Abstract
Systemic sclerosis (SSc or scleroderma) is a progressive and highly debilitating autoimmune disorder characterized by inflammation, vasculopathy, and extensive fibrosis. SSc is highly heterogeneous in its clinical presentation, extent and severity of skin and internal organ involvement, and clinical course and has the highest fatality rate among connective tissue diseases. While clinical outcomes have improved in recent years, no current therapy is able to reverse or slow the natural progression of SSc, a reflection of its complex pathogenesis. Although activation of the immune system has long been recognized, the mechanisms responsible for the initiation of autoimmunity and the role of immune effector pathways in the pathogenesis of SSc remain incompletely understood. This review summarizes recent progress in disease pathogenesis with particular focus on the immunopathogenetic mechanisms of SSc.
Introduction
Systemic sclerosis (SSc) is a rare disease with a prevalence ranging from 150 to 300 cases per million.Citation1,Citation2 Although SSc has a worldwide distribution, prevalence varies substantially around the world, with lower estimates (<150 per million) in Northern Europe and Japan and higher estimates (276–443 per million) in Southern Europe, North America, and Australia.Citation2 As in many other autoimmune diseases, women are at higher risk than men (4:1 ratio over men),Citation3–Citation5 and ethnicity plays a critical role in disease manifestations and mortality.Citation6 The etiology of SSc remains elusive, but it likely involves an interaction between environmental factors in a genetic predisposing background. Although SSc is not an inherited disease,Citation7 genetic factors contribute to its susceptibility,Citation8,Citation9 as shown by a 60-fold higher occurrence of the disease in families (1.6%) than in the general population (0.026%).Citation8 Genetic linkage studies and genome-wide association studies have identified polymorphisms associated with the predisposition of patients to develop SSc.Citation10–Citation15 These include genes of the major histocompatibility complex (MHC) class II,Citation9,Citation14,Citation16,Citation17 as well as non-MHC genes,Citation13,Citation18–Citation24 such as genes associated with the metabolism of extracellular matrix (ECM) moleculesCitation25–Citation27 and genes coding for proteins involved in the control of innate immunity, macrophage activation, and T-cell functions.Citation10,Citation14,Citation28–Citation32 Although progress has been made in the identification of genetic risk factors in SSc, the corresponding functional mechanisms remain elusive, except for the contribution of MHC class II to autoantibody specificity.Citation33–Citation38 Functional studies of associated loci are thus an area of current focus. Environmental factors have been implicated as early triggers of disease processes. Viruses, including human cytomegalovirus,Citation39 parvovirus B19,Citation40 and Epstein–Barr virus,Citation41 are hypothesized to contribute to the development of SSc by inducing vascular damage and fibroblast proliferation.Citation42 Other environmental factors, such as drugs as well as environmental and occupational exposures to organic solvents including vinyl chloride, silica,Citation43 and nanoparticles from traffic-derived pollution,Citation44 have also been implicated as potential causative agents. SSc exhibits an extensive patient-to-patient variability. Heterogeneity has been observed in its clinical manifestations, clinical course, response to treatment, and survival.Citation3 Based on the extent of skin fibrosis and the pattern of internal organ involvement, patients with SSc are commonly classified into diffuse cutaneous SSc (dcSSc) and limited cutaneous SSc (lcSSc) subsets.Citation45–Citation47 Patients with dcSSc have rapidly progressive fibrosis of the skin, lungs, and other internal organs and present early development of visceral organ complications. In contrast, in lcSSc, the most prominent features are vascular manifestations, with generally mild skin and internal organ fibrosis. Classification criteria for SSc have been recently updated by a joint committee of the American College of Rheumatology and the European League Against Rheumatism.Citation48 The American College of Rheumatology/European League Against Rheumatism classification criteria are more sensitive and specific than the previous criteria and now include patients in the early stages of SSc and lcSSc. The expectation is that earlier and more specific diagnosis will enable timely treatment before irreversible organ damage occurs.
Mechanisms of pathogenesis
The pathogenesis of SSc is poorly understood, which has hampered the development of effective therapeutics for this complex connective tissue disease. Research effort in understanding the key pathogenetic pathways, cell types, and mediators underlying disease manifestations is crucial for the early diagnosis of SSc as well as for the development of targeted therapies. Pathogenesis of SSc is characterized by three hallmarks: small-vessel vasculopathy, dysregulation of innate and adaptive immunity, and extensive fibrosis of the skin and visceral organs. Fibrosis is a major contributor to the high level of morbidity and mortality in SSc and is believed to result from the interaction of immune mediators and other growth factors with responsive tissue fibroblasts, resulting in increased deposition of ECM in the skin and internal organs.Citation49,Citation50 Although cutaneous fibrosis is the most characteristic feature of SSc, fibrosis of visceral organs results in organ damage and poor clinical outcome.
Clinical symptoms and histological data indicate that vascular injury and endothelial damage are the earliest pathogenic events in SSc,Citation51,Citation52 possibly initiated by viruses, autoantibodies, chemicals, or oxidative products.Citation52,Citation53 Activated endothelial cells upregulate the expression of adhesion molecules,Citation54 such as vascular cell adhesion protein 1, intercellular adhesion molecule, and E-selectin, as well as chemokines, such as MCP-1, MIP-1α, and MIP-1β, resulting in the recruitment of inflammatory cells. Endothelial cells also produce endothelin-1 and connective tissue growth factor, which stimulate vascular smooth-muscle cell proliferation and ECM production.Citation55,Citation56 Progressive thickening of the vessel wall results in a narrowing of the lumen of the capillaries and in the loss of the microvasculature, which leads to tissue hypoxia and oxidative stress.Citation51 Moreover, vascular repair and angiogenesis are found defective in SSc, promoting the chronic disease state.Citation57 Infiltration of inflammatory cells is prominent in patients with early-stage diseaseCitation58,Citation59 and is often seen in a perivascular distribution and preceding the development of vasculopathy and fibrosis.
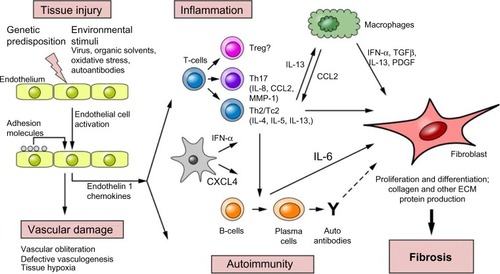
A schematic representation of SSc pathogenesis is illustrated in . This review focuses on the immune dysregulation processes associated with SSc pathogenesis and discusses the recent advances.
Figure 1 Etiopathogenesis of SSc.
Abbreviations: ECM, extracellular matrix; IFN, interferon; IL, interleukin; MMP-1, matrix metalloproteinases-1; PDGF, platelet-derived growth factor; SSc, systemic sclerosis; TGFβ, transforming growth factor beta; Treg, T-regulatory cell; ?, role unknown.
Immunopathogenesis of SSc
Immunological abnormalities of innate and adaptive immune system have long been recognized in SSc, including chronic mononuclear cell infiltration of affected tissues, dysregulation of cytokine and growth factor production, and production of autoantibodies.Citation60,Citation61 In addition, numerous genetic association studies have identified several polymorphisms in genes relevant for innate and adaptive immune responses that confer susceptibility to SSc.Citation10,Citation14 Polymorphisms in genes of the innate immune system include PLD4,Citation62 toll-like receptor (TLR)2,Citation31 NLRP1,Citation63 and ATG5.Citation13 Other polymorphisms associated with SSc are in genes that play important roles in T-cell differentiation, proliferation, and/or activation. Among those are STAT4,Citation64,Citation65 TBX21,Citation65 PTPN22,Citation66 tumor necrosis factor (TNF)SF4,Citation67,Citation68 interleukin (IL)-21,Citation69 CD247,Citation28,Citation70 and CD226.Citation71 Polymorphisms in gene regulators of interferon (IFN) types I and II, such as IFN-regulatory factor (IRF) 5Citation28,Citation72 and IRF8,Citation11 are also associated with SSc susceptibility. Other cytokines and chemokine genes associated with SSc include TNFAIP3,Citation73,Citation74 MIF,Citation75 IL-6,Citation76 CXCL8,Citation77 and CCR6.Citation78 However, the mechanisms responsible for the initiation of autoimmunity leading to fibrosis and the role of immune effector pathways in the pathogenesis of SSc remain incompletely understood.
Mononuclear cell infiltrates
Histological studies indicate that a perivascular inflammatory infiltrate accompanies endothelial cell damage in very early stages of SSc.Citation58,Citation59 Macrophages and T-lymphocytes are the predominant inflammatory cell types and are believed to produce cytokines and other immune mediators with proinflammatory and profibrotic function. Interestingly, in situ hybridization studies have demonstrated that collagen-synthesizing fibroblasts are located in close proximity to small blood vessels and to the perivascular inflammatory infiltrate,Citation60,Citation79 consistent with the hypothesis that inflammatory cells provide important stimuli that drive collagen synthesis in fibroblasts. Indeed, multiple studies in patients with early disease have demonstrated an association between macrophages, inflammation, and skinCitation80 and lung fibrosis.Citation81 Tissue-resident macrophages become profibrotic through “alternative activation” by type 2 cytokines, such as IL-13 (M2 macrophages), and produce transforming growth factor beta (TGFβ) with profibrotic function.Citation82 Indeed, increased levels of soluble CD163, a marker for M2 macrophages, were found in the blood and in the affected tissues of patients with early SSc.Citation83 Infiltrating T-cells in SSc-affected tissues exhibit increased expression of activation markers and express an oligoclonal T-cell receptor repertoire suggestive of an antigen-driven expansion.Citation84,Citation85 While their antigen specificity is not known, T-cell-derived cytokines have been implicated in the induction of fibrosis.Citation86 T-cells have also been found necessary for the production of autoantibodiesCitation87 and in driving inflammatory responses, which can involve concurrently fibroblasts as well as endothelial and epithelial cells. CD4+ and CD8+ T-cell subsets were both found in the skinCitation88 and lungsCitation89 of patients with SSc. However, we observed that CD8+ lymphocytes are more abundant than CD4+ T-cells in the skin of patients with early SSc, while in late-stage disease more CD4+ lymphocytes are found,Citation88 suggesting that CD8+ T-cells are involved in early disease processes. We and others established that SSc CD4+ and CD8+ are characterized by a predominant type 2 phenotypeCitation86,Citation88 and produce type 2 cytokines, such as IL-13. Moreover, we demonstrated that IL-13-producing CD8+ lymphocytes are abundant in the skin lesions of patients with early-stage disease and induce a profibrotic phenotype in fibroblasts.Citation88 Although Th17 cells have been found in the skin of patients,Citation90 several studies indicate that they do not play a direct role in skin fibrosis but contribute in boosting the inflammatory response in SSc.Citation91
Dysfunction of T-regulatory cells (Tregs) also seems to contribute to altered immune homeostasis in SSc, with some studies implicating a Treg-deficient suppressive functionCitation92–Citation94 and/or reduced numberCitation95–Citation97 and other studies indicating a redirected function of Tregs favoring fibrosis.Citation98
An activated B-cell signature has been found in lesional skin and affected the lung tissues of patients with SSc,Citation99,Citation100 with upregulation of cell-surface expression of CD19 and CD21,Citation101 costimulatory molecules, such as CD80 and CD86,Citation101 and B-cell activating factor,Citation102 a B-cell stimulatory molecule that induces B-cell proliferation, and immunoglobulin secretion.Citation103 CD19 associates with CD21 and positively regulates B-cell function.Citation104 CD19 overexpression induces the production of autoantibodiesCitation105 and skin fibrosisCitation106 in transgenic mouse models. Significantly, a single nucleotide polymorphism in the CD19 gene promoter (−499G>T) has been associated with higher CD19 expression in B-cells and with susceptibility to SSc,Citation107 consistent with a role in B-cell activation in SSc. Furthermore, recent studies have shown that SSc B-cells can induce contact-dependent human dermal fibroblasts activation and upregulation of type I collagenCitation108 and that depletion of B-cells in a mouse model of scleroderma led to reduced fibrosis.Citation109 Therefore, B-cell activation and overactivity is not only involved in autoantibodies production in SSc but might also contribute to the fibrotic process.
Several recent studies implicate TLR signaling as one of the early steps during inflammatory and fibrotic processes of SSc.Citation110,Citation111 TLR activation in SSc innate immune cells is believed to be triggered by microbial and endogenous ligands, such as products released from cells upon damage, necrosis, or stress.Citation110 A recent study showed that TLR2 is upregulated in SSc fibroblasts and responds to the acute-phase reactant serum amyloid A, resulting in increased IL-6 secretion by fibroblasts.Citation112,Citation113 Interestingly, a rare polymorphism in the gene for TLR2 is associated with the SSc phenotype and induces the production of inflammatory mediators.Citation31 TLR4 is overexpressed in SSc skin and lung biopsies,Citation114–Citation116 and its levels correlate with progressive skin disease.Citation114 Although the canonical ligand of TLR4 is LPS, numerous endogenous ligands have been shown to activate TLR4.Citation117 TLR4 can also respond to the alternative spliced fibronectin domain A (Fn-EDA), which is markedly upregulated in response to tissue damage and wound healing.Citation118 Interestingly, Fn-EDA was shown to be upregulated in the serum and skin biopsies of patients with SSc.Citation116 High levels of Fn-EDA were also found in idiopathic pulmonary fibrosisCitation119 and cardiac allograft fibrosis.Citation120 Significantly, in vitro and in vivo studies demonstrated that Fn-EDA promoted cutaneous fibrosis through TLR4 signaling, whereas its blockade led to reduced experimental fibrosis,Citation115,Citation116 supporting a model of endogenous Fn-EDA–TLR4 signaling axis in cutaneous fibrosis. Intracellular TLRs, such as TLR3, TLR7, TLR8, and TLR9, are sensors for nucleic acids, often of viral origin,Citation121 and have been implicated in driving inflammation and fibrosis in SSc.Citation110,Citation111 Of interest, Farina et alCitation41 reported an association between Epstein–Barr virus infection of SSc dermal fibroblasts and endothelial cells, activation of TLR, and upregulation of selected IRFs, IFN-stimulated genes, TGFβ, and several markers of fibroblast activation, such as smooth-muscle actin and endothelin-1. These results suggest that persistent injury following a viral infection of nonimmune cells might cause chronic inflammation and fibrosis. TLR activation in SSc immune cells triggers the production of several inflammatory cytokines, particularly type I IFNs. Indeed, an increased gene expression IFN “signature” has been found in peripheral blood mononuclear cells and in the skin of patients with SScCitation31,Citation122,Citation123 along with evidence suggesting TLR activity in SSc sera.Citation124 Moreover, TLR activation of dendritic cells and macrophages also stimulates IL-1, TNFα, and IL-6 production, and these or other undefined mediators might drive inflammation and fibrosis in SSc.
Immune mediators
Several cytokines and growth factors are released by immune cells and are believed to play a critical role in the inflammatory and fibrotic processes of SSc.Citation125,Citation126 Abnormal levels of cytokines, such as TGFβ,Citation127 TNFα, and IL-6, IL-10,Citation128 IL-17,Citation129 IL-4, and IL-13,Citation130 have been found in the serum and affected tissues of patients with SSc. Among other functions, these cytokines are thought to promote overproduction of collagen by fibroblasts, resulting in excessive fibrosis.Citation131 Monocytes and macrophages mainly produce TGFβ, IFN-α, IL-13, TNFα, and IL-1. Lesional macrophages are also a main source of platelet-derived growth factor (PDGF). Numerous studies have implicated PDGF working in concert with TGFβ in the development of organ fibrosis in SSc. These findings demonstrated the existence of a TGFβ and IL-1α-dependent autocrine PDGF-A/PDGF receptor α signaling loop in scleroderma skin and lung fibroblasts, which promotes fibrogenesis.Citation132,Citation133
IL-6 is also involved in the pathogenesis of SSc. A recent study reported that serum and skin levels of IL-6 are significantly increased in patients with early dcSSc and that a monoclonal anti-IL-6-receptor antibody prevents the development of bleomycin-induced dermal fibrosis in mice.Citation134 A clinical trial with an anti-IL-6-receptor antibody (tocilizumab) in SSc is completed, but the results have not been released (NCT01532869). B-cells are a main source of IL-6 in SSc,Citation135 but endothelial cells and fibroblasts also produce high levels of IL-6.Citation136
Recent studies have focused on TGFβ and to a lesser extent on IL-13 as major profibrotic factors in the pathogenesis of SSc. Recent advances in these studies are outlined below.
TGFβ has long been implicated in the pathogenesis of SSc.Citation137 Based on extensive in vitro and animal data and the correlation observed between disease activity and increased expression of TGFβ-regulated genes in fibrotic skin and lungs of patients with SSc,Citation137 TGFβ is considered as a key mediator of fibrosis in SSc. TGFβ promotes collagen synthesis, secretion, processing, and cross-linking,Citation137 as well as secretion of other matrix molecules, such as fibronectin and thrombospondin.Citation137 Although inhibition of TGFβ represents an ideal therapeutic approach in SSc, a recent clinical trial using the anti-TGFβ mAb CAT-192 failed to show any change in skin thickening, measured by the Modified Rodnan Skin Score, between treatment groups.Citation138 However, striking results were recently obtained in an open-label trial that used fresolimumab, a high-affinity neutralizing antibody, that targets all three TGFβ isoforms.Citation139 Patients with early-stage dcSSc treated with fresolimumab showed a rapid and significant decrease in Modified Rodnan Skin Score, which correlated closely with the inhibition of TGFβ-regulated gene expression. Thus, this study shows that fresolimumab reverses markers of skin fibrosis and holds promise as a potent antifibrotic agent.
Multiple studies indicate that the immunopathological response in SSc is dominated by type 2 cytokines, such as IL-4 and IL-13.Citation86,Citation126 Type 2 cytokines are important regulators of ECM remodeling, leading to enhanced collagen deposition and tissue fibrosis. Animal studies provide support for the role of a polarized immune response in the pathogenesis of fibrosis.Citation140,Citation141 Transcriptome analysis in animal models of inflammation has shown that genes involved in wound healing and fibrosis are associated with Th2-polarized responses,Citation142,Citation143 and IL-13 was shown to have an important role in the mouse model of bleomycin-induced fibrosis.Citation141 Increased levels of type 2 cytokines have been found in the serum and affected tissues of patients,Citation88,Citation125,Citation130 and we and others established that T-lymphocytesCitation88 and macrophagesCitation144 are the major cellular source in SSc. We demonstrated that dysregulated production of profibrotic IL-13 by peripheral blood effector CD8+ T-cells correlates with more severe skin thickening in SScCitation145 and is associated with defects in the molecular control of IL-13 production, such as the aberrant expression of the transcription factor GATA-3.Citation146 Circulating CD8+IL-13+ T-cells express skin-homing receptors and induce a profibrotic phenotype in normal dermal fibroblasts, which is inhibited by an anti-IL-13 antibody.Citation88 High number of CD8+IL-13+ T-cells were also found in the skin lesions of patients, particularly in the early inflammatory phase of the diseaseCitation88 and potentially contributing to the development of sustained profibrotic and inflammatory autoimmune responses. Two Phase II double-blind, randomized, placebo-controlled trials were started in SSc-related interstitial lung disease (ILD) and IPF with a fully human monoclonal antibody against human IL-13 (Clinical Trial Registration Number: NCT00581997). However, the study was terminated early due to concerns with risks associated with the bronchoscopy procedure involved, and no results ensued.
Chemokines play a crucial role in the inflammatory, vascular, and fibrotic processes of SSc. In all cases, chemokines provide a chemotactic signal to cells by binding to their specific cell-surface receptors. Chemokines, such as CCL18, CCL19, and CXCL13, were found upregulated in the skin of patients with dcSSc. Expression of CCL19 correlated with markers of vascular inflammation and macrophage recruitment and may represent a marker for the perivascular inflammation and immune cell recruitment in dcSSc skin disease.Citation147 Serum and tissue levels of CCL2, CCL3, and IL-8 are also increased in patients with SSc and correlate with disease severity and can predict progression.Citation148–Citation150 Plasmacytoid dendritic cells from patients were found to secrete high levels of CXCL4 (or platelet factor 4),Citation151 a chemokine with antiangiogenic function. Plasma levels of CXCL4 are increased in SSc and correlate with disease severity,Citation151 including lung fibrosis and pulmonary arterial hypertension.Citation151 A recent study shows that the expression and function of CCR1, CCR2, and CCR3 are upregulated in monocytes from patients with SSc via molecular mechanisms involving caveolin-1, Src/Lyn, and MEK/ERK signaling and represent promising targets for novel treatments for fibrotic diseases, such as SSc.Citation152
Autoantibodies
Serum autoantibodies directed against a variety of intracellular antigens are present in nearly all patients and are considered a hallmark of SSc.Citation153 Many of these autoantibodies are specific to nuclear antigens and play no role in the pathogenesis of the disease. However, they represent important diagnostic and prognostic agents and exhibit a strong association with distinct clinical subsets, which has been confirmed in many independent patient cohorts.Citation153 More recently, autoantibodies targeting cell-surface antigens and/or extracellular proteins have been detected in the serum of patients with SSc. These autoantibodies have been shown in some studies to be functional, as they were capable of triggering receptor activation and eliciting profibrotic responses. Several patients have been reported to have circulating autoantibodies against the PDGF receptor.Citation154 These antibodies were shown to generate reactive oxygen species and stimulate myofibroblast differentiation and type 1 collagen production. Autoantibodies against the angiotensin II receptor type 1 and endothelin receptor type A have been recently identified in patients with SSc and are believed to stimulate production of IL-18 and CCL18 by mononuclear blood cells.Citation155 Antiendothelial cell antibodies have been detected in the sera of some patients with SSc and have been shown to induce endothelial cell apoptosis in vitro.Citation156–Citation158 Finally, antibodies against fibroblasts,Citation159,Citation160 fibrillin,Citation161 and matrix metalloproteinases-1Citation162 and -3Citation163 were found, which are also believed to carry biological activities.
Immunosuppressive and immunomodulatory therapies in SSc
Therapeutic options in SSc are limited due to the multisystem involvement of this disease and the wide spectrum of clinical features. Current therapeutic strategies include general immunosuppression and organ-based therapies for the improvement of symptoms. More specific therapies for SSc are currently unavailable. Current or completed clinical studies of immunotherapeutic candidates are reported in .
Table 1 Clinical studies of immunotherapeutic candidates in systemic sclerosis
Immunosuppressive therapy has been commonly used to control the inflammatory phase of patients with progressive or early-stage disease. However, multiple studies have demonstrated the inefficacy of this therapy in affecting the fibrotic manifestations and the potential for severe secondary effects. Immunosuppressive agents have been used on more aggressive forms of SSc skin disease, such as early dcSSc.Citation164 However, no conclusive trials are currently available to guide management of SSc skin involvement. This is due to the lack of sensitive and specific outcome measures and to the normally variable history of SSc.Citation165 An improvement in skin score was observed in two multicenter, randomized, controlled trials in which methotrexateCitation166 and cyclophosphamideCitation167 were used. In addition, two case series at scleroderma centers indicated the efficacy of mycophenolate mofetil in the treatment of skin disease.Citation168,Citation169
Immunosuppressive therapy has shown benefits in the treatment of SSc-ILD in those patients with severe lung involvement. Several clinical trials have shown the efficacy of cyclophosphamide in improvingCitation167,Citation170,Citation171 and/or stabilizingCitation172–Citation176 lung function parameters. Other frequently used immunosuppressants in ILD include mycophenolate mofetil and azathioprine.Citation177 While immunosuppressive therapy is advised for patients with early stage, progressive SSc, lung transplantation can be considered for end-stage disease.Citation178
Imatinib is a powerful inhibitor of PDGF and TGFβ signaling pathways and has been evaluated in multiple clinical studies to establish effectiveness on skinCitation179,Citation180 and lungCitation181,Citation182 fibrosis. Outcomes from these studies provided controversial results on efficacy and demonstrated the poor tolerability of this drug. In a recent study, low-dose imatinib was used in a cohort of patients with SSc-ILD with active pulmonary disease and unresponsive to cyclophosphamide.Citation182 Of note, 73% of the 30 patients treated had improved or stabilized pulmonary disease after 6 months’ treatment. Despite these encouraging results, the risk/benefit ratio for the use of imatinib needs to be determined in larger controlled trials.
Rituximab, an inhibitor of B-cell function, has also shown promise as a new therapeutic option in various manifestations of SSc, particularly ILD. In an open-label clinical trial, rituximab treatment improved skin scores and preserved the pulmonary function of patients with early progressive dcSSc.Citation183 Moreover, rituximab was well tolerated by patients even after repeated courses of treatment.Citation183 As no control group was used in this study, a double-blind, randomized control trial is going on to confirm these results. Efficacy on skin thickness and lung function was also observed after rituximab treatment in a case–control study using the European Scleroderma Trial and Research cohort.Citation184
Intravenous immunoglobulin (IVIG) is another potential agent for skin involvement. The role of IVIG in SSc is currently unknown. However, IVIG has demonstrated to have immunomodulatory and anti-inflammatory effects in other autoimmune disorders as well as an antifibrotic effect in several animal models.Citation185 Multiple courses of IVIG treatment were employed in a multicenter, randomized, controlled clinical trial.Citation186 The outcome of this study demonstrated a beneficial effect on skin score. Similarly, improvement in skin involvement was also observed in a single-center retrospective study, in which patients with active refractory dcSSc received monthly courses of IVIG (with or without immunosuppressive therapies).Citation187
High-dose immunosuppressive therapy followed by autologous hematopoietic stem-cell transplantation (HSCT) is an emerging treatment option for patients with early progressive SSc who are refractory to conventional treatments.Citation177–Citation181 Clinical trials, such as the American Scleroderma Stem Cell versus Immune Suppression TrialCitation188 and the Autologous Stem-Cell Transplantation International Scleroderma trial,Citation189 have shown efficacy in preventing disease progression. In both trials, HSCT was shown to cause an improvement in skin and lung involvement as well as vasculopathy and was able to correct immune abnormalities. Despite its potential benefits, HSCT is a dangerous therapeutic option, which is associated with a high risk of treatment-related mortality and an increase in serious adverse events. Its use, therefore, is limited to severe cases of SSc and administered only as a part of a research protocol. Two large multicenter trials are going on. One trial compares monthly intravenous cyclophosphamide to myeloablation with cyclophosphamide and total body irradiation (Scleroderma: Cyclophosphamide or Transplantation) (ClinicalTrials.gov identifier NCT00114530). The second trial (Scleroderma Treatment with Autologous Transplant) includes myeloablation followed by HSC transplantation and long-term immunosuppression (mycophenylate) for dcSSc (ClinicalTrials. gov identifier NCT01413100).
Mesenchymal stem cell (MSC)-based therapy represents an alternative potential therapeutic approach for SSc, with fewer long-term side effects.Citation190–Citation192 Several in vitro studies have demonstrated that MSCs display specific immunomodulatory and immunosuppressive properties as well as regenerative potential.Citation193–Citation195 Their most important immunosuppressive effects are on T-cell proliferation and dendritic cell differentiationCitation191,Citation192,Citation196–Citation199 as well as the production of immunosuppressive mediators, such as TGFβ,Citation200 prostaglandin E2, and indoleamine 2,3-deoxygenase.Citation201 A recent report demonstrated that MSCs from patients with SSc while supporting normal hemopoiesis and retaining their immunosuppressive properties on T-cells also exhibit an increased expression of TGFβ receptor type II compared to MSCs from healthy donors, which leads to increased activation of TGFβ signaling and synthesis of COL1A1,Citation200,Citation202 thereby contributing to SSc pathogenesis. While this defect limits the clinical use of autologous MSCs in SSc, it supports the use of allogeneic MSCs instead. Two recent clinical case studiesCitation203,Citation204 describe the use of allogeneic MSCs in patients with severe refractory SSc. In one study, a significant decrease in the number of digital ulcers and skin thickness was observed after 3 months and 6 months, respectively, from intravenous injection of a patient with MSCs.Citation203 The second study reported skin improvement in two out of the four cases analyzed and observed no major side effects for several months from MSCs’ injections.Citation204 Although these results are encouraging, no conclusions about the efficacy of allogeneic MSCs in SSc can be yet drawn because of the limited number of patients tested. Moreover, additional studies are necessary to better understand the underlying MSC-immunomodulatory mechanisms as well as the role of MSCs in the pathogenesis of SSc. Furthermore, preclinical and clinical data that underlie the therapeutic potential of MSCs in patients with SSc are also necessary.
Conclusion
SSc is a complex multisystem disorder with heterogeneous clinical features that results from individual genetic background and exposure to environmental triggers. Pathogenesis of SSc is dominated by a complex interrelation between vascular, immunologic, and fibrotic processes, and it is poorly understood. Clinical outcomes in SSc have improved considerably in recent years, which may reflect improvements in the early detection and better management of significant complications, such as renal crisis or pulmonary arterial hypertension. However, SSc continues to exhibit high mortality, and it is still considered an incurable disease. Research efforts toward understanding the cellular and molecular basis of scleroderma aim to reveal novel molecular targets and diagnostic agents, leading to early and accurate diagnosis and innovative therapies against this disease. Next-generation sequencing and other cutting-edge technologies applied to affected tissues or cells will be crucial for the identification of biomarkers and pathways that are uniquely expressed in patients and are associated with disease form and/or stage. The development of preclinical models, including animal models that accurately recapitulate human disease, will be essential tools for the ultimate goal of finding a cure for this disease.
Personalized medicine offers interesting opportunities in SSc. Genomic and proteomic studies coupled with novel computational approaches have led to the identification of several biomarker signatures in patients with SSc,Citation15,Citation205–Citation208 which allowed the classification of related patients for more specific treatment. Advances in personalized medicine could be used for objective assessment of responses to clinical trials as well as for developing more effective therapies tailored to a patient’s genome or to the molecular and cellular contents.
Disclosure
The author reports no conflicts of interest in this work.
References
- GabrielliAAvvedimentoEVKriegTSclerodermaN Engl J Med2009360191989200319420368
- BarnesJMayesMDEpidemiology of systemic sclerosis: incidence, prevalence, survival, risk factors, malignancy, and environmental triggersCurr Opin Rheumatol201224216517022269658
- MedsgerTAJrNatural history of systemic sclerosis and the assessment of disease activity, severity, functional status, and psychologic well-beingRheum Dis Clin North Am2003292255273vi12841294
- MedsgerTAJrClassification, prognosisClementsPJFurstDESystemic Sclerosis2nd edPhiladelphiaLippincott Williams and Williams20041728
- MayesMDScleroderma epidemiologyRheum Dis Clin North Am200329223925412841293
- GelberACMannoRLShahAARace and association with disease manifestations and mortality in scleroderma: a 20-year experience at the Johns Hopkins Scleroderma Center and review of the literatureMedicine201392419120523793108
- Feghali-BostwickCMedsgerTAJrWrightTMAnalysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodiesArthritis Rheum20034871956196312847690
- ArnettFCChoMChatterjeeSAguilarMBReveilleJDMayesMDFamilial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohortsArthritis Rheum20014461359136211407695
- LuoYWangYWangQXiaoRLuQSystemic sclerosis: genetics and epigeneticsJ Autoimmun20134116116723415078
- RamosPSSilverRMFeghali-BostwickCAGenetics of systemic sclerosis: recent advancesCurr Opin Rheumatol201527652152926317679
- GorlovaOMartinJERuedaBIdentification of novel genetic markers associated with clinical phenotypes of systemic sclerosis through a genome-wide association strategyPLoS Genet201177e100217821779181
- MartinJEAssassiSDiaz-GalloLMA systemic sclerosis and systemic lupus erythematosus pan-meta-GWAS reveals new shared susceptibility lociHum Mol Genet201322194021402923740937
- MayesMDBossini-CastilloLGorlovaOImmunochip analysis identifies multiple susceptibility loci for systemic sclerosisAm J Hum Genet2014941476124387989
- JinJChouCLimaMZhouDZhouXSystemic sclerosis is a complex disease associated mainly with immune regulatory and inflammatory genesOpen Rheumatol J20148294225328554
- MahoneyJMTaroniJMartyanovVSystems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphismsPLoS Comput Biol2015111e100400525569146
- ArnettFCGourhPSheteSMajor histocompatibility complex (MHC) class II alleles, haplotypes and epitopes which confer susceptibility or protection in systemic sclerosis: analyses in 1300 Caucasian, African-American and Hispanic cases and 1000 controlsAnn Rheum Dis201069582282719596691
- GladmanDDKungTNSiannisFPellettFFarewellVTLeePHLA markers for susceptibility and expression in sclerodermaJ Rheumatol20053281481148716078323
- PattanaikDBrownMPostlethwaiteBCPostlethwaiteAEPathogenesis of systemic sclerosisFront Immunol2015627226106387
- AgarwalSKThe genetics of systemic sclerosisDiscov Med2010105113414320807474
- MartinJEBossini-CastilloLMartinJUnraveling the genetic component of systemic sclerosisHum Genet201213171023103722218928
- ZochlingJNewellFCharlesworthJCAn Immunochip-based interrogation of scleroderma susceptibility variants identifies a novel association at DNASE1L3Arthritis Res Ther201416543825332064
- AllanoreYSaadMDieudéPGenome-wide scan identifies TNIP1, PSORS1C1, and RHOB as novel risk loci for systemic sclerosisPLoS Genet201177e100209121750679
- Bossini-CastilloLMartinJEBroenJConfirmation of TNIP1 but not RHOB and PSORS1C1 as systemic sclerosis risk factors in a large independent replication studyAnn Rheum Dis201372460260722896740
- AssassiSRadstakeTRMayesMDMartinJGenetics of scleroderma: implications for personalized medicine?BMC Med201311923311619
- FonsecaCLindahlGEPonticosMA polymorphism in the CTGF promoter region associated with systemic sclerosisN Engl J Med2007357121210122017881752
- SalazarGMayesMDGenetics, epigenetics, and genomics of systemic sclerosisRheum Dis Clin North Am201541334536626210123
- WipffJDieudePAvouacJAssociation of metalloproteinase gene polymorphisms with systemic sclerosis in the European Caucasian populationJ Rheumatol201037359960220110530
- RadstakeTRGorlovaORuedaBGenome-wide association study of systemic sclerosis identifies CD247 as a new susceptibility locusNat Genet201042542642920383147
- López-IsacEBossini-CastilloLSimeonCPA genome-wide association study follow-up suggests a possible role for PPARG in systemic sclerosis susceptibilityArthritis Res Ther2014161R624401602
- López-IsacEBossini-CastilloLGuerraSGIdentification of IL12RB1 as a novel systemic sclerosis susceptibility locusArthritis Rheumatol201466123521352325199642
- BroenJCBossini-CastilloLvan BonLA rare polymorphism in the gene for Toll-like receptor 2 is associated with systemic sclerosis phenotype and increases the production of inflammatory mediatorsArthritis Rheum201264126427121905008
- ArismendiMGiraudMRuzehajiNIdentification of NF-kappaB and PLCL2 as new susceptibility genes and highlights on a potential role of IRF8 through interferon signature modulation in systemic sclerosisArthritis Res Ther2015177125880423
- ReveilleJDOwerbachDGoldsteinRMoredaRIsernRAArnettFCAssociation of polar amino acids at position 26 of the HLA-DQB1 first domain with the anticentromere autoantibody response in systemic sclerosis (scleroderma)J Clin Invest1992894120812131556182
- ReveilleJDDurbanEMacLeod-St ClairMJAssociation of amino acid sequences in the HLA-DQB1 first domain with antitopoisomerase I autoantibody response in scleroderma (progressive systemic sclerosis)J Clin Invest19929039739801326003
- KuwanaMOkanoYKaburakiJInokoHHLA class II genes associated with anticentromere antibody in Japanese patients with systemic sclerosis (scleroderma)Ann Rheum Dis199554129839878546531
- FalknerDWilsonJFertigNClawsonKMedsgerTAJrMorelPAStudies of HLA-DR and DQ alleles in systemic sclerosis patients with autoantibodies to RNA polymerases and U3-RNP (fibrillarin)J Rheumatol20002751196120210813287
- JohnsonRWTewMBArnettFCThe genetics of systemic sclerosisCurr Rheumatol Rep2002429910711890874
- NguyenBMayesMDArnettFCHLA-DRB1*0407 and *1304 are risk factors for scleroderma renal crisisArthritis Rheum201163253053421280007
- LunardiCBasonCNavoneRSystemic sclerosis immunoglobulin G autoantibodies bind the human cytomegalovirus late protein UL94 and induce apoptosis in human endothelial cellsNat Med20006101183118611017152
- ZakrzewskaKCorcioliFCarlsenKMHuman parvovirus B19 (B19V) infection in systemic sclerosis patientsIntervirology200952527928219672101
- FarinaACironeMYorkMEpstein-Barr virus infection induces aberrant TLR activation pathway and fibroblast-myofibroblast conversion in sclerodermaJ Invest Dermatol2014134495496424129067
- FerriCGiuggioliDColaciMViral infections and systemic sclerosisClin Exp Rheumatol2014326 suppl 86S–229
- NietertPJSilverRMSystemic sclerosis: environmental and occupational risk factorsCurr Opin Rheumatol200012652052611092202
- MastrofrancescoAAlfèMRosatoEProinflammatory effects of diesel exhaust nanoparticles on scleroderma skin cellsJ Immunol Res2014201413875124982919
- LeRoyECBlackCFleischmajerRScleroderma (systemic sclerosis): classification, subsets and pathogenesisJ Rheumatol19881522022053361530
- LeRoyECMedsgerTAJrCriteria for the classification of early systemic sclerosisJ Rheumatol20012871573157611469464
- IngegnoliFGualtierottiRA systematic overview on the use and relevance of capillaroscopy in systemic sclerosisExpert Rev Clin Immunol20139111091109724147535
- van den HoogenFKhannaDFransenJ2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiativeArthritis Rheum201365112737274724122180
- KatsumotoTRWhitfieldMLConnollyMKThe pathogenesis of systemic sclerosisAnnu Rev Pathol2011650953721090968
- JinninMMechanisms of skin fibrosis in systemic sclerosisJ Dermatol2010371112520175837
- TrojanowskaMCellular and molecular aspects of vascular dysfunction in systemic sclerosisNat Rev Rheumatol20106845346020585340
- VargaJAbrahamDSystemic sclerosis: a prototypic multisystem fibrotic disorderJ Clin Invest2007117355756717332883
- KahalehMBRaynaud phenomenon and the vascular disease in sclerodermaCurr Opin Rheumatol200416671872215577610
- GruschwitzMvon den DrieschPKellnerIHornsteinOPSterryWExpression of adhesion proteins involved in cell-cell and cell-matrix interactions in the skin of patients with progressive systemic sclerosisJ Am Acad Dermatol1992272 Pt 11691771430352
- KawaguchiYSuzukiKHaraMIncreased endothelin-1 production in fibroblasts derived from patients with systemic sclerosisAnn Rheum Dis19945385065107944634
- Shi-WenXDentonCPDashwoodMRFibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1J Invest Dermatol2001116341742511231316
- CiprianiPDi BenedettoPCapeceDImpaired Cav-1 expression in SSc mesenchymal cells upregulates VEGF signaling: a link between vascular involvement and fibrosisFibrogenesis Tissue Repair201471325237397
- RoummADWhitesideTLMedsgerTAJrRodnanGPLymphocytes in the skin of patients with progressive systemic sclerosis. Quantification, subtyping, and clinical correlationsArthritis Rheum19842766456536375682
- FleischmajerRPerlishJSReevesJRCellular infiltrates in scleroderma skinArthritis Rheum1977204975984861067
- JimenezSADerkCTFollowing the molecular pathways toward an understanding of the pathogenesis of systemic sclerosisAnn Intern Med20041401375014706971
- SakkasLIPlatsoucasCDIs systemic sclerosis an antigen-driven T cell disease?Arthritis Rheum20045061721173315188347
- TeraoCOhmuraKKawaguchiYPLD4 as a novel susceptibility gene for systemic sclerosis in a Japanese populationArthritis Rheum201365247248023124809
- DieudéPGuedjMWipffJNLRP1 influences the systemic sclerosis phenotype: a new clue for the contribution of innate immunity in systemic sclerosis-related fibrosing alveolitis pathogenesisAnn Rheum Dis201170466867421149496
- RuedaBBroenJSimeonCThe STAT4 gene influences the genetic predisposition to systemic sclerosis phenotypeHum Mol Genet200918112071207719286670
- GourhPAgarwalSKDivechaDPolymorphisms in TBX21 and STAT4 increase the risk of systemic sclerosis: evidence of possible gene-gene interaction and alterations in Th1/Th2 cytokinesArthritis Rheum200960123794380619950257
- RieckMArechigaAOnengut-GumuscuSGreenbaumCConcannonPBucknerJHGenetic variation in PTPN22 corresponds to altered function of T and B lymphocytesJ Immunol200717974704471017878369
- GourhPArnettFCTanFKAssociation of TNFSF4 (OX40L) polymorphisms with susceptibility to systemic sclerosisAnn Rheum Dis201069355055519778912
- Bossini-CastilloLBroenJCSimeonCPA replication study confirms the association of TNFSF4 (OX40L) polymorphisms with systemic sclerosis in a large European cohortAnn Rheum Dis201170463864121187296
- Diaz-GalloLMSimeonCPBroenJCImplication of IL-2/IL-21 region in systemic sclerosis genetic susceptibilityAnn Rheum Dis20137271233123823172754
- DieudéPBoileauCGuedjMIndependent replication establishes the CD247 gene as a genetic systemic sclerosis susceptibility factorAnn Rheum Dis20117091695169621474487
- DieudéPGuedjMTruchetetMEAssociation of the CD226 Ser(307) variant with systemic sclerosis: evidence of a contribution of costimulation pathways in systemic sclerosis pathogenesisArthritis Rheum20116341097110521162102
- DieudePDawidowiczKGuedjMPhenotype-haplotype correlation of IRF5 in systemic sclerosis: role of 2 haplotypes in disease severityJ Rheumatol201037598799220231204
- DieudéPGuedjMWipffJAssociation of the TNFAIP3 rs5029939 variant with systemic sclerosis in the European Caucasian populationAnn Rheum Dis201069111958196420511617
- KoumakisEGiraudMDieudéPBrief report: candidate gene study in systemic sclerosis identifies a rare and functional variant of the TNFAIP3 locus as a risk factor for polyautoimmunityArthritis Rheum20126482746275222488580
- WuSPLengLFengZMacrophage migration inhibitory factor promoter polymorphisms and the clinical expression of sclerodermaArthritis Rheum200654113661366917075815
- CénitMCSimeónCPVonkMCInfluence of the IL6 gene in susceptibility to systemic sclerosisJ Rheumatol201239122294230223027890
- SalimPHJobimMBredemeierMCombined effects of CXCL8 and CXCR2 gene polymorphisms on susceptibility to systemic sclerosisCytokine201260247347722763041
- KoumakisEBouazizMDieudéPA regulatory variant in CCR6 is associated with susceptibility to antitopoisomerase-positive systemic sclerosisArthritis Rheum201365123202320823983073
- ScharffetterKLankat-ButtgereitBKriegTLocalization of collagen mRNA in normal and scleroderma skin by in-situ hybridizationEur J Clin Invest19881819173130266
- Higashi-KuwataNJinninMMakinoTCharacterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosisArthritis Res Ther2010124R12820602758
- ChristmannRBSampaio-BarrosPStifanoGAssociation of Interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosisArthritis Rheumatol201466371472524574232
- MurrayPJAllenJEBiswasSKMacrophage activation and polarization: nomenclature and experimental guidelinesImmunity2014411142025035950
- NakayamaWJinninMMakinoKSerum levels of soluble CD163 in patients with systemic sclerosisRheumatol Int201232240340721120485
- SakkasLIXuBArtlettCMLuSJimenezSAPlatsoucasCDOligoclonal T cell expansion in the skin of patients with systemic sclerosisJ Immunol200216873649365911907131
- KalogerouAGelouEMountantonakisSSettasLZafiriouESakkasLEarly T cell activation in the skin from patients with systemic sclerosisAnn Rheum Dis20056481233123516014686
- ChizzoliniCT cells, B cells, and polarized immune response in the pathogenesis of fibrosis and systemic sclerosisCurr Opin Rheumatol200820670771218946333
- KuwanaMMedsgerTAJrWrightTMT and B cell collaboration is essential for the autoantibody response to DNA topoisomerase I in systemic sclerosisJ Immunol19951555270327147650398
- FuschiottiPLarreginaATHoJFeghali-BostwickCMedsgerTAJrInterleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosisArthritis Rheum201365123624623001877
- LuzinaIGAtamasSPWiseROccurrence of an activated, profibrotic pattern of gene expression in lung CD8+ T cells from scleroderma patientsArthritis Rheum20034882262227412905481
- TruchetetMEBrembillaNCMontanariEInterleukin-17A+ cell counts are increased in systemic sclerosis skin and their number is inversely correlated with the extent of skin involvementArthritis Rheum20136551347135623335253
- BrembillaNCMontanariETruchetetMERaschiEMeroniPChizzoliniCTh17 cells favor inflammatory responses while inhibiting type I collagen deposition by dermal fibroblasts: differential effects in healthy and systemic sclerosis fibroblastsArthritis Res Ther2013155R15124289089
- RadstakeTRvan BonLBroenJIncreased frequency and compromised function of T regulatory cells in systemic sclerosis (SSc) is related to a diminished CD69 and TGFbeta expressionPLoS One200946e598119543397
- AntigaEQuaglinoPBellandiSRegulatory T cells in the skin lesions and blood of patients with systemic sclerosis and morphoeaBr J Dermatol201016251056106320105169
- SlobodinGAhmadMSRosnerIRegulatory T cells (CD4(+) CD25(bright)FoxP3(+)) expansion in systemic sclerosis correlates with disease activity and severityCell Immunol20102612778020096404
- MathianAParizotCDorghamKActivated and resting regulatory T cell exhaustion concurs with high levels of interleukin-22 expression in systemic sclerosis lesionsAnn Rheum Dis20127171227123422696687
- KleinSKretzCCRulandVReduction of regulatory T cells in skin lesions but not in peripheral blood of patients with systemic sclerodermaAnn Rheum Dis20117081475148121097800
- WangYYWangQSunXHDNA hypermethylation of the forkhead box protein 3 (FOXP3) promoter in CD4+ T cells of patients with systemic sclerosisBr J Dermatol20141711394724641670
- MacDonaldKGDawsonNAHuangQDunneJVLevingsMKBroadyRRegulatory T cells produce profibrotic cytokines in the skin of patients with systemic sclerosisJ Allergy Clin Immunol20151354e946e949
- WhitfieldMLFinlayDRMurrayJISystemic and cell type-specific gene expression patterns in scleroderma skinProc Natl Acad Sci U S A200310021123191232414530402
- LafyatisRO’HaraCFeghali-BostwickCAMattesonEB cell infiltration in systemic sclerosis-associated interstitial lung diseaseArthritis Rheum20075693167316817763433
- SatoSFujimotoMHasegawaMTakeharaKAltered blood B lymphocyte homeostasis in systemic sclerosis: expanded naive B cells and diminished but activated memory B cellsArthritis Rheum20045061918192715188368
- MatsushitaTHasegawaMYanabaKKoderaMTakeharaKSatoSElevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytesArthritis Rheum200654119220116385515
- MackayFBrowningJLBAFF: a fundamental survival factor for B cellsNat Rev Immunol20022746547512094221
- TedderTFInaokiMSatoSThe CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunityImmunity1997621071189047233
- SatoSHasegawaMFujimotoMTedderTFTakeharaKQuantitative genetic variation in CD19 expression correlates with autoimmunityJ Immunol2000165116635664311086109
- YoshizakiAIwataYKomuraKCD19 regulates skin and lung fibrosis via Toll-like receptor signaling in a model of bleomycin-induced sclerodermaAm J Pathol200817261650166318467694
- TsuchiyaNKurokiKFujimotoMAssociation of a functional CD19 polymorphism with susceptibility to systemic sclerosisArthritis Rheum200450124002400715593213
- FrancoisAChatelusEWachsmannDB lymphocytes and B-cell activating factor promote collagen and profibrotic markers expression by dermal fibroblasts in systemic sclerosisArthritis Res Ther2013155R16824289101
- HasegawaMHamaguchiYYanabaKB-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosisAm J Pathol2006169395496616936269
- BhattacharyyaSVargaJEmerging roles of innate immune signaling and toll-like receptors in fibrosis and systemic sclerosisCurr Rheumatol Rep201517147425604573
- FullardNO’ReillySRole of innate immune system in systemic sclerosisSemin Immunopathol201537551151726159672
- O’ReillySCantRCiechomskaMSerum amyloid A induces interleukin-6 in dermal fibroblasts via Toll-like receptor 2, interleukin-1 receptor-associated kinase 4 and nuclear factor-kappaBImmunology2014143333134024476318
- LakotaKCarnsMPodluskySSerum amyloid A is a marker for pulmonary involvement in systemic sclerosisPLoS One2015101e011082025629975
- StifanoGAffandiAJMathesALChronic Toll-like receptor 4 stimulation in skin induces inflammation, macrophage activation, transforming growth factor beta signature gene expression, and fibrosisArthritis Res Ther2014164R13624984848
- BhattacharyyaSKelleyKMelichianDSToll-like receptor 4 signaling augments transforming growth factor-beta responses: a novel mechanism for maintaining and amplifying fibrosis in sclerodermaAm J Pathol2013182119220523141927
- BhattacharyyaSTamakiZWangWFibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signalingSci Transl Med20146232232ra250
- ChenGYNuñezGSterile inflammation: sensing and reacting to damageNat Rev Immunol2010101282683721088683
- OkamuraYWatariMJerudESThe extra domain A of fibronectin activates Toll-like receptor 4J Biol Chem200127613102291023311150311
- MuroAFMorettiFAMooreBBAn essential role for fibronectin extra type III domain A in pulmonary fibrosisAm J Respir Crit Care Med2008177663864518096707
- BoothAJWoodSCCornettAMRecipient-derived EDA fibronectin promotes cardiac allograft fibrosisJ Pathol2012226460961821960174
- KawaiTAkiraSToll-like receptors and their crosstalk with other innate receptors in infection and immunityImmunity201134563765021616434
- TanFKZhouXMayesMDSignatures of differentially regulated interferon gene expression and vasculotrophism in the peripheral blood cells of systemic sclerosis patientsRheumatology200645669470216418202
- YorkMRNagaiTManginiAJLemaireRvan SeventerJMLafyatisRA macrophage marker, Siglec-1, is increased on circulating monocytes in patients with systemic sclerosis and induced by type I interferons and toll-like receptor agonistsArthritis Rheum20075631010102017328080
- KimDPeckASanterDInduction of interferon-alpha by scleroderma sera containing autoantibodies to topoisomerase I: association of higher interferon-alpha activity with lung fibrosisArthritis Rheum20085872163217318576347
- BarautJMichelLVerrecchiaFFargeDRelationship between cytokine profiles and clinical outcomes in patients with systemic sclerosisAutoimmun Rev2010102657320713187
- FuschiottiPRole of IL-13 in systemic sclerosisCytokine201156354454921920770
- DentonCPAbrahamDJTransforming growth factor-beta and connective tissue growth factor: key cytokines in scleroderma pathogenesisCurr Opin Rheumatol200113650551111698729
- SatoSHasegawaMTakeharaKSerum levels of interleukin-6 and interleukin-10 correlate with total skin thickness score in patients with systemic sclerosisJ Dermatol Sci200127214014611532378
- KurasawaKHiroseKSanoHIncreased interleukin-17 production in patients with systemic sclerosisArthritis Rheum200043112455246311083268
- HasegawaMFujimotoMKikuchiKTakeharaKElevated serum levels of interleukin 4 (IL-4), IL-10, and IL-13 in patients with systemic sclerosisJ Rheumatol19972423283329034992
- KissinEYKornJHFibrosis in sclerodermaRheum Dis Clin North Am200329235136912841299
- BonnerJCRegulation of PDGF and its receptors in fibrotic diseasesCytokine Growth Factor Rev200415425527315207816
- TrojanowskaMRole of PDGF in fibrotic diseases and systemic sclerosisRheumatology200847suppl 5v2v418784131
- DesallaisLAvouacJFréchetMTargeting IL-6 by both passive or active immunization strategies prevents bleomycin-induced skin fibrosisArthritis Res Ther2014164R15725059342
- BoselloSLDe LucaGRuccoMLong-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosisSemin Arthritis Rheum201544442843625300701
- KochAEKronfeld-HarringtonLBSzekaneczZIn situ expression of cytokines and cellular adhesion molecules in the skin of patients with systemic sclerosis. Their role in early and late diseasePathobiology1993615–62392467507681
- LafyatisRTransforming growth factor beta – at the centre of systemic sclerosisNat Rev Rheumatol2014101270671925136781
- DentonCPMerkelPAFurstDERecombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192Arthritis Rheum200756132333317195236
- RiceLMPadillaCMMcLaughlinSRFresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patientsJ Clin Invest201512572795280726098215
- LakosGMelichianDWuMVargaJIncreased bleomycin-induced skin fibrosis in mice lacking the Th1-specific transcription factor T-betPathobiology200673522423717314493
- AliprantisAOWangJFathmanJWTranscription factor T-bet regulates skin sclerosis through its function in innate immunity and via IL-13Proc Natl Acad Sci U S A200710482827283017307869
- HoffmannKFMcCartyTCSegalDHDisease fingerprinting with cDNA microarrays reveals distinct gene expression profiles in lethal type 1 and type 2 cytokine-mediated inflammatory reactionsFASEB J200115132545254711641263
- SandlerNGMentink-KaneMMCheeverAWWynnTAGlobal gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repairJ Immunol200317173655366714500663
- GreenblattMBSargentJLFarinaGInterspecies comparison of human and murine scleroderma reveals IL-13 and CCL2 as disease subset-specific targetsAm J Pathol201218031080109422245215
- FuschiottiPMedsgerTAJrMorelPAEffector CD8+ T cells in systemic sclerosis patients produce abnormally high levels of interleukin-13 associated with increased skin fibrosisArthritis Rheum20096041119112819333920
- MedsgerTAJrIvancoDEKardavaLMorelPALucasMRFuschiottiPGATA-3 upregulation in CD8+ T cells is a biomarker of immune dysfunction in systemic sclerosis, resulting in excess IL-13 productionArthritis Rheum20116361738174721638273
- MathesALChristmannRBStifanoGGlobal chemokine expression in systemic sclerosis (SSc): CCL19 expression correlates with vascular inflammation in SSc skinAnn Rheum Dis201473101864187223873879
- BandinelliFDel RossoAGabrielliACCL2, CCL3 and CCL5 chemokines in systemic sclerosis: the correlation with SSc clinical features and the effect of prostaglandin E1 treatmentClin Exp Rheumatol2012302 Suppl 71S44S4922691208
- HasegawaMAsanoYEndoHSerum chemokine levels as prognostic markers in patients with early systemic sclerosis: a multicenter, prospective, observational studyMod Rheumatol20132361076108423180322
- TievKPHua-HuyTKettanehASerum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosisEur Respir J20113861355136021778167
- van BonLAffandiAJBroenJProteome-wide analysis and CXCL4 as a biomarker in systemic sclerosisN Engl J Med2014370543344324350901
- LeeRReeseCPerryBEnhanced chemokine-receptor expression, function, and signaling in healthy African American and scleroderma-patient monocytes are regulated by caveolin-1Fibrogenesis Tissue Repair201581126322128
- SteenVDAutoantibodies in systemic sclerosisSemin Arthritis Rheum2005351354216084222
- BaroniSSSantilloMBevilacquaFStimulatory autoantibodies to the PDGF receptor in systemic sclerosisN Engl J Med2006354252667267616790699
- GüntherJKillABeckerMOAngiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patientsArthritis Res Ther2014162R6524612997
- CarvalhoDSavageCOBlackCMPearsonJDIgG antiendothelial cell autoantibodies from scleroderma patients induce leukocyte adhesion to human vascular endothelial cells in vitro. Induction of adhesion molecule expression and involvement of endothelium-derived cytokinesJ Clin Invest19969711111198550821
- AhmedSSTanFKArnettFCJinLGengYJInduction of apoptosis and fibrillin 1 expression in human dermal endothelial cells by scleroderma sera containing anti-endothelial cell antibodiesArthritis Rheum20065472250226216802364
- SgoncRGruschwitzMSBoeckGSeppNGruberJWickGEndothelial cell apoptosis in systemic sclerosis is induced by antibody-dependent cell-mediated cytotoxicity via CD95Arthritis Rheum200043112550256211083280
- ChizzoliniCRaschiERezzonicoRAutoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosisArthritis Rheum20024661602161312115192
- RondaNRaschiETestoniCAnti-fibroblast antibodies in systemic sclerosisIsr Med Assoc J2002411 Suppl85886412455161
- TanFKArnettFCAntohiSAutoantibodies to the extracellular matrix microfibrillar protein, fibrillin-1, in patients with scleroderma and other connective tissue diseasesJ Immunol199916321066107210395706
- SatoSHayakawaIHasegawaMFujimotoMTakeharaKFunction blocking autoantibodies against matrix metalloproteinase-1 in patients with systemic sclerosisJ Invest Dermatol2003120454254712648215
- NishijimaCHayakawaIMatsushitaTAutoantibody against matrix metalloproteinase-3 in patients with systemic sclerosisClin Exp Immunol2004138235736315498049
- NagarajaVDentonCPKhannaDOld medications and new targeted therapies in systemic sclerosisRheumatology201554111944195325065013
- AmjadiSMaranianPFurstDECourse of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trialsArthritis Rheum20096082490249819644851
- PopeJEBellamyNSeiboldJRA randomized, controlled trial of methotrexate versus placebo in early diffuse sclerodermaArthritis Rheum20014461351135811407694
- TashkinDPElashoffRClementsPJCyclophosphamide versus placebo in scleroderma lung diseaseN Engl J Med2006354252655266616790698
- MendozaFANagleSJLeeJBJimenezSAA prospective observational study of mycophenolate mofetil treatment in progressive diffuse cutaneous systemic sclerosis of recent onsetJ Rheumatol20123961241124722467932
- LeENWigleyFMShahAABoinFHummersLKLong-term experience of mycophenolate mofetil for treatment of diffuse cutaneous systemic sclerosisAnn Rheum Dis20117061104110721378404
- TashkinDPElashoffRClementsPJEffects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung diseaseAm J Respir Crit Care Med2007176101026103417717203
- HoylesRKEllisRWWellsburyJA multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in sclerodermaArthritis Rheum200654123962397017133610
- BeckerMOSchoheAWeinertKResponders to cyclophosphamide: results of a single-centre analysis among systemic sclerosis patientsAnn Rheum Dis201271122061206222689320
- EspinosaGSimeonCPPlasinMAEfficacy of cyclophospamide in the treatment of interstitial lung disease associated with systemic sclerosisArch Bronconeumol201147523924521458128
- DomicianoDSBonfáEBorgesCTA long-term prospective randomized controlled study of non-specific interstitial pneumonia (NSIP) treatment in sclerodermaClin Rheumatol201130222322920544245
- AbhishekAYazdaniRPearceFOutcome of systemic sclerosis associated interstitial lung disease treated with intravenous cyclophosphamideClin Rheumatol20113081099110421484227
- PoormoghimHMoradi LakehMMohammadipourMSodagariFToofaninjedNCyclophosphamide for scleroderma lung disease: a systematic review and meta-analysisRheumatol Int20123282431244421691743
- PoormoghimHRezaeiNSheidaieZSystemic sclerosis: comparison of efficacy of oral cyclophosphamide and azathioprine on skin score and pulmonary involvement-a retrospective studyRheumatol Int201434121691169924801572
- SchachnaLMedsgerTAJrDauberJHLung transplantation in scleroderma compared with idiopathic pulmonary fibrosis and idiopathic pulmonary arterial hypertensionArthritis Rheum200654123954396117133609
- PreySEzzedineKDoussauAImatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multi-centre randomized double-blinded controlled trialBr J Dermatol201216751138114423039171
- PopeJMcBainDPetrlichLImatinib in active diffuse cutaneous systemic sclerosis: results of a six-month, randomized, double-blind, placebo-controlled, proof-of-concept pilot study at a single centerArthritis Rheum201163113547355121769850
- KhannaDSaggarRMayesMDA one-year, phase I/IIa, open-label pilot trial of imatinib mesylate in the treatment of systemic sclerosis-associated active interstitial lung diseaseArthritis Rheum201163113540354621769849
- FraticelliPGabrielliBPomponioGLow-dose oral imatinib in the treatment of systemic sclerosis interstitial lung disease unresponsive to cyclophosphamide: a phase II pilot studyArthritis Res Ther2014164R14425007944
- BoselloSDe SantisMLamaGB cell depletion in diffuse progressive systemic sclerosis: safety, skin score modification and IL-6 modulation in an up to thirty-six months follow-up open-label trialArthritis Res Ther2010122R5420338043
- JordanSDistlerJHMaurerBEffects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) groupAnn Rheum Dis20157461188119424442885
- MannoRBoinFImmunotherapy of systemic sclerosisImmunotherapy20102686387821091117
- TakeharaKIhnHSatoSA randomized, double-blind, placebo-controlled trial: intravenous immunoglobulin treatment in patients with diffuse cutaneous systemic sclerosisClin Exp Rheumatol2013312 Suppl 7615115623910617
- PoelmanCLHummersLKWigleyFMAndersonCBoinFShahAAIntravenous immunoglobulin may be an effective therapy for refractory, active diffuse cutaneous systemic sclerosisJ Rheumatol201542223624225433527
- BurtRKShahSJDillKAutologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trialLancet2011378979049850621777972
- van LaarJMFargeDSontJKAutologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trialJAMA2014311242490249825058083
- CrasAFargeDCarmoiTLatailladeJJWangDDSunLUpdate on mesenchymal stem cell-based therapy in lupus and sclerodermaArthritis Res Ther20151730126525582
- LargheroJFargeDBracciniAPhenotypical and functional characteristics of in vitro expanded bone marrow mesenchymal stem cells from patients with systemic sclerosisAnn Rheum Dis200867444344917526552
- Bocelli-TyndallCBracciLSpagnoliGBone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous-and allogeneic-stimulated lymphocytes in vitroRheumatology200746340340816920750
- Le BlancKTammikCRosendahlKZetterbergERingdénOHLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cellsExp Hematol2003311089089614550804
- CaplanAIDennisJEMesenchymal stem cells as trophic mediatorsJ Cell Biochem20069851076108416619257
- DazziFHorwoodNJPotential of mesenchymal stem cell therapyCurr Opin Oncol200719665065517906466
- Di NicolaMCarlo-StellaCMagniMHuman bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuliBlood200299103838384311986244
- JiangXXZhangYLiuBHuman mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cellsBlood2005105104120412615692068
- NautaAJKruisselbrinkABLurvinkEWillemzeRFibbeWEMesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cellsJ Immunol200617742080208716887966
- RamasamyRFazekasovaHLamEWSoeiroILombardiGDazziFMesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycleTransplantation2007831717617220794
- VanneauxVFarge-BancelDLecourtSExpression of transforming growth factor beta receptor II in mesenchymal stem cells from systemic sclerosis patientsBMJ Open201331e001890
- MeiselRZibertALaryeaMGöbelUDäubenerWDillooDHuman bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradationBlood2004103124619462115001472
- CiprianiPDi BenedettoPLiakouliVMesenchymal stem cells (MSCs) from scleroderma patients (SSc) preserve their immunomodulatory properties although senescent and normally induce T regulatory cells (Tregs) with a functional phenotype: implications for cellular-based therapyClin Exp Immunol2013173219520623607751
- ChristopeitMSchendelMFollJMullerLPKeysserGBehreGMarked improvement of severe progressive systemic sclerosis after transplantation of mesenchymal stem cells from an allogeneic haploidentical-related donor mediated by ligation of CD137LLeukemia20082251062106417972956
- KeyszerGChristopeitMFickSTreatment of severe progressive systemic sclerosis with transplantation of mesenchymal stromal cells from allogeneic related donors: report of five casesArthritis Rheum20116382540254221547891
- MilanoAPendergrassSASargentJLMolecular subsets in the gene expression signatures of scleroderma skinPLoS One200837e269618648520
- PendergrassSALemaireRFrancisIPMahoneyJMLafyatisRWhitfieldMLIntrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsiesJ Invest Dermatol201213251363137322318389
- JohnsonMEMahoneyJMTaroniJExperimentally-derived fibroblast gene signatures identify molecular pathways associated with distinct subsets of systemic sclerosis patients in three independent cohortsPLoS One2015101e011401725607805
- MartyanovVWhitfieldMLMolecular stratification and precision medicine in systemic sclerosis from genomic and proteomic dataCurr Opin Rheumatol2016281838826555452
