Abstract
Background
Anaphylaxis is a serious, potentially life-threatening condition. Adequate preparation for anaphylaxis management is imperative for school personnel. This descriptive pilot study assessed preparedness of US schools to manage anaphylactic reactions.
Methods
An exploratory, cross-sectional, web-based, pilot survey assessed the occurrence and characteristics of anaphylactic events, as well as training provided to school personnel for the recognition and treatment of anaphylaxis. Eligible US schools were participants in the EpiPen4Schools® program during the 2013–2014 school year. EpiPen4Schools provides EpiPen® (epinephrine injection) Auto-Injectors and training materials to qualifying US schools. Survey data were parsed by US Census Bureau region and state and were evaluated using descriptive statistics.
Results
Schools from all 50 states and the District of Columbia participated in the survey (N=6,019). Among schools that provided information on anaphylactic events, 11% (607/5,683) reported the occurrence of one or more events, with significant variability in incidence across census regions and among states. A total of 5,613 schools provided information regarding which staff members were trained to recognize the signs and symptoms of anaphylaxis. Thirty-six percent of schools (2,022/5,613) indicated that only the school nurse and select staff were trained in anaphylaxis recognition. The proportion of schools in which most or all school staff received such training differed by region/state (range, 13%–100%). A total of 5,578 schools provided information on which staff were permitted to administer epinephrine. The majority of schools (54%; 3,024/5,578) permitted only the school nurse and select staff to administer epinephrine, although percentages varied by region/state (range, 4%–100%).
Conclusion
Schools differed substantially in their preparedness to manage anaphylaxis, with significant disparities in staff training and permission to treat. Given the ramifications of delayed treatment, removing barriers to the recognition and treatment of anaphylactic events in schools is an important public health goal.
Background
The prevention and management of anaphylaxis in the school setting is increasingly recognized as a public health concern as the incidence of food allergies, a major trigger for anaphylaxis, appears to be on the rise in children.Citation1 From 1997 to 2007, the prevalence of reported food allergy in the US increased by 18% in children <18 years of age.Citation2 Unfortunately, many times the school is unaware that a child has a life-threatening allergy before the occurrence of an anaphylactic event.Citation3 Likewise, it is not uncommon for a child to experience a first-time anaphylactic attack in the school setting.Citation4
Often, children who experience an anaphylactic reaction do not receive epinephrine, the first-line treatment option, before presentation to the emergency department.Citation5,Citation6 Delay in the recognition and treatment of anaphylaxis has serious ramifications from hospitalizationCitation5 and biphasic reactionsCitation7 (ie, a reoccurrence of anaphylaxis symptoms hours after the index event) to death.Citation8,Citation9 Indeed, a majority of fatalities due to anaphylaxis that have been observed among adolescents and young adults have involved a lack of timely access to epinephrine.Citation9 Hospitalization also has serious repercussions as it is disruptive to school attendance and daily activities and is a source of financial burden. In the US, hospitalizations for anaphylaxis are estimated to cost $4,500 per event,Citation10 and anaphylaxis is estimated to have total direct expenditures ranging from $288 million to $3.7 billion.Citation11 To prevent or mitigate these adverse sequelae, adequate training programs are needed to properly educate school personnel to recognize and treat anaphylactic reactions.
The results presented here from a pilot survey among US schools participating in the EpiPen4Schools® program (Mylan Specialty L.P., Canonsburg, PA, USA) describe the occurrence of anaphylactic reactions and the training and personnel resources available to manage these events. Evaluating these aspects of anaphylaxis management will allow for a better assessment of school preparedness to deal with these serious and potentially life-threatening events.
Methods
Data source
Eligible schools were participants in the EpiPen4Schools program, which provides EpiPen® (epinephrine injection) Auto-Injectors (Mylan Specialty L.P.) and training materials to qualifying public and private kindergarten, elementary, middle, and high schools in the US. Participating schools received two EpiPen Auto-Injector 2-packs, two EpiPen Jr® Auto-Injector 2-packs, or one of each 2-pack free of charge. Of the >40,000 schools enrolled in the program, 32,387 schools had available contact information and were invited to participate in the survey.
The 15-question, web-based survey was to be completed by the person best qualified to provide information on anaphylactic events and preparedness, which was most often the school nurse.Citation12 Responses were limited to events that occurred during the 2013–2014 academic school year. A total of 6,019 surveys were received between May 21, 2014 and July 9, 2014. Study design and survey methodology have been previously described in greater detail.Citation12
Data analysis
Descriptive statistics were used to report the characteristics of participating schools (eg, census region, state), anaphylactic events, and staff training. Statistics were unweighted and, therefore, did not account for potential variation in individual school populations. Most questions included a count of missing data, as respondents were not required to answer every question. Therefore, percentages calculated for the descriptive statistics were derived using the total number of responses per question.
Ethics
The study was submitted to the RTI institutional review board (IRB) for approval, which determined that the research activity did not constitute research involving human subjects as defined by the US Code of Federal Regulations (45 CFR 46.102). The approval of these activities by the RTI IRB was not necessary; therefore, an exemption was granted.
Results
Respondents
The number of schools that enrolled in the EpiPen4Schools program varied by region and state, with the largest number of participating schools in the Northeast and the greatest single-state participation rate in New York (). Individual state survey participation rates ranged from 1% to 52%. There was no apparent association between the number of schools within a state that enrolled in the EpiPen4Schools program and percentage of schools that responded to the survey. However, survey participation rates were the lowest among the largest school districts and, in some cases, large districts were unable to participate at all because of requirements for research approval that could not be completed within the time frame for the study.
Table 1 School participation by region and state
Anaphylactic events
Of the 6,019 schools that participated in the survey, 5,683 provided information on the occurrence of anaphylactic events, with 607 (11%) reporting that one or more anaphylactic events occurred during the 2013–2014 school year. A total of 919 events were described.Citation12 On a state-by-state basis, the number of anaphylactic events ranged from no events to a high of 103 events. When calculating the mean number of anaphylactic events per school (ie, number of anaphylactic events in a given state divided by number of responding schools in that state), the values ranged from 0 to 0.29 ().
Table 2 Percentage of anaphylactic events (relative to responding schools) and staff training by region and state
School staff trained to recognize anaphylaxis
Of the 6,019 schools that participated in the survey, 5,613 schools (93%) provided information regarding staff members who were trained to recognize anaphylaxis. In response to the question, “Who in your school is trained to recognize the signs and symptoms of anaphylaxis?”, the most common answer (reported by 36% of schools that provided data [2,022/5,613]) was that only the school nurse and select staff were trained (). Less than a third of schools (1,730/5,613) trained all staff, and 29% (1,621/5,613) trained most staff in anaphylaxis recognition.
Figure 1 School staff training and permission.
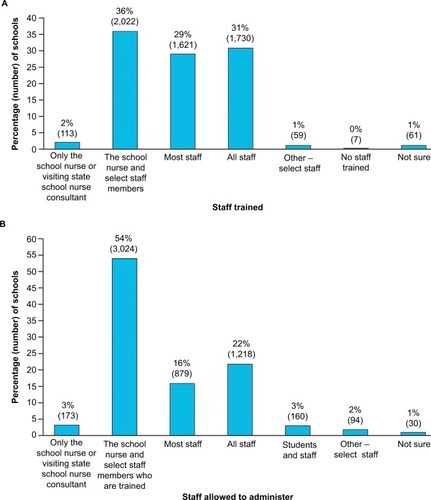
When analyzed by state, the percentage of schools that trained most or all staff in anaphylaxis recognition was highly variable (). All represented schools from Iowa, North Dakota, and Washington, as well as the District of Columbia, reported training most or all staff. However, most or all staff training rates as low as 13% were also observed.
School staff who were permitted to administer epinephrine
A total of 5,578 schools provided information on the types of staff who were permitted to administer epinephrine via an epinephrine auto-injector (EAI). The majority of schools (54%; 3,024/5,578) permitted the school nurse and select staff to administer epinephrine (). All or most staff were permitted to administer epinephrine in 38% of schools, and just 3% of schools limited EAI use to the school nurse or visiting state school nurse consultant (ie, a nurse who rotates among multiple schools to provide services as needed). Three percent of responding schools allowed both students and staff to administer epinephrine.
Regional and state data demonstrated a high level of diversity in the types of staff who were permitted to administer epinephrine (). In almost all cases, the proportion of schools that allowed most or all staff to administer epinephrine was lower than the proportion of schools that trained most or all staff to recognize anaphylaxis. There was no apparent correlation between most/all staff training levels and most/all staff administration levels. For example, the states listed above as having 100% training for recognition of signs and symptoms for most or all staff had comparatively low levels (25%–38%) of permission for EAI administration by most or all staff. However, in general, states with a high proportion of schools in which most or all school staff were permitted to administer epinephrine had a high proportion of schools that trained most or all staff on anaphylaxis recognition. For example, the five states listed as having >65% permission for EAI administration by most or all staff (Alaska, Florida, Idaho, Montana, and Wyoming) also had relatively high rates of training for recognition of anaphylaxis signs and symptoms for most or all staff (67%–83%). (Note: the rate of permission was 100% for the District of Columbia, but there were only two schools in the District of Columbia that responded to the survey.)
Discussion
The results of this descriptive pilot study show that schools’ preparedness for managing anaphylaxis varies substantially. Training in the recognition of anaphylaxis predominantly included the school nurse and select staff, and regional/state proportions of schools in which most or all staff were trained ranged from 13% to 100%. In general, fewer staff were permitted to administer epinephrine than were trained to recognize ana-phylaxis signs and symptoms. In the majority of cases (54%), only the school nurse and select staff members were allowed to administer epinephrine. On a regional/state basis, the proportion of schools in which most or all school staff were permitted to treat anaphylactic events with EAIs ranged from 0% to 100%.
Few studies have evaluated school staff training in anaphylaxis recognition, anaphylaxis management, or both. When such studies have been conducted, they have typically involved a geographically limited sample. For example, a recent survey of food allergy management readiness included 78 schools from southeastern Wisconsin. Forty nine of the schools (66%) reported that they provided staff training on anaphylaxis and epinephrine.Citation13
By expanding training and the ability to treat anaphylaxis, schools are better able to provide a timely response and, hence, increase the potential for a more favorable outcome. Evaluations of pediatric emergency department records have shown that less than half of children presenting with anaphylaxis received treatment with epinephrine in the pre-hospital setting.Citation5,Citation6 One of these studies reported that 12% of the analyzed anaphylactic reactions occurred at school.Citation6 Of those, 69% of children were administered epinephrine by the school nurse. With the increasingly limited number of full-time school nursesCitation6 and the potential for events to occur during field trips, before/after school hours, or during extracurricular activities,Citation14 depending on a limited pool of trained responders may put children at risk.
Along with the need for adequate staff training, it is important for schools to stock epinephrine because of the risk of reactions in individuals without previously known life-threatening allergiesCitation3 and because children and adolescents with known risk factors often fail to carry an unexpired EAICitation15 or do not have ready access to an EAI.Citation16 Additionally, the majority of individuals at high risk of anaphylaxis do not receive personal prescriptions for EAIs.Citation17 In the EpiPen4Schools survey, 22% of reported anaphylactic events occurred in individuals with no known allergies.Citation12 The majority of anaphylactic events (75%) reported were treated using EAIs; of these events, 49% were treated with stock EpiPen Auto-Injectors from the EpiPen4Schools program.Citation12 Most of the events that were not treated with an EAI were instead treated with antihistamines.Citation12
Most states have legislation in place that allows schools to stock epinephrine.Citation18 Nine states – California, Delaware, Maryland, Michigan, Nebraska, Nevada, New Jersey, North Carolina, and Virginia – have legislation that requires schools to stock epinephrine.Citation18 Nonetheless, in a survey of school nurses from California, where stocking epinephrine and administration training for unlicensed assistive personnel are permitted, only 13% reported that their school had a stock epinephrine program in place.Citation19 Moreover, despite the large presence of children with known food allergies, only 43% of schools from a Houston, Texas, school district reported having a stock EAI.Citation20 Schools with lower socioeconomic status were less likely than schools with higher socioeconomic status to stock EAIs. Most states have laws regarding students carrying and self-administering prescribed epinephrine.Citation21 While typically allowed by law, special approval is usually required. Since only 3% of responding schools said they allowed both students and staff to administer epinephrine, this suggests that, in practice, self-administration of EAIs by students may not be broadly permitted.
As a voluntary survey that was open to a subset of US schools (ie, those schools with a potentially higher allergy awareness information base as they were already participating in the EpiPen4Schools program), the interpretation of these results is subject to inherent limitations (eg, reporting bias, respondent recall, variance related to interpretation of meaning). In addition, survey results were not adjusted for potential confounding factors such as regional variations in the size of the student populations and types of schools. Future studies may benefit from deeper analyses of the details surrounding each event, including specifics of treatment administered, location of the event, and hospital transport and follow-up; demographic data such as race/ethnicity and sex may also be probed. Moreover, additional questions pertaining to the reasons for limited staff training or permission to treat, including regulations enacted by state or local governing bodies such as school boards, could provide greater insight into barriers that may prevent more extensive staff involvement in anaphylaxis management.
Conclusion
The risks and costs associated with anaphylaxis warrant that schools be prepared to act quickly both in the recognition of an anaphylactic event and in its treatment. Broader training of school staff, expanded staff involvement in treatment, and ready availability of medication are necessary to achieve this goal. The extent to which US schools have met these criteria is highly variable, both among schools and regions/states. To provide the best possible outcomes for children, barriers to their timely care within the school setting need to be identified and resolved.
Acknowledgments
This study was supported by Mylan Specialty L.P. Editorial assistance was provided under the direction of the authors by Crystal Murcia, PhD, Todd Parker, PhD, and Jennifer Rossi, MA, MedThink SciCom, and supported by Mylan Specialty L.P. BioRidge Pharma maintains the database of schools registered with the EpiPen4Schools program and provided logistical mailing services for survey invitation letters sent to identified contacts at participating schools. The authors would also like to acknowledge Margaret J Wooddell for her contributions to conception and critical feedback of early drafts.
Disclosure
Susan L Hogue, Diana Goss, Kelly Hollis, and Suyapa Silvia have no conflicts of interest in this work. Martha V White has served as a consultant for Mylan Specialty L.P. and Merck; has served as a board member for the Allergy and Asthma Network Mothers of Asthmatics; and has received grants, fees, or support from Mylan Specialty L.P., Merck, CSL Behring, AstraZeneca, Shire, Salix, Novartis, Teva, GlaxoSmithKline, Dyax, and Optinose.
References
- WeissCMuñoz-FurlongAFurlongTJArbitJImpact of food allergies on school nursing practiceJ Sch Nurs200420526827815469377
- BranumAMLukacsSLFood allergy among children in the United StatesPediatrics200912461549155519917585
- McIntyreCLSheetzAHCarrollCRYoungMCAdministration of epinephrine for life-threatening allergic reactions in school settingsPediatrics200511651134114016264000
- PowersJBergrenMDFinneganLComparison of school food allergy emergency plans to the Food Allergy and Anaphylaxis Network’s standard planJ Sch Nurs200723525225817894522
- HuangFChawlaKJärvinenKMNowak-WęgrzynAAnaphylaxis in a New York City pediatric emergency department: triggers, treatments, and outcomesJ Allergy Clin Immunol20121291162.e1168.e322018905
- TiyyaguraGKArnoldLConeDCLanghanMPediatric anaphy-laxis management in the prehospital settingPrehosp Emerg Care2014181465124028748
- AlqurashiWStiellIChanKNetoGAlsadoonAWellsGEpidemiology and clinical predictors of biphasic reactions in children with anaphylaxisAnn Allergy Asthma Immunol2015115321722326112147
- SasakiKSugiuraSMatsuiTA workshop with practical training for anaphylaxis management improves the self-efficacy of school personnelAllergol Int201564215616025838091
- BockSAMuñoz-FurlongASampsonHAFurther fatalities caused by anaphylactic reactions to food, 2001–2006J Allergy Clin Immunol200711941016101817306354
- PatelDAHoldfordDAEdwardsECarrollNVEstimating the economic burden of food-induced allergic reactions and anaphylaxis in the United StatesJ Allergy Clin Immunol2011128111011521489610
- DunnJDSclarDAAnaphylaxis: a payor’s perspective on epinephrine autoinjectorsAm J Med20141271 SupplS45S5024384137
- WhiteMVHogueSLBennettMEEpiPen4Schools pilot survey: occurrence of anaphylaxis, triggers, and epinephrine administration in a U.S. school settingAllergy Asthma Proc201536430631225898241
- EldredgeCPattersonLWhiteBSchellhaseKAssessing the readiness of a school system to adopt food allergy management guidelinesWMJ2014113415516125211803
- AllenKHenselmanKLairdBQuiñonesAReutzelTPotential life-threatening events in schools involving rescue inhalers, epinephrine autoinjectors, and glucagon delivery devices: reports from school nursesJ Sch Nurs2012281475521878573
- SpinaJLMcIntyreCLPulciniJAAn intervention to increase high school students’ compliance with carrying auto-injectable epinephrine: a MASNRN studyJ Sch Nurs201228323023722217467
- DeMuthKAFitzpatrickAMEpinephrine autoinjector availability among children with food allergyAllergy Asthma Proc201132429530021781405
- SclarDALiebermanPLAnaphylaxis: underdiagnosed, underreported, and undertreatedAm J Med20141271 supplS1S524384132
- Foodallergy.org [homepage on the Internet]McLean, VAFood Allergy Research & Educationc2015 [updated October 30, 2015; cited July 17, 2015]. Available from: http://www.foodallergy.org/advocacy/epinephrine/mapAccessed July 17, 2015
- MorrisPBakerDBelotCEdwardsAPreparedness for students and staff with anaphylaxisJ Sch Health201181847147621740432
- ShahSSParkerCLO’Brian SmithEDavisCMDisparity in the availability of injectable epinephrine in a large, diverse US school districtJ Allergy Clin Immunol Pract201423288.e1293.e124811019
- BaulsirBInnissBSummary Matrix of State Laws Addressing Epi-Pen Use in SchoolsSt Paul, MNThe Network for Public Health Law2015
