Abstract
Adipose tissue has traditionally been defined as connective tissue that stores excess calories in the form of triacylglycerol. However, the physiologic functions attributed to adipose tissue are expanding, and it is now well established that adipose tissue is an endocrine gland. Among the endocrine factors elaborated by adipose tissue are the adipokines; hormones, similar in structure to cytokines, produced by adipose tissue in response to changes in adipocyte triacylglycerol storage and local and systemic inflammation. They inform the host regarding long-term energy storage and have a profound influence on reproductive function, blood pressure regulation, energy homeostasis, the immune response, and many other physiologic processes. The adipokines possess pro- and anti-inflammatory properties and play a critical role in integrating systemic metabolism with immune function. In calorie restriction and starvation, proinflammatory adipokines decline and anti-inflammatory adipokines increase, which informs the host of energy deficits and contributes to the suppression of immune function. In individuals with normal metabolic status, there is a balance of pro- and anti-inflammatory adipokines. This balance shifts to favor proinflammatory mediators as adipose tissue expands during the development of obesity. As a consequence, the proinflammatory status of adipose tissue contributes to a chronic low-grade state of inflammation and metabolic disorders associated with obesity. These disturbances are associated with an increased risk of metabolic disease, type 2 diabetes, cardiovascular disease, and many other pathological conditions. This review focuses on the impact of energy homeostasis on the adipokines in immune function.
Introduction
It is now well recognized that adipose serves as a depot for excess energy storage and as an endocrine gland that produces several biological mediators known to regulate blood pressure, reproductive function, appetite, glucose homeostasis, angiogenesis, and immune function.Citation1 Adipose tissue produces both pro- and anti-inflammatory mediators that influence local and systemic inflammation. Among these mediators are the adipokines, proteins produced by cells within white adipose tissue that function as hormones.Citation2 As a family of mediators, the adipokines consist of true adipokines that are predominantly produced by pre- and mature adipocytes and classical cytokines that are produced by adipocytes as well as immune cells found in the stromal vascular fraction (SVF) of adipose tissue and many other cell types outside adipose tissue depots. The balance of pro- and anti-inflammatory adipokines is dictated by many different factors, including the nutritional/metabolic status of the host, the presence of infection or systemic inflammation, oxidative stress, smoking status, age, and sex.Citation3–Citation9 Most importantly, adipokines play a major role in the regulation of the inflammatory response in adipose tissue during the development of obesity and in response to infection or systemic inflammation. This review focuses on the ability of adipokines to regulate the inflammatory response in the setting of chronic calorie restriction and obesity.Citation10–Citation13
Cellular composition of adipose tissue
Adipose tissue is composed of mature adipocytes, preadipocytes, mesenchymal cells, and cells within the SVF that include vascular endothelial and smooth muscle cells, fibroblasts, and several different leukocyte subsets (). Interestingly, nearly all immune cells, such as resident macrophages, mast cells, monocytes, dendritic cells, natural killer cells, B-cells, T-cells, neutrophils, and eosinophils, have been found in adipose tissue.Citation14–Citation18 These cells play a critical role in adipose tissue remodeling and repair in lean mice and humans. Although their function in calorie restriction is poorly understood, immune cell populations in general decline during calorie restriction and increase in obesity. Resident and recruited macrophages are the most abundant type of immune cells in adipose tissue. These cells have been characterized as having M1 (classically activated) or M2 (alternatively activated) phenotypes. M1 macrophages appear to be primed for host defense against infection, while M2 macrophages are thought to play an important role in tissue remodeling and repair. Recent evidence suggests that this dichotomous classification may be an oversimplification, since macrophages may exhibit different phenotypes that span a spectrum of activation states.Citation19,Citation20 They also play a critical role in orchestrating the inflammatory response in obesity and type 2 diabetes (T2D).Citation21
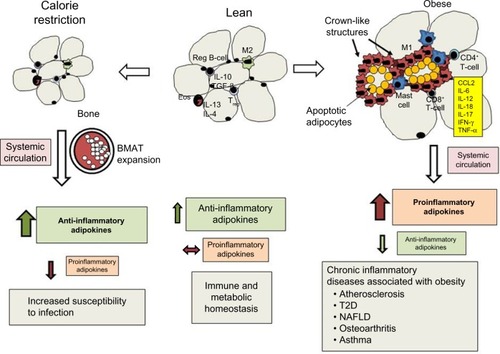
Figure 1 Effects of calorie restriction and obesity on adipose tissue leukocyte populations, adipokine secretion, and chronic inflammation.
Abbreviations: BMAT, bone marrow adipose tissue; T2D, type 2 diabetes; NAFLD, nonalcoholic fatty liver disease; SCAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; Eos, eosinophils.
Mast cells, which are known to mediate acute inflammation in type 1 hypersensitivity responses and host defense against parasitic organisms, are also found in adipose tissue.Citation22 Dendritic cells are professional antigen-presenting cells that recognize foreign antigens and present them to T-cells via major histocompatibility-complex molecules. Adipose tissue dendritic cells have been found in mice and humans and may play an important role in T-helper (TH)-17 cell responses.Citation18,Citation23 The most abundant granulocyte found in blood, the neutrophil, can also be found in adipose tissue. While these cells play a prominent role in host defense against bacterial infections, their function in adipose tissue is not clear. These cells transiently infiltrate murine adipose tissue with the initiation of high-fat-diet feeding and mediate insulin resistance in mice.Citation24,Citation25 Natural killer (NK) cells, best known for their role in the early host response to viral infections and in killing tumor cells, have recently been shown to promote macrophage proliferation and polarization in adipose tissue and insulin resistance in mice fed a high-fat diet.Citation26
B-cells are most commonly studied for their contribution to host defense against infection and autoimmune disease. In adipose tissue, several different B-cell subsets have been identified, and these cells contribute to local and systemic inflammation by secreting cytokines, producing antibodies, and presenting antigens to T-cells.Citation27 Finally, several different T-cell subsets, such as CD4+ TH cells, which include Treg, TH1, and TH2, and CD8+ T-cells, have been identified in adipose tissue, where they regulate local inflammation through the secretion of cytokines that influence the differentiation and polarization of macrophages.Citation28,Citation29 The activation state, differentiation, and proliferation of immune cells in adipose tissue are profoundly influenced by anti- and proinflammatory cytokines, lipid mediators, and adipokines secreted within local fat pads and in circulation.Citation30–Citation34
Anti-inflammatory adipokines
Adiponectin, C1q/TNF-related proteins (CTRPs), omentin, and secreted frizzled-related protein 5 (SFRP5) are anti-inflammatory adipokines produced by adipose tissue.Citation35–Citation38 Adiponectin is the best-known and most abundant adipokine found in human serum, with concentrations typically in the µg/mL range.Citation39 Unlike other adipokines, which are found in greatest quantities in visceral and subcutaneous adipose tissue (SCAT), it is predominantly produced by bone marrow adipose tissue (BMAT).Citation40 Calorie restriction, aging, estrogen deficiency, T1D, and treatment with thiazolidinediones increase, while obesity, T2D, oxidative stress, and cigarette-smoke exposure decrease serum adiponectin levels.Citation41–Citation46 Adiponectin is a complex molecule that forms low-, intermediate-, and high molecular weight complexes in circulation. Its effects are mediated through the AdipoR1 and AdipoR2 receptors, which activate AMPK in immune cells and tissues.Citation47 In particular, the high-molecular-weight complex has anti-inflammatory properties known to inhibit inflammation by blocking NF-κB activation and reducing such cytokines as TNFα, IL-6, and IL-18.Citation2,Citation48–Citation50
Adiponectin-knockout mice exhibit enhanced inflammatory responses, suggesting a major role for adiponectin in suppressing systemic and tissue inflammation.Citation31,Citation51–Citation53 For example, adiponectin attenuates vascular inflammation, which may play a protective role against aortic aneurism.Citation51 The protective effects of adiponectin in the vasculature may be mediated by locally produced perivascular adipose tissue.Citation54 Moreover, the lungs of adiponectin-knockout animals exhibit an emphysematous phenotype, with enlarged air spaces and activated alveolar macrophages capable of producing higher levels of TNFα and MMP12.Citation31 These animals also develop pulmonary arterial hypertension characterized by perivascular inflammation.Citation53 Adiponectin plays a protective role in acute lung injury and myocardial ischemia, where it reduces cellular infiltration.Citation52,Citation55–Citation57 In contrast to these studies showing a protective anti-inflammatory effect, adiponectin may play a proinflammatory role in arthritic joints by promoting COX2 expression and the synthesis of PGE2, which increases inflammation and pain.Citation58
Much less is known regarding other adipokines with anti-inflammatory properties, such as CTRP, omentin, and SFRP5. The CTRPs are structurally similar to adiponectin, and at least 15 isoforms have been described.Citation59 CTRP3 has been shown to reduce cytokine production in human monocytes and adipocytes stimulated with lipopolysaccharide and free fatty acids by inhibiting TLR4 activation.Citation59 In addition, CTRP13 inhibits inflammation in lipid-loaded hepatocytes and improves insulin sensitivity.Citation60 Omentin is a novel adipokine that inhibits TNFα-induced endothelial cell COX2 expression and induces endothelial nitric oxide synthase.Citation61 SFRP5 has anti-inflammatory effects in adipose tissue and in macrophages that are mediated through the suppression of noncanonical Wnt5a/JNK signaling, which ultimately inhibits macrophage TNFα, IL-1β, and CCL2–MCP1 synthesis.Citation62 Clearly, there is a need to increase our understanding of the biology of these novel anti-inflammatory adipokines and their role in opposing the effects of proinflammatory mediators and adipokines.
Proinflammatory adipokines
Leptin is the best known proinflammatory adipokine that increases in proportion to white adipose tissue mass, and was first described as a satiety hormone.Citation11 The long form of the leptin receptor, which is expressed by nearly all immune cells, initiates intracellular signaling to activate the tyrosine kinase JAK2, the latent transcription factor STAT3, MAPK, ERK1/2, and PI3K pathways that activate the innate immune response.Citation63 Leptin can directly enhance the production of several proinflammatory cytokines, such as IL-6, IL-12, IL-18, and TNFα, the chemokines IL-8 and CCL2/MCP-1, and the lipid mediators PGE2, cysteinyl leukotrienes (cysLTs), and leukotriene B4 (LTB4) in peripheral blood monocytes and resident tissue macrophages in mice and humans.Citation8,Citation64–Citation67 Leptin can also induce the production of reactive oxygen intermediates in macrophages, neutrophils, and endothelial cells and potentiate IFNγ-induced expression of nitric oxide synthase.Citation68–Citation70 Leptin enhances platelet aggregation and promotes leukocyte–endothelial cell interactions by increasing the expression of adhesion molecules on myeloid cells and vascular endothelial cells.Citation68,Citation71–Citation73 TH1 and TH17 responses are enhanced with leptin, which can also prevent T-cell apoptosis.Citation74
Resistin and resistin-like molecules were first characterized in mice as mediators of insulin resistance, T2D, and metabolic syndrome.Citation75 While adipose tissue is the primary source of this adipokine in mice, monocytes and macrophages are the most important sources of resistin in humans.Citation76 In addition, there are substantial differences between mouse and human resistin amino acid sequence homology, indicating that the physiological actions of this adipokine may differ in mice and humans.Citation77 To address these controversial issues, Qatanani et al created a humanized mouse that expressed human resistin in macrophages but not adipose tissue that has provided a robust system to substantiate the importance of human resistin in T2D and obesity.Citation76 The proinflammatory effects of resistin are mediated through CAP1, recently identified as a receptor for resistin. It initiates cAMP-mediated PKA activation and NF-κB-related transcription of inflammatory cytokines in human monocytes.Citation78
There are several other less studied proinflammatory adipokines that have been implicated in the promotion of inflammation associated with obesity, including chemerin, retinol binding protein 4 (RBP4), and lipocalin 2 (LCN2).Citation2 Chemerin is an adipocyte-derived chemoattractant for monocytes and dendritic cells that is produced by mature adipocytes. It is secreted as a preprohormone that requires enzymatic cleavage by extracellular proteases.Citation79 RBP4 is a member of the lipocalin family of proteins that transports retinol from the liver to the peripheral tissues.Citation80 RBP4 is produced by the liver, adipose tissue, and macrophages. RBP4 may contribute to inflammation by activating adipose tissue antigen-presenting cells that promote TH1-cell polarization.Citation81 LCN2 is produced by adipocytes and is induced by inflammatory stimuli that activate NF-κB in adipose tissue.Citation82 LCN2, also referred to as neutrophil gelatinase-associated lipocalin, binds and transports hydrophobic molecules, such as retinoids, arachidonic acid, LTB4, platelet-activating factor, and steroids.Citation82,Citation83 LCN2 also plays an important role in host defense against bacterial infections by sequestering iron.Citation84 Through the elaboration of anti- and proinflammatory adipokines that spill over into the systemic circulation, adipose tissue plays a critical role in regulating the inflammatory response in the setting of calorie restriction and obesity.
The impact of adipokines on immune function during calorie restriction
Prolonged calorie restriction reduces the amount of energy in the form of triacylglycerol (TAG) stored in adipose tissue. The size of individual adipocytes within visceral adipose tissue (VAT) declines. If caloric restriction is extended, SCAT declines as well, but this response requires more time.Citation85 In contrast, BMAT expands during calorie restriction, replacing active hematopoietic cells.Citation40 Calorie restriction also has a profound impact on the adipokines and mediators of inflammation produced within adipose tissue.
The production of anti-inflammatory adipokines, such as adiponectin, CTRP, omentin, and SFRP5, increases.Citation40,Citation86–Citation88 Unlike other adipokines, more adiponectin is produced by BMAT than by SCAT or VAT.Citation40 Adiponectin plays an important role in adipogenesis and insulin sensitivity via AMPK signaling, which promotes fatty acid oxidation and glucose uptake in skeletal muscle and liver.Citation89,Citation90 The increase in omentin that accompanies calorie restriction may be protective against cardiovascular disease.Citation37 Omentin may reduce vascular inflammation associated with atherosclerosis, since it reduces macrophage adhesion to endothelial cells in vitro by inhibiting ICAM1 and VCAM1 expression.Citation37 SFRP5 may also play a protective role in cardiovascular disease.Citation62 The increased secretion of SFRP5 following calorie restriction in obese patients may contribute to improvements in atherosclerosis and protect against myocardial ischemia–reperfusion injury.Citation62 IL-10 has also been shown to increase during calorie restriction. It is produced by regulatory B-cells within adipose tissue, where it suppresses inflammatory cytokine production by resident CD8+ T-cells.Citation91 Tregs elaborate TGFβ and IL-10, which helps maintain an anti-inflammatory environment.Citation28 In contrast, the production of proinflammatory adipokines (leptin, resistin, RBP4), cytokines (IL-1β, IL-6. IL-17, TNFα), chemokines (CCL2 and MIP2), and lipid mediators (cysLTs and LTB4) decline.Citation3,Citation10,Citation33,Citation76,Citation92,Citation93 While calorie restriction has been shown to suppress inflammation, protect against cardiovascular disease, improve glucose homeostasis in T2D, and increase the life span of mice, it also has negative consequences on host defense against infection.Citation3,Citation94–Citation96
Studies by Gardner et al evaluated the impact of a calorie-restricted diet on host defense against a mouse adaptive influenza virus, PR8, in young and aged mice.Citation94,Citation95 In these studies, young and aged mice that consumed 40% fewer calories than their ad libitum-fed counterparts exhibited greater weight loss, mortality, lung viral titers, and impaired NK-cell cytotoxic function following influenza virus infection.Citation94,Citation95 Reduced IFNα and IFNβ production was also associated with increased viral burdens of young calorie-restricted mice.Citation95 In a similar study, refeeding calorie-restricted mice improved survival and nearly restored NK-cell numbers and cytotoxic function at all time points following influenza infection. While refeeding calorie-restricted mice reconstituted adipose tissue, it did not restore leptin to levels observed in ad libitum-fed animals, and this may have accounted for the differences in NK-cell numbers and function.Citation96
Acute calorie restriction, a common occurrence in critically ill patients and starvation, rapidly mobilizes energy stores from adipose tissue, shrinking the size of VAT and SCAT adipose tissue. In general, both anti- and proinflammatory adipokines decline with prolonged fasting. For example, serum leptin levels decline rapidly and are disproportionately lower than would be expected for a given fat mass in humans and mice.Citation4,Citation97 Unlike leptin, serum adiponectin levels remain stable after 3 days of fasting and decline slightly during a prolonged fast.Citation98,Citation99 The decline of leptin in adaptation to starvation has been examined in murine models to determine the impact of leptin in thymic atrophy and pneumococcal pneumonia.Citation3,Citation10,Citation92,Citation100 As leptin levels decline during fasting, glucocorticoid levels rise, and this contributes to peripheral blood T- and B-lymphocyte apoptosis and diminished thymic and bone marrow cellularity. These events are prevented if leptin levels are maintained during fasting.Citation92,Citation101
Fasting and chronic energy malnutrition are known to suppress the immune response to infection, and leptin levels may predict survival in severe acute childhood malnutrition.Citation102 In mice infected with Streptococcus pneumoniae, 48 hours of fasting reduced pulmonary bacterial clearance, and this was associated with reduced neutrophil counts in peripheral blood and bronchoalveolar lavage. Lung-homogenate cytokine (IL-6), chemokine (MIP2), and LTB4 synthesis in alveolar macrophages were also reduced by fasting. Alveolar macrophages obtained from mice after fasting exhibited defective phagocytosis and killing of S. pneumoniae. Interestingly, all of these responses were restored when exogenous leptin was administered during fasting.Citation3 Using a similar model of leptin depletion by starvation and lipopolysaccharide-induced sepsis, Faggioni et al demonstrated that leptin improved survival, and this improvement was associated with lower levels of systemic TNFα.Citation100 These studies demonstrate the physiologic importance of the adipokines generated in calorie restriction and starvation, which play a crucial role in the host response to infection and sepsis. Since infectious disease remains a leading cause of morbidity and mortality in undernourished patients and in severely malnourished children in low-income nations, more research is needed to understand the role of adipokines and immunosuppression associated with energy malnutrition.Citation103
Hyperplasia and hypertrophy of adipocytes in VAT and SCAT during obesity
In contrast to calorie restriction and starvation, obesity is characterized by energy excess and the expansion of white-adipose tissue that contributes to a chronic state of low-grade inflammation and increased risk of chronic disease (). Adipose tissue expansion occurs as excess energy is stored in the form of TAG. Under conditions of normal metabolic homeostasis, the expansion of adipose tissue can buffer excess dietary lipids.Citation85 As excess energy accumulates, the adipose tissue must expand to store excess TAG through the process of adipocyte hyperplasia and hypertrophy.Citation104,Citation105 Hyperplasia occurs through the differentiation of preadipocytes into mature adipocytes, and this process occurs in all adipose-tissue depots, but is more prominent in SCAT.Citation106 The enlargement of SCAT around the femoral gluteal area, also known as the gynoid pattern of obesity, is well suited for lipid buffering, and this helps maintain homeostasis during fluctuation of dietary lipids.Citation106,Citation107 With regard to the role of adipokines in this process, adiponectin promotes adipogenesis and hyperplasia through the activation of PPARγ. SFRP5 expression increases during the expansion of adipose tissue, where it seems to play an important role in suppressing oxidative metabolism and adipocyte growth during obesity.Citation108 The expansion of SCAT via adipocyte hyperplasia is associated with a lower risk of metabolic disease, higher levels of adiponectin, and lower levels of proinflammatory adipokines.Citation109–Citation111 In contrast, the expansion of adipose tissue through the process of hypertrophy is associated with adipose tissue inflammation.Citation2 While adipocyte hypertrophy occurs in all adipose tissue depots, its appearance in VAT is highly correlated with the accumulation of immune cells, proinflammatory adipokines, and metabolic dysfunction.Citation2,Citation112
Changes in immune cell composition and phenotype in adipose tissue during obesity
As adipose tissue expands, the number of eosinophils and levels of IL-13 and IL-4 decline in mice and humans.Citation113 The population of T-cells changes as well, with a decline T reg cells and increases in CD4+ and CD8+ T-cells.Citation28,Citation29,Citation114 In addition to a decline in anti-inflammatory cytokine and adipokine levels, the number of CD4+ T-cells that secrete the TH1 cytokine IFNγ and CD8+ T-cells increases, promoting the differentiation of resident macrophages into the classically activated or M1-macrophage phenotype.Citation21,Citation29 A substantial increase in adipose tissue macrophages occurs as a consequence of the recruitment of peripheral blood monocytes that respond to chemokines (CCL2, CXCL5).Citation14 Neutrophils are also recruited to adipose tissue following dietary high-fat feeding in mice, and the chemokines for their recruitment have not been identified.Citation115 Finally, mast cells also increase in the SVF of VAT in obese mice and human subjects, and these cells contribute to the proinflammatory state by releasing TNFα, IL-6, and lipid mediators, such as cysLTs.Citation16,Citation116
Upon further expansion, the proinflammatory cytokines IL-6, TNFα, and IL-18 and adipokines leptin and resistin promote a proinflammatory environment and contribute to metabolic dysfunction.Citation11,Citation117–Citation121 The expansion of individual adipocytes is limited by the extracellular matrix and hypoxia resulting from rarefaction.Citation122 As a consequence, these cells undergo apoptosis and eventually necrosis.Citation104 Crown-like structures are frequently found in adipose tissue sections from obese mice and humans, and these are necrotic adipocytes surrounded by M1 macrophages.Citation13,Citation104 There are distinct differences in the type and number of inflammatory cells within adipose tissue depots in VAT and SCAT of lean and obese human subjects, and these differences are correlated with metabolic disease risk.Citation123 Compared with VAT, there are fewer macrophages, mast cells, and other immune cells in SCAT, suggesting less adipose tissue inflammation in SCAT compared with VAT.Citation16 Crown-like structures in obese mice are also more prevalent in VAT versus SCAT.Citation16 In total, the location and quality of excess adipose tissue may profoundly influence the inflammatory state of obese individuals.
Adipose tissue inflammation
Inflammation most often occurs in response to infection, irritation, allergic and autoimmune responses, or tissue trauma and is classically characterized by the cardinal signs of inflammation, which include heat, redness, swelling, pain, and loss of function.Citation124 These responses develop as a consequence of the production of proinflammatory mediators, such as lipid mediators (prostaglandins and leukotrienes), cytokines, chemokines, and cellular debris resulting from the release of intracellular constituents or “danger” signals.Citation124 These mediators can induce vasodilation, increase microvascular permeability, activate local immune cells, increase the production of leukocytes from the bone marrow, recruit peripheral blood leukocytes to the site of inflammation, and trigger nociceptors to induce the sensation of pain. Unlike the classic inflammatory response that is activated by pattern-recognition receptors during an infection, the inflammatory response in obesity is initiated by intrinsic signals, such as nutrient sensing, the unfolded protein response, and endoplasmic reticulum stress, and is often referred to as meta-inflammation.Citation125 These intrinsic signals promote an inflammatory response through the activation of the NF-κB-signaling pathway, the production of proinflammatory cytokines, generation of reactivate oxygen species, and proinflammatory adipokine synthesis by adipocytes and immune cells within adipose tissue.Citation125 Conversely, anti-inflammatory adipokine synthesis, eg, adiponectin, declines as a consequence of the unfolded protein response and endoplasmic reticulum stress.Citation126 Under these circumstances, resident and recruited leukocytes within adipose tissue elaborate proinflammatory mediators that not only contribute to local inflammation but spill over into the systemic circulation, causing a chronic state of low-grade inflammation.
Contribution of adipokines to inflammation associated with obesity
Proinflammatory adipokines, which increase in obese individuals, contribute to systemic inflammation and diseases associated with obesity. For example, leptin levels are correlated with the severity of illness in several diseases, such as osteoarthritis, multiple sclerosis, nonalcoholic fatty liver disease, hepatic fibrosis, renal disease, atherosclerosis, and thrombosis.Citation34,Citation127–Citation135 Resistin levels are elevated in obese humans and are associated with a greater risk of cardiovascular disease.Citation106 Resistin may promote chronic inflammation and insulin resistance by enhancing monocyte recruitment through the induction of CCL2 in inflamed VAT in humans and atherosclerosis by promoting monocyte foam cell formation and endothelial cell interactions with the vascular endothelium.Citation136,Citation137 Likewise, chemerin inhibits the maturation of preadipocytes and alters the metabolic functions of mature adipocyte cell lines in vitro.Citation138 While chemerin levels in human subjects are correlated with obesity and metabolic syndrome, a causal role for chemerin in adipose tissue inflammation and obesity-associated disease has not been reported.Citation139 In addition, RBP4 is produced by the liver, adipose tissue, and macrophages, and its expression increases with increasing body mass index, waist circumference, and visceral adiposity in human subjects.Citation140,Citation141 It inhibits intracellular signaling events induced by insulin, such as phosphorylation of IRS1, and contributes to insulin resistance in T2D.Citation142,Citation143 RBP4 may contribute to inflammation by activating adipose tissue antigen-presenting cells that promote TH1-cell polarization.Citation81 Finally, LCN2 may play a critical role in cardiovascular disease associated with obesity, since it is markedly elevated in atherosclerotic plaques.Citation144 However, a causal role for LCN2 in vascular tissue remodeling in atherosclerotic lesions associated with obesity has not been established, and additional research is needed to understand the role of LCN2 in diseases associated with obesity.
Conclusion
Adipose tissue produces several endocrine factors, cytokines, and chemokines that regulate physiologic processes and immune function. Among these mediators are the adipokines, hormones produced by adipocytes, and cells within the SVF of adipose tissue. There are pro- and anti-adipokines that provide a means of communication between energy stored in adipose tissue and several organ systems to integrate metabolism with several physiologic functions. As adipose tissue shrinks during calorie restriction, anti-inflammatory adipokines rise and proinflammatory adipokines decline, resulting in increased insulin sensitivity and suppressed immune function. As adipose tissue expands during obesity, there is an increase in proinflammatory and a reduction in anti-inflammatory adipokines, which contributes to local and systemic inflammation and disturbances in glucose homeostasis. Future therapeutic interventions may target adipokines and their intracellular signaling cascades to enhance immune function in calorie restriction or ameliorate chronic inflammation and T2D in obese patients.
Disclosure
The author reports no conflicts of interest in this work.
References
- SchererPEAdipose tissue: from lipid storage compartment to endocrine organDiabetes20065561537154516731815
- OuchiNParkerJLLugusJJWalshKAdipokines in inflammation and metabolic diseaseNat Rev Immunol2011112859721252989
- MancusoPHuffnagleGBOlszewskiMAPhippsJPeters-GoldenMLeptin corrects host defense defects following acute starvation in murine pneumococcal pneumoniaAm J Respir Crit Care Med2006173221221816210671
- AhimaRSPrabakaranDMantzorosCRole of leptin in the neuroendocrine response to fastingNature199638265882502528717038
- SullJWKimHJYunJEParkEJKimGJeeSHSerum adiponectin is associated with smoking status in healthy Korean menEndocr J2009561737818840926
- IsidoriAMStrolloFMorèMLeptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weightsJ Clin Endocrinol Metab20008551954196210843181
- SomechRReifSGolanderASpirerZLeptin and C-reactive protein levels correlate during minor infection in childrenIsr Med Assoc J200792767817348475
- MancusoPGottschalkAPhareSMPeters-GoldenMLukacsNWHuffnagleGBLeptin-deficient mice exhibit impaired host defense in Gram-negative pneumoniaJ Immunol200216884018402411937559
- NakanishiSYamaneKKameiNNojimaHOkuboMKohnoNA protective effect of adiponectin against oxidative stress in Japanese Americans: the association between adiponectin or leptin and urinary isoprostaneMetabolism200554219419915690313
- LordGMMatareseGHowardJKBakerRJBloomSRLechlerRILeptin modulates the T-cell immune response and reverses starvation-induced immunosuppressionNature199839466968979019732873
- FriedmanJMHalaasJLLeptin and the regulation of body weight in mammalsNature199839567047637709796811
- TakabatakeNNakamuraHAbeSCirculating leptin in patients with chronic obstructive pulmonary diseaseAm J Respir Crit Care Med19991594 Pt 11215121910194168
- LumengCNBodzinJLSaltielARObesity induces a phenotypic switch in adipose tissue macrophage polarizationJ Clin Invest2007117117518417200717
- WeisbergSPMcCannDDesaiMRosenbaumMLeibelRLFerranteAWJrObesity is associated with macrophage accumulation in adipose tissueJ Clin Invest2003112121796180814679176
- DeiuliisJShahZShahNVisceral adipose inflammation in obesity is associated with critical alterations in T regulatory cell numbersPLoS One201161e1637621298111
- AltintasMMAzadANayerBMast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in miceJ Lipid Res201152348048821148461
- OhmuraKIshimoriNOhmuraYNatural killer T cells are involved in adipose tissues inflammation and glucose intolerance in diet-induced obese miceArterioscler Thromb Vasc Biol201030219319919910631
- BedfordPATodorovicVWestcottEDAdipose tissue of human omen-tum is a major source of dendritic cells, which lose MHC class II and stimulatory function in Crohn’s diseaseJ Leukoc Biol200680354655416822853
- CildirGAkincilarSCTergaonkarVChronic adipose tissue inflammation: all immune cells on the stageTrends Mol Med201319848750023746697
- GinhouxFSchultzeJLMurrayPJOchandoJBiswasSKNew insights into the multidimensional concept of macrophage ontogeny, activation and functionNat Immunol2015171344026681460
- LumengCNDeyoungSMSaltielARMacrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteinsAm J Physiol Endocrinol Metab20072921E166E17416926380
- PoglioSDe Toni-CostesFArnaudEAdipose tissue as a dedicated reservoir of functional mast cell progenitorsStem Cells201028112065207220845475
- BertolaACiucciTRousseauDIdentification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patientsDiabetes20126192238224722596049
- TalukdarSOhDYBandyopadhyayGNeutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastaseNat Med20121891407141222863787
- MeakinPJMorrisonVLSneddonCCMice lacking β2-integrin function remain glucose tolerant in spite of insulin resistance, neutrophil infiltration and inflammationPLoS One2015109e013887226405763
- WensveenFMJelenčićVValentićSNK cells link obesity-induced adipose stress to inflammation and insulin resistanceNat Immunol201516437638525729921
- WinerDAWinerSChngMHShenLEnglemanEGB lymphocytes in obesity-related adipose tissue inflammation and insulin resistanceCell Mol Life Sci20137161033104324127133
- FeuererMHerreroLCipollettaDLean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parametersNat Med200915893093919633656
- NishimuraSManabeINagasakiMCD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesityNat Med200915891492019633658
- De RosaVProcacciniCCalìGA key role of leptin in the control of regulatory T cell proliferationImmunity200726224125517307705
- SummerRLittleFFOuchiNAlveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient miceAm J Physiol Lung Cell Mol Physiol20082946L1035L104218326826
- FarooqiISMatareseGLordGMBeneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiencyJ Clin Invest200211081093110312393845
- RodriguezLGranielJOrtizREffect of leptin on activation and cytokine synthesis in peripheral blood lymphocytes of malnourished infected childrenClin Exp Immunol2007148347848517355247
- MatareseGCarrieriPBLa CavaALeptin increase in multiple sclerosis associates with reduced number of CD4+CD25+ regulatory T cellsProc Natl Acad Sci U S A2005102145150515515788534
- Gutiérrez-VidalRVega-BadilloJReyes-FermínLMSFRP5 hepatic expression is associated with non-alcoholic liver disease in morbidly obese womenAnn Hepatol201514566667426256895
- OhashiKShibataRMuroharaTOuchiNRole of anti-inflammatory adipokines in obesity-related diseasesTrends Endocrinol Metab201425734835524746980
- TanYLZhengXLTangCKThe protective functions of omentin in cardiovascular diseasesClin Chim Acta20154489810626079253
- AuguetTQuinteroYRiescoDNew adipokines vaspin and omentin: circulating levels and gene expression in adipose tissue from morbidly obese womenBMC Med Genet2011126021526992
- SchererPEWilliamsSFoglianoMBaldiniGLodishHFA novel serum protein similar to C1q, produced exclusively in adipocytesJ Biol Chem19952704526746267497592907
- CawthornWPSchellerELLearmanBSBone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restrictionCell Metab201420236837524998914
- CombsTPBergAHRajalaMWSexual differentiation, pregnancy, calorie restriction, and aging affect the adipocyte-specific secretory protein adiponectinDiabetes200352226827612540596
- FazeliPKHorowitzMCMacDougaldOAMarrow fat and bone – new perspectivesJ Clin Endocrinol Metab201398393594523393168
- HongSCYooSWChoGJCorrelation between estrogens and serum adipocytokines in premenopausal and postmenopausal womenMenopause200714583584017667144
- ImagawaAFunahashiTNakamuraTElevated serum concentration of adipose-derived factor, adiponectin, in patients with type 1 diabetesDiabetes Care20022591665166612196453
- YuJGJavorschiSHevenerALThe effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjectsDiabetes200251102968297412351435
- YuanHWongLSBhattacharyaMThe effects of second-hand smoke on biological processes important in atherogenesisBMC Cardiovasc Disord20077117210084
- YamauchiTKamonJItoYCloning of adiponectin receptors that mediate antidiabetic metabolic effectsNature2003423694176276912802337
- YokotaTOritaniKTakahashiIAdiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophagesBlood20009651723173210961870
- YamaguchiNArguetaJGMasuhiroYAdiponectin inhibits Toll-like receptor family-induced signalingFEBS Lett2005579306821682616325814
- ChandrasekarBBoylstonWHVenkatachalamKWebsterNJPrabhuSDValenteAJAdiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-κB/PTEN suppressionJ Biol Chem200828336248892489818632660
- YoshidaSFusterJJWalshKAdiponectin attenuates abdominal aortic aneurysm formation in hyperlipidemic miceAtherosclerosis2014235233934624911638
- KonterJMParkerJLBaezEAdiponectin attenuates lipopoly-saccharide-induced acute lung injury through suppression of endothelial cell activationJ Immunol2012188285486322156343
- MedoffBDOkamotoYLeytonPAdiponectin-deficiency increases allergic airway inflammation and pulmonary vascular remodelingAm J Respir Cell Mol Biol200941439740619168697
- RuanCCGeQLiYComplement-mediated macrophage polarization in perivascular adipose tissue contributes to vascular injury in deoxycorticosterone acetate-salt miceArterioscler Thromb Vasc Biol201535359860625573852
- ShoreSATerryRDFlyntLXuAHugCAdiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in miceJ Allergy Clin Immunol2006118238939516890763
- VerboutNGBeneditoLWilliamsASImpact of adiponectin overexpression on allergic airways responses in miceJ Allergy (Cairo)2013201334952023861690
- ShibataRSatoKPimentelDRAdiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanismsNat Med200511101096110316155579
- BasSFinckhAPuskasGJAdipokines correlate with pain in lower limb osteoarthritis: different associations in hip and kneeInt Orthop201438122577258325005460
- KoppABalaMBuechlerCC1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissueEndocrinology2010151115267527820739398
- WeiZPetersonJMWongGWMetabolic regulation by C1q/TNF-related Protein-13 (CTRP13): activation of AMP-activated protein kinase and suppression of fatty acid-induced JNK signalingJ Biol Chem201128618156521566521378161
- YamawakiHKuramotoJKameshimaSUsuiTOkadaMHaraYOmentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cellsBiochem Biophys Res Commun2011408233934321514279
- NakamuraKSanoSFusterJJSecreted fizzled-related protein 5 diminishes cardiac inflammation and protects the heart from ischemia-reperfusion injuryJ Biol Chem201629162566257526631720
- La CavaAMatareseGThe weight of leptin in immunityNat Rev Immunol20044537137915122202
- LoffredaSYangSLinHLeptin regulates proinflammatory immune responsesFASEB J199812157659438411
- GainsfordTWillsonTAMetcalfDLeptin can induce proliferation, differentiation, and functional activation of hemopoietic cellsProc Natl Acad Sci U S A1996932514564145688962092
- MancusoPCanettiCGottschalkATithofPKPeters-GoldenMLeptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2γ) protein expressionAm J Physiol Lung Cell Mol Physiol20042873L497L50215145787
- YamagishiSIEdelsteinDDuXLKanedaYGuzmánMBrownleeMLeptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase AJ Biol Chem200127627250962510011342529
- RasoGMPacilioMEspositoECoppolaADi CarloRMeliRLeptin potentiates IFN-γ-induced expression of nitric oxide synthase and cyclo-oxygenase-2 in murine macrophage J774A.1Br J Pharmacol2002137679980412411410
- Caldefie-ChezetFPoulinAVassonMPLeptin regulates functional capacities of polymorphonuclear neutrophilsFree Radic Res200337880981414567439
- BouloumieAMarumoTLafontanMBusseRLeptin induces oxidative stress in human endothelial cellsFASEB J199913101231123810385613
- KonstantinidesSSchäferKKoschnickSLoskutoffDJLeptin-dependent platelet aggregation and arterial thrombosis suggests a mechanism for atherothrombotic disease in obesityJ Clin Invest2001108101533154011714745
- Santos-AlvarezJGobernaRSánchez-MargaletVHuman leptin stimulates proliferation and activation of human circulating monocytesCell Immunol1999194161110357875
- Zarkesh-EsfahaniHPockleyGMetcalfeRAHigh-dose leptin activates human leukocytes via receptor expression on monocytesJ Immunol200116784593459911591788
- ReisBSLeeKFanokMHLeptin receptor signaling in T cells is required for Th17 differentiationJ Immunol2015194115253526025917102
- SteppanCMBaileySTBhatSThe hormone resistin links obesity to diabetesNature2001409681830731211201732
- QatananiMSzwergoldNRGreavesDRAhimaRSLazarMAMacrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in miceJ Clin Invest2009119353153919188682
- YangRZHuangQXuAComparative studies of resistin expression and phylogenomics in human and mouseBiochem Biophys Res Commun2003310392793514550293
- LeeSLeeHCKwonYWAdenylyl cyclase-associated protein 1 is a receptor for human resistin and mediates inflammatory actions of human monocytesCell Metab201419348449724606903
- RourkeJLDranseHJSinalCJTowards an integrative approach to understanding the role of chemerin in human health and diseaseObes Rev201314324526223216632
- NewcomerMERetinoid-binding proteins: structural determinants important for functionFASEB J1995922292397781925
- Moraes-VieiraPMYoreMMDwyerPMSyedIAryalPKahnBBRBP4 activates antigen-presenting cells, leading to adipose tissue inflammation and systemic insulin resistanceCell Metab201419351252624606904
- WangHHWuMMChanMWPuYSChenCJLeeTCLong-term low-dose exposure of human urothelial cells to sodium arsenite activates lipocalin-2 via promoter hypomethylationArch Toxicol20148881549155924570342
- BrattTOhlsonSBorregaardNInteractions between neutrophil gelatinase-associated lipocalin and natural lipophilic ligandsBiochim Biophys Acta199914721–226226910572948
- FloTHSmithKDSatoSLipocalin 2 mediates an innate immune response to bacterial infection by sequestrating ironNature2004432701991792115531878
- GoossensGHThe role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistancePhysiol Behav200894220621818037457
- UrbanováMDostálováITrachtaPSerum concentrations and subcutaneous adipose tissue mRNA expression of omentin in morbid obesity and type 2 diabetes mellitus: the effect of very-low-calorie diet, physical activity and laparoscopic sleeve gastrectomyPhysiol Res201463220721824397804
- SchulteDMMüllerNNeumannKPro-inflammatory Wnt5a and anti-inflammatory sFRP5 are differentially regulated by nutritional factors in obese human subjectsPLoS One201272e3243722384249
- ClémentKViguerieNPoitouCWeight loss regulates inflammation-related genes in white adipose tissue of obese subjectsFASEB J200418141657166915522911
- YeRRSchererPEAdiponectin, driver or passenger on the road to insulin sensitivity?Mol Metab20132313314124049728
- YamauchiT1KamonJMinokoshiYAdiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinaseNat Med20028111288129512368907
- NishimuraSManabeITakakiSAdipose natural regulatory B cells negatively control adipose tissue inflammationCell Metab2013185759766
- HowardJLordGMatareseGLeptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob miceJ Clin Invest199910481051105910525043
- HarveyAELashingerLMHaysDCalorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-κB activation, and inflammation-related gene expression in an insulin-like growth factor-1–dependent mannerPLoS One201495e9415124804677
- GardnerEMCaloric restriction decreases survival of aged mice in response to primary influenza infectionJ Gerontol A Biol Sci Med Sci200560668869415983169
- RitzBWAktanINogusaSGardnerEMEnergy restriction impairs natural killer cell function and increases the severity of influenza infection in young adult male C57BL/6 miceJ Nutr2008138112269227518936230
- ClinthorneJFAdamsDJFentonJIRitzBWGardnerEMShort-term re-feeding of previously energy-restricted C57BL/6 male mice restores body weight and body fat and attenuates the decline in natural killer cell function after primary influenza infectionJ Nutr201014081495150120534876
- BodenGChenXMozzoliMRyanIEffect of fasting on serum leptin in normal human subjectsJ Clin Endocrinol Metab1996819341934238784108
- MerlVPetersAOltmannsKMSerum adiponectin concentrations during a 72-hour fast in over- and normal-weight humansInt J Obes Relat Metab Disord20052989981001
- FazeliPKLunMKimSMFGF21 and the late adaptive response to starvation in humansJ Clin Invest2015125124601461126529252
- FaggioniRMoserAFeingoldKRGrunfeldCReduced leptin levels in starvation increase susceptibility to endotoxic shockAm J Pathol200015651781178710793089
- FujitaYYanagidaHMimoriTPrevention of fasting-mediated bone marrow atrophy by leptin administrationCell Immunol20122731525822196379
- BartzSModyAHornikCSevere acute malnutrition in childhood: hormonal and metabolic status at presentation, response to treatment, and predictors of mortalityJ Clin Endocrinol Metab20149962128213724606092
- KatonaPKatona-ApteJClinical practice: the interaction between nutrition and infectionClin Infect Dis200846101582158818419494
- CintiSMitchellGBarbatelliGAdipocyte death defines macrophage localization and function in adipose tissue of obese mice and humansJ Lipid Res200546112347235516150820
- BergerEHéraudSMojallalAPathways commonly dysregulated in mouse and human obese adipose tissue: FAT/CD36 modulates differentiation and lipogenesisAdipocyte20154316118026257990
- DroletRRichardCSnidermanADHypertrophy and hyperplasia of abdominal adipose tissues in womenInt J Obes (Lond)200832228329117726433
- FraynKNAdipose tissue as a buffer for daily lipid fluxDiabetologia20024591201121012242452
- MoriHPrestwichTCReidMASecreted Frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibitionJ Clin Invest201212272405241622728933
- SeidellJCPérusseLDesprésJPBouchardCWaist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family StudyAm J Clin Nutr200174331532111522554
- MilewiczAJędrzejukDDunajskaKLwowFWaist circumference and serum adiponectin levels in obese and non-obese postmenopausal womenMaturitas201065327227520004538
- GarauletMPerex-LlamasFFuenteTZamoraSTebarFJAnthropometric, computed tomography and fat cell data in an obese population: relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormonesEur J Endocrinol2000143565766611078990
- YusufSHawkenSOunpuuSObesity and the risk of myocardial infarction in 27 000 participants from 52 countries: a case-control studyLancet200536694971640164916271645
- WuDMolofskyABLiangHEEosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasisScience2011332602624324721436399
- PacificoLDi RenzoLAnaniaCIncreased T-helper interferon-γ-secreting cells in obese childrenEur J Endocrinol2006154569169716645016
- TalukdarSOhDYBandyopadhyayGNeutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastaseNat Med20121891407141222863787
- TheoharidesTCSismanopoulosNDelivanisDAZhangBHatziagelakiEEKalogeromitrosDMast cells squeeze the heart and stretch the gird: their role in atherosclerosis and obesityTrends Pharmacol Sci201132953454221741097
- FriedSKBunkinDAGreenbergASOmental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoidJ Clin Endocrinol Metab19988338478509506738
- HotamisligilGSShargillNSSpiegelmanBMAdipose expression of tumor necrosis factor-α: direct role in obesity-linked insulin resistanceScience1993259509187917678183
- WoodISWangBJenkinsJRTrayhurnPThe pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFα in human adipocytesBiochem Biophys Res Commun2005337242242916188228
- NeteaMGJoostenLALewisEDeficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistanceNat Med200612665065616732281
- HuangFDel-Río-NavarroBEPérez-OntiverosJAEffect of six-month lifestyle intervention on adiponectin, resistin and soluble tumor necrosis factor-α receptors in obese adolescentsEndocr J201461992193125029953
- PasaricaMSeredaORRedmanLMReduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic responseDiabetes200958371872519074987
- HarlevAAMacrophage infiltration and stress-signaling in omental and subcutaneous adipose tissue in diabetic pregnanciesJ Matern Fetal Neonatal Med201427121189119424111719
- MurphyKBasic concepts of immunologyJaneway’s Immunobiology8th edLondonTaylor and Francis2012136
- HotamisligilGSErbayENutrient sensing and inflammation in metabolic diseasesNat Rev Immunol200881292393419029988
- HosogaiNFukuharaAOshimaKAdipose tissue hypoxia in obesity and its impact on adipocytokine dysregulationDiabetes200756490191117395738
- Karvonen-GutierrezCAHarlowSDMancusoPJacobsonJde LeonCFNanBLeptin levels are associated with radiographic knee osteoarthritis among a cohort of mid-life womenArthritis Care Res (Hoboken)201365693694423281224
- Karvonen-GutierrezCAHarlowSDJacobsonJMancusoPJiangYThe relationship between longitudinal serum leptin measures and measures of magnetic resonance imaging-assessed knee joint damage in a population of mid-life womenAnn Rheum Dis201473588388923576710
- DattaroyDPourhoseiniSDasSMicro-RNA 21 inhibition of SMAD7 enhances fibrogenesis via leptin-mediated NADPH oxidase in experimental and human nonalcoholic steatohepatitisAm J Physiol Gastrointest Liver Physiol20153084G298G31225501551
- BodaryPFWestrickRJWickenheiserKJShenYEitzmanDTEffect of leptin on arterial thrombosis following vascular injury in miceJAMA2002287131706170911926895
- LimCCTeoBWTaiESElevated serum leptin, adiponectin and leptin to adiponectin ratio is associated with chronic kidney disease in Asian adultsPLoS one2015103e012200925793395
- Hasan-AliHAbd El-MottalebNAHamedHBAbd-ElsayedASerum adiponectin and leptin as predictors of the presence and degree of coronary atherosclerosisCoron Artery Dis201122426426921383620
- ShamsuzzamanASWinnickiMWolkRIndependent association between plasma leptin and C-reactive protein in healthy humansCirculation2004109182181218515117839
- PetersenKFOralEADufourSLeptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophyJ Clin Invest2002109101345135012021250
- CochranEYoungJRSebringNDePaoliAOralEAGordenPEfficacy of recombinant methionyl human leptin therapy for the extreme insulin resistance of the Rabson-Mendenhall syndromeJ Clin Endocrinol Metab20048941548155415070911
- HsuWYChaoYWTsaiYLResistin induces monocyte-endothelial cell adhesion by increasing ICAM-1 and VCAM-1 expression in endothelial cells via p38MAPK-dependent pathwayJ Cell Physiol201122682181218821520070
- VermaSLiSHWangCHResistin promotes endothelial cell activation: further evidence of adipokine-endothelial interactionCirculation2003108673674012874180
- GoralskiKBMcCarthyTCHannimanEAChemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolismJ Biol Chem200728238281752818817635925
- ErnstMCIssaMGoralskiKBSinalCJChemerin exacerbates glucose intolerance in mouse models of obesity and diabetesEndocrinology201015151998200720228173
- KlötingNGrahamTEBerndtJSerum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat massCell Metab200761798717618858
- BrochMRamírezRAuguetMTMacrophages are novel sites of expression and regulation of retinol binding protein-4 (RBP4)Physiol Res201059229930319537932
- YangQGrahamTEModyNSerum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetesNature2005436704935636216034410
- SamarasKBotelhoNKChisholmDJLordRVSubcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetesObesity (Silver Spring)201018588488920019678
- HemdahlALGabrielsenAZhuCExpression of neutrophil gelatinase-associated lipocalin in atherosclerosis and myocardial infarctionArterioscler Thromb Vasc Biol200626113614216254208
