model for research on the relationship between gut microbiota and the hostVet Microbiol20121571–21722079217
- CashHLWhithamCVBehrendtCLHooperLVSymbiotic bacteria direct expression of an intestinal bactericidal lectinScience200631357901126113016931762
- LindénSKFlorinTHMcGuckinMAMucin dynamics in intestinal bacterial infectionPLoS One2008312e395219088856
- Rakoff-NahoumSPaglinoJEslami-VarzanehFEdbergSMedzhitovRRecognition of commensal microflora by toll-like receptors is required for intestinal homeostasisCell2004118222924115260992
- CordingSFleissnerDHeimesaatMMCommensal microbiota drive proliferation of conventional and Foxp3(+) regulatory CD4(+) T cells in mesenteric lymph nodes and Peyer’s patchesEur J Microbiol Immunol (Bp)20133111024265914
- ShawMHKamadaNKimYGNúñezGMicrobiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestineJ Exp Med2012209225125822291094
- AtarashiKTanoueTShimaTInduction of colonic regulatory T cells by indigenous Clostridium speciesScience2011331601533734121205640
- MarcobalABarbozaMFroehlichJWConsumption of human milk oligosaccharides by gut-related microbesJ Agric Food Chem20105895334534020394371
- TrompetteAGollwitzerESYadavaKGut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesisNat Med201420215916624390308
- WongJMde SouzaRKendallCWEmamAJenkinsDJColonic health: fermentation and short chain fatty acidsJ Clin Gastroenterol200640323524316633129
- ParkJKimMKangSGShort-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathwayMucosal Immunol201581809324917457
- SmithPMHowittMRPanikovNThe microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasisScience2013341614556957323828891
- AbrahamssonTRJakobssonHEAnderssonAFBjörksténBEngstrandLJenmalmMCLow gut microbiota diversity in early infancy precedes asthma at school ageClin Exp Allergy201444684285024330256
- AzadMBKonyaTGuttmanDSCHILD Study InvestigatorsInfant gut microbiota and food sensitization: associations in the first year of lifeClin Exp Allergy201545363264325599982
- Hoskin-ParrLTeyhanABlockerAHendersonAJAntibiotic exposure in the first two years of life and development of asthma and other allergic diseases by 7.5 yr: a dose-dependent relationshipPediatr Allergy Immunol201324876277124299467
- KummelingIStelmaFFDagneliePCEarly life exposure to antibiotics and the subsequent development of eczema, wheeze, and allergic sensitization in the first 2 years of life: the KOALA Birth Cohort StudyPediatrics20071191e225e23117200248
- JakobssonHEAbrahamssonTRJenmalmMCDecreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean sectionGut201463455956623926244
- PendersJThijsCVinkCFactors influencing the composition of the intestinal microbiota in early infancyPediatrics2006118251152116882802
- ArslanogluSMoroGESchmittJTandoiLRizzardiSBoehmGEarly dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of lifeJ Nutr200813861091109518492839
- PendersJGerholdKStobberinghEEEstablishment of the intestinal microbiota and its role for atopic dermatitis in early childhoodJ Allergy Clin Immunol20131323601607.e823900058
- MulloolyJPSchulerRBarrettMMaherJEVaccines, antibiotics, and atopyPharmacoepidemiol Drug Saf200716327528816794993
- OrtqvistAKLundholmCKielerHAntibiotics in fetal and early life and subsequent childhood asthma: nationwide population based study with sibling analysisBMJ2014349g697925432937
- SearsMRGreeneJMWillanARLong-term relation between breastfeeding and development of atopy and asthma in children and young adults: a longitudinal studyLancet2002360933790190712354471
- AlmqvistCCnattingiusSLichtensteinPLundholmCThe impact of birth mode of delivery on childhood asthma and allergic diseases – a sibling studyClin Exp Allergy20124291369137622925323
- MaitraASherriffAStrachanDHendersonJTeamASMode of delivery is not associated with asthma or atopy in childhoodClin Exp Allergy20043491349135515347366
- KramerMSMatushLVanilovichIPromotion of Breastfeeding Intervention Trial (PROBIT) Study GroupEffect of prolonged and exclusive breast feeding on risk of allergy and asthma: cluster randomised trialBMJ2007335762481517855282
- ForsythePInmanMDBienenstockJOral treatment with live Lactobacillus reuteri inhibits the allergic airway response in miceAm J Respir Crit Care Med2007175656156917204726
- SchabussovaIHufnaglKWildCDistinctive anti-allergy properties of two probiotic bacterial strains in a mouse model of allergic poly-sensitizationVaccine201129101981199021216308
- LyonsAO’MahonyDO’BrienFBacterial strain-specific induction of Foxp3+ T regulatory cells is protective in murine allergy modelsClin Exp Allergy201040581181920067483
- OlszakTAnDZeissigSMicrobial exposure during early life has persistent effects on natural killer T cell functionScience2012336608048949322442383
- ArnoldICDehzadNReuterSHelicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cellsJ Clin Invest201112183088309321737881
- RussellSLGoldMJWillingBPThorsonLMcNagnyKMFinlayBBPerinatal antibiotic treatment affects murine microbiota, immune responses and allergic asthmaGut Microbes20134215816423333861
- RussellSLGoldMJReynoldsLAPerinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseasesJ Allergy Clin Immunol2015135110010925145536
- ArrietaMCStiemsmaLTAmenyogbeNBrownEMFinlayBThe intestinal microbiome in early life: health and diseaseFront Immunol2014542725250028
- MolodeckyNASoonISRabiDMIncreasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic reviewGastroenterol201214214654.e42 quiz e30
- SellonRKTonkonogySSchultzMResident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient miceInfect Immun19986611522452319784526
- MadsenKLDoyleJSTaverniniMMJewellLDRennieRPFedorakRNAntibiotic therapy attenuates colitis in interleukin 10 gene-deficient miceGastroenterol2000118610941105
- BamiasGMariniMMoskalukCADown-regulation of intestinal lymphocyte activation and Th1 cytokine production by antibiotic therapy in a murine model of Crohn’s diseaseJ Immunol200216995308531412391251
- HoentjenFHarmsenHJBraatHAntibiotics with a selective aerobic or anaerobic spectrum have different therapeutic activities in various regions of the colon in interleukin 10 gene deficient miceGut200352121721172714633949
- ShawSYBlanchardJFBernsteinCNAssociation between the use of antibiotics and new diagnoses of Crohn’s disease and ulcerative colitisAm J Gastroenterol2011106122133214221912437
- HviidASvanströmHFrischMAntibiotic use and inflammatory bowel diseases in childhoodGut2011601495420966024
- ShawSYBlanchardJFBernsteinCNAssociation between the use of antibiotics in the first year of life and pediatric inflammatory bowel diseaseAm J Gastroenterol2010105122687269220940708
- KoidoSOhkusaTKajiuraTLong-term alteration of intestinal microbiota in patients with ulcerative colitis by antibiotic combination therapyPLoS One201491e8670224489770
- RogierEWFrantzALBrunoMESecretory antibodies in breast milk promote long-term intestinal homeostasis by regulating the gut microbiota and host gene expressionProc Natl Acad Sci U S A201411183074307924569806
- BarclayARRussellRKWilsonMLGilmourWHSatsangiJWilsonDCSystematic review: the role of breastfeeding in the development of pediatric inflammatory bowel diseaseJ Pediatr2009155342142619464699
- KarvonenMViik-KajanderMMoltchanovaELibmanILaPorteRTuomilehtoJIncidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project GroupDiabetes Care200023101516152611023146
- DIAMOND Project GroupIncidence and trends of childhood Type 1 diabetes worldwide 1990–1999Diabet Med200623885786616911623
- D’AngeliMAMerzonEValbuenaLFTirschwellDParisCAMuellerBAEnvironmental factors associated with childhood-onset type 1 diabetes mellitus: an exploration of the hygiene and overload hypothesesArch Pediatr Adolesc Med2010164873273820679164
- VirtanenSMTakkinenHMNwaruBIMicrobial exposure in infancy and subsequent appearance of type 1 diabetes mellitus-associated autoantibodies: a cohort studyJAMA Pediatr2014168875576324957949
- BrugmanSVisserJTJHillebrandsJLBosNARozingJProlonged exclusive breastfeeding reduces autoimmune diabetes incidence and increases regulatory T-cell frequency in bio-breeding diabetes-prone ratsDiabetes Metab Res Rev200925438038719334008
- CardwellCRSteneLCJonerGCaesarean section is associated with an increased risk of childhood-onset type 1 diabetes mellitus: a meta-analysis of observational studiesDiabetologia200851572673518292986
- BonifacioEWarnckeKWinklerCWallnerMZieglerAGCesarean section and interferon-induced helicase gene polymorphisms combine to increase childhood type 1 diabetes riskDiabetes201160123300330622110093
- Mejía-LeónMEPetrosinoJFAjamiNJDomínguez-BelloMGde la BarcaAMFecal microbiota imbalance in Mexican children with type 1 diabetesSci Rep20144381424448554
- ValladaresRSankarDLiNLactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP ratsPLoS One201055e1050720463897
- WenLLeyREVolchkovPYInnate immunity and intestinal microbiota in the development of Type 1 diabetesNature200845572161109111318806780
- KriegelMASefikEHillJAWuHJBenoistCMathisDNaturally transmitted segmented filamentous bacteria segregate with diabetes protection in nonobese diabetic miceProc Natl Acad Sci U S A201110828115481155321709219
- HaraNAlkananiAKIrDPrevention of virus-induced type 1 diabetes with antibiotic therapyJ Immunol201218983805381422988033
- HviidASvanströmHAntibiotic use and type 1 diabetes in childhoodAm J Epidemiol200916991079108419318617
- ApiwattanakulNThomasPGIversonARMcCullersJAChronic helminth infections impair pneumococcal vaccine responsesVaccine201432425405541025131738
- BobatSDarbyMMrdjenDNatural and vaccine-mediated immunity to Salmonella typhimurium is impaired by the helminth Nippostrongylus brasiliensisPLoS Negl Trop Dis2014812e334125474738
- CooperPJEspinelIParedesWGuderianRHNutmanTBImpaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10J Infect Dis19981784113311389806045
- CooperPJChicoMSandovalCHuman infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgRInfect Immun20016931574158011179329
- SabinEAAraujoMICarvalhoEMPearceEJImpairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoniJ Infect Dis199617312692728537675
- FlemingJOWeinstockJVClinical trials of helminth therapy in autoimmune diseases: rationale and findingsParasite Immunol201537627729225600983
- SummersRWElliottDEUrbanJFJrThompsonRWeinstockJVTrichuris suis therapy in Crohn’s diseaseGut2005541879015591509
- SandbornWJElliottDEWeinstockJRandomised clinical trial: the safety and tolerability of Trichuris suis ova in patients with Crohn’s diseaseAliment Pharmacol Ther201338325526323730956
- CroeseJO’NeilJMassonJA proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donorsGut200655113613716344586
- SummersRWElliottDEQadirKUrbanJFJrThompsonRWeinstockJVTrichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel diseaseAm J Gastroenterol20039892034204114499784
- FearyJVennABrownASafety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility studyClin Exp Allergy20093971060106819400893
- SummersRWElliottDEUrbanJFJrThompsonRAWeinstockJVTrichuris suis therapy for active ulcerative colitis: a randomized controlled trialGastroenterol20051284825832
- HewitsonJPGraingerJRMaizelsRMHelminth immunoregulation: the role of parasite secreted proteins in modulating host immunityMol Biochem Parasitol2009167111119406170
- McSorleyHJO’GormanMTBlairNSutherlandTEFilbeyKJMaizelsRMSuppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrusEur J Immunol201242102667268222706967
- McSorleyHJBlairNFSmithKAMcKenzieANMaizelsRMBlockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergyMucosal Immunol2014751068107824496315
- RuyssersNEDe WinterBYDe ManJGTherapeutic potential of helminth soluble proteins in TNBS-induced colitis in miceInflamm Bowel Dis200915449150019023900
- CançadoGGFiuzaJAde PaivaNCHookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c miceInflamm Bowel Dis201117112275228621290484
- SchnoellerCRauschSPillaiSA helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophagesJ Immunol200818064265427218322239
- FerreiraISmythDGazeSHookworm excretory/secretory products induce interleukin-4 (IL-4)+ IL-10+ CD4+ T cell responses and suppress pathology in a mouse model of colitisInfect Immun20138162104211123545299
- ZacconePFehérváriZJonesFMSchistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetesEur J Immunol20033351439144912731071
- CulleyFJBrownAConroyDMSabroeIPritchardDIWilliamsTJEotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivoJ Immunol2000165116447645311086084
- GraingerJRSmithKAHewitsonJPHelminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF- β pathwayJ Exp Med2010207112331234120876311
- RauschSHeldJFischerASmall intestinal nematode infection of mice is associated with increased enterobacterial loads alongside the intestinal tractPLoS One201389e7402624040152
- ReynoldsLASmithKAFilbeyKJCommensal-pathogen interactions in the intestinal tract: lactobacilli promote infection with, and are promoted by, helminth parasitesGut Microbes20145452253225144609
- WalkSTBlumAMEwingSAWeinstockJVYoungVBAlteration of the murine gut microbiota during infection with the parasitic helminth Heligmosomoides polygyrusInflamm Bowel Dis201016111841184920848461
- SchultzMVeltkampCDielemanLALactobacillus plantarum 299V in the treatment and prevention of spontaneous colitis in interleukin-10-deficient miceInflamm Bowel Dis200282718011854603
- Nanda KumarNSBalamuruganRJayakanthanKPulimoodAPugazhendhiSRamakrishnaBSProbiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfateJ Gastroenterol Hepatol200823121834183919120873
- CalcinaroFDionisiSMarinaroMOral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouseDiabetologia20054881565157515986236
- DuanFFLiuJHMarchJCEngineered commensal bacteria reprogram intestinal cells into glucose-responsive insulin-secreting cells for the treatment of diabetesDiabetes20156451794180325626737
- TangMLPonsonbyALOrsiniFAdministration of a probiotic with peanut oral immunotherapy: a randomized trialJ Allergy Clin Immunol20151353737744.e825592987
- RosenfeldtVBenfeldtENielsenSDEffect of probiotic Lactobacillus strains in children with atopic dermatitisJ Allergy Clin Immunol2003111238939512589361
- OlivaSDi NardoGFerrariFRandomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitisAliment Pharmacol Ther201235332733422150569
- KruisWFricPPokrotnieksJMaintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazineGut200453111617162315479682
- AbrahamssonTRJakobssonTBjörksténBOldaeusGJenmalmMCNo effect of probiotics on respiratory allergies: a seven-year follow-up of a randomized controlled trial in infancyPediatr Allergy Immunol201324655656123902407
- BoyleRJBath-HextallFJLeonardi-BeeJMurrellDFTangMLProbiotics for the treatment of eczema: a systematic reviewClin Exp Allergy20093981117112719573037
- FeigheryLMSmithPO’MahonyLFallonPGBraydenDJEffects of Lactobacillus salivarius 433118 on intestinal inflammation, immunity status and in vitro colon function in two mouse models of inflammatory bowel diseaseDig Dis Sci20085392495250618157694
- CummingsJHMacfarlaneGTEnglystHNPrebiotic digestion and fermentationAm J Clin Nutr2001732 Suppl415S420S11157351
- VosAPvan EschBCStahlBDietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in miceInt Immunopharmacol20077121582158717920536
- KolevaPTValchevaRSSunXGänzleMGDielemanLAInulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic ratsBr J Nutr201210891633164322243836
- BenjaminJLHedinCRKoutsoumpasARandomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s diseaseGut201160792392921262918
- de KivitSSaelandEKraneveldADGalectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humansAllergy201267334335222229637
- van der AaLBHeymansHSvan AalderenWMSynbad Study GroupEffect of a new synbiotic mixture on atopic dermatitis in infants: a randomized-controlled trialClin Exp Allergy201040579580420184604
- van der AaLBvan AalderenWMHeymansHSSynbad Study GroupSynbiotics prevent asthma-like symptoms in infants with atopic dermatitisAllergy201166217017720560907
- FurrieEMacfarlaneSKennedyASynbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trialGut200554224224915647189
- LueKHSunHLLuKHA trial of adding Lactobacillus johnsonii EM1 to levocetirizine for treatment of perennial allergic rhinitis in children aged 7–12 yearsInt J Pediatr Otorhinolaryngol2012767994100122513081
- XiaoJZKondoSYanagisawaNClinical efficacy of probiotic Bifidobacterium longum for the treatment of symptoms of Japanese cedar pollen allergy in subjects evaluated in an environmental exposure unitAllergol Int2007561677517259812
