Abstract
Asthma is a heterogeneous syndrome with numerous underlining molecular and inflammatory mechanisms contributing to the wide spectrum of clinical phenotypes. Multiple therapies targeting severe asthma with type 2 (T2) high inflammation are or soon will be available. T2 high inflammation is defined as inflammation associated with atopy or eosinophilia or an increase in cytokines associated with T-helper 2 lymphocytes. Omalizumab is a humanized anti-IgE monoclonal antibody and the first biologic therapy approved for moderate–severe allergic asthma. Despite the specificity of biologic therapies like omalizumab, clinical response is variable, with approximately 50% of treated patients achieving the primary outcome. A prior identification of the ideal candidate for therapy would improve patient outcomes and optimize the use of health care resources. As the number of biologic therapies for asthma increases, the goal is identification of biomarkers or clinical phenotypes likely to respond to a specific therapy. This review focuses on potential biomarkers and clinical history that may identify responders to omalizumab therapy for asthma.
Introduction
Asthma is a complicated chronic disease that affects 7%–13% of the population, resulting in increasing morbidity and annual utilization of US$19.7 billion in health care.Citation1 The current literature states that 10% of patients with asthma are poorly controlled, contributing to high utilization of health care resources and negative impact on quality of life.Citation2 Severe asthma or exacerbation-prone asthma is defined in various ways. The European Respiratory Society and American Thoracic Society have defined severe asthma as a “condition that requires treatment with high doses of inhaled corticosteroids [ICSs] plus a second controller and/or systemic corticosteroids to prevent it from becoming ‘uncontrolled’ or from remaining ‘uncontrolled’ despite this therapy”.Citation1,Citation3 In other studies, severe asthma is defined as subjects with refractory symptoms and exacerbation while on three controller medications. Despite current definitions, guidelines, and management algorithms, some subjects with persistent asthma are uncontrolled. Choosing the correct therapy to achieve control in severe persistent asthma remains a challenge in the clinical setting. The phenotype, clinical presentation, and the endotype, molecular mechanism of inflammation in the lungs, play an important role in selecting appropriate targeted therapy.
Various phenotypic manifestations of refractory asthma based on triggers, radiographic findings, severity, response to treatment, viral infections, obesity, or cigarette smoking are discussed in the literature. Cluster analysis of severe or uncontrolled asthma, as developed in SARP and other studies, illustrate that early (childhood)-onset asthma is associated with atopic disease and allergic conditions, whereas late- or adult-onset asthma is more commonly associated with obesity and female sex. In general, severity of airflow obstruction correlates with frequency of exacerbations.Citation4,Citation5 Despite their providing some insight into the complexity of persistent asthma, the clinical value of such cluster analyses in improving asthma outcomes is not apparent.Citation6 The link between clinical phenotype and molecular mechanism could be the key in adding appropriate add-on therapy and optimization of control in severe asthma.
Allergic asthma is an observable clinical phenotype. IgE is integral to the pathophysiology of allergic asthma. Omalizumab (Xolair; Genentech, San Francisco, CA, and Novartis, Basel, Switzerland) is a humanized monoclonal antibody with specificity for the IgE molecule at the binding site for the high-affinity IgE receptor. Omalizumab is approved as an add-on therapy for moderate–severe allergic asthma in children (6–18 years old, with pediatric dosing for 6–<12 years of age and subsequent dose adjustment for adolescents aged 12 years and older) and adults in the US, Europe, and many other territories.Citation7 The reality of the situation is that treatment with omalizumab is expensive and cost-effective only in a subset of treated patients. It is imperative but challenging for clinicians to identify individuals most likely to have therapeutic benefit. This review focuses on individualized therapy and patient selection for omalizumab. The intent is to highlight current measurable molecular mechanisms, clinical characteristics, and available biomarkers that may assist in identifying ideal candidates for therapy.
Types of inflammation in asthma
The current hypothesis suggests two major inflammatory categories of asthma: type 2 (T2) high and T2 low. T2 low includes a heterogeneous group of asthma phenotypes including paucigranular, TH9 high, and TH17 high (neutrophilic). Neutrophilic infiltration in T2 low is attributed to increased expression of TH17 cytokines, including IL17, IL21, and IL22,Citation8 whereas in T2 high a predominance of eosinophils in the sputum and airways occurs with increased expression of type 2 cytokines, including IL4, IL5, and IL13.Citation9 For the purpose of this review, we focus on molecular mechanisms of T2 high inflammation and associated biomarkers, since this is the more likely group for which omalizumab will be effective.
Allergic asthma results from a response to an exogenous peptide or antigen in a sensitized individual. Genetic and environmental factors impact sensitization. Through exposure to an allergen, an inflammatory cascade occurs, suggestive of T2 high inflammation (). Allergen exposure contributes to the production of IL33, IL25, and TSLP by epithelial cells. These alarmins stimulate ILC2, TH2, and mast cells. Simultaneously, the antigen is processed by dendritic cells and presented to naïve T cells, contributing to further differentiation to TH2 cells. This complex cross talk among cells allows for an environment rich in IL4, IL5, and IL13.Citation9,Citation10 IL4 and IL13 promote the production of IgE by B cells,Citation9 IL5 affects eosinophil biology, and IL13 may be important in airway remodeling.
Figure 1 Complex cross talk in T2 high inflammation.
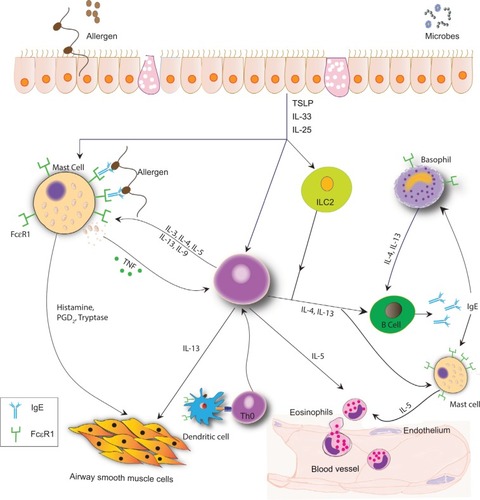
It is important to acknowledge IgE as one of the key factors in T2 high inflammation, and the presence of specific IgE identifies an individual as atopic. Two primary IgE receptors are known: 1) the high-affinity receptor, FcεRI, predominantly found on mast cells and basophils in tetrameric form and in trimeric form on antigen-presenting cells; and 2) the low-affinity receptor, FcεRII (CD23), found on B cells and other hematopoietic cells.Citation11–Citation13 The density of both receptors is correlated directly with the concentration of blood IgE. Allergen sensitization results in specific IgE binding to FcεRI on mast cells and basophils. Subsequent exposure to allergens cross-links the membrane-bound IgE on mast cells and basophils, resulting in the release of intracellular mediators, as well as contributing to T2 high inflammation.Citation1,Citation13,Citation14
Role of omalizumab in T2 high inflammation
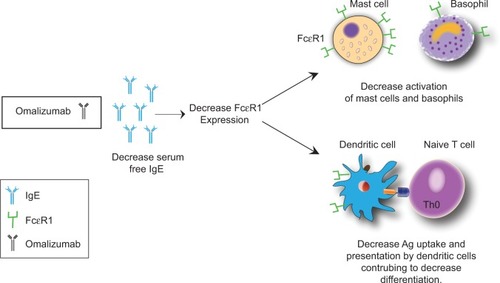
Omalizumab blocks the interaction of IgE with FcεRI on mast cells, antigen-presenting cells, and basophils by selectively binding to free IgE.Citation15,Citation16 Omalizumab indirectly downregulates FcεRI expression on basophils, mast cells, and dendritic cells, affecting type 2 cytokine production and inhibiting T2 inflammation ().Citation14 In 2013, Chan et al found that omalizumab also targeted IgE-bearing B cells, causing a state of anergy and unresponsiveness to antigenic/allergen stimulation.Citation17
Figure 2 Omalizumab causes a decrease in serum free IgE.
Multiple studies have demonstrated the efficacy of omalizumab in reducing asthma exacerbations by 30%–60% with minimal change in lung function and modest symptom improvement. Omalizumab treatment reduces the need for rescue systemic CS therapy, but does not reduce maintenance systemic CS therapy.Citation18,Citation19 It is the first asthma therapy to target a specific subtype of asthma: moderate–severe asthma with blood IgE in a specific range and evidence of sensitization to perennial allergens. Despite the knowledge of the mechanism of action and selection of treated subjects based upon total and specific IgE, clinical studies and 13 years of use demonstrate that the response is variable and not readily predicted. In a retrospective analysis of the EXTRA trial, Hanania et al identified possible biomarkers predictive of omalizumab response, including fractional exhaled nitric oxide (FeNO), peripheral blood eosinophils, and serum periostin.Citation20 This was the first study of omalizumab therapy that separated patients into categories of T2 high vs T2 low inflammation. Significant reduction in asthma exacerbations was noted in the T2 high and but not in the T2 low subgroups, providing some guidance in identifying individuals for omalizumab treatment.
Biomarkers that predict responsiveness to omalizumab
Understanding T2 high molecular inflammation has opened the doors to pursuing more targeted therapy in severe allergic asthma. Clinical biomarkers have long been used in medicine to evaluate the risk of a disease state, responsiveness to medications, and even progression of disease. Biomarkers may be divided into three types: type 0 assess the natural history of the disease state, type 1 predict responsiveness to treatment, and type 2 identify risk for particular diseases.Citation21 Type 0 biomarkers are not identified for asthma. In T2 high asthma, several biomarkers have been identified and studied with respect to responsiveness of drug therapy. These include increased FeNO; blood eosinophils, periostin, total IgE, and antigen-specific IgE; and low forced expiratory volume in 1 second (FEV1).Citation20
Eosinophils
Elevated blood eosinophils have been associated with more severe asthma and predict a higher risk of asthma exacerbation.Citation21–Citation23 In fact, in a large UK cohort, patients with blood eosinophil counts ≥400 cells/mL experienced more severe asthma exacerbations and had poorer asthma control than those with <400 cells/mL.Citation24 Obtaining peripheral eosinophil counts is relatively easy, and while not an optimal surrogate for airway eosinophils, it is suggestive of possible underlying T2 high inflammation. Treatment with omalizumab may decrease peripheral blood eosinophil counts.Citation25 In a recent pooled analysis of the two pivotal Phase III double-blind, placebo-controlled registration trials of omalizumab in allergic asthma, eosinophil counts >300 cells/mL were associated with higher disease activity and better response to omalizumab.Citation26 Similar findings were demonstrated by Busse et al in a prospective 24-week multicenter parallel-group, double-blind randomized placebo-control trial, which showed that omalizumab-treated individuals with an eosinophil count ≥300/mL or more had a 59% reduction in protocol-defined exacerbations compared to placebo.Citation27 In a randomized double-blind multicenter study evaluating persistent response to omalizumab, Ledford et al reported subjects treated with omalizumab therapy with higher peripheral blood eosinophil counts were more likely to suffer asthma exacerbations if omalizumab was discontinued.Citation16 Therefore, omalizumab seems to have greater efficacy in patients with increased baseline peripheral eosinophil counts, and blood eosinophil quantification is a practical biomarker in predicting relapse if omalizumab is discontinued.
Other markers of eosinophils may be useful measures of respiratory disease. For example, eosinophil peroxidase, an eosinophil-granule component, is strongly correlated with induced sputum eosinophils.Citation28 Bioactive strips that measure eosinophil peroxidase as a point-of-care tool in a clinical setting is in development.Citation29
Fractional exhaled nitric oxide
Measurement of FeNO is a noninvasive way to potentially quantify T2 high airway inflammation. Similar to sputum and blood eosinophils, elevated FeNO is predictive of asthma exacerbations and asthma severity. In the lung, oxidation of the amino acid l-arginine through NO synthase (NOS) produces NO. Three isoforms of NOS have been described: neural, endothelial, and inducible. A variety of cells – epithelial, inflammatory (macrophages, mast cells, and neutrophils), neuronal airway, and vascular endothelial – express the various isoforms of NOS. Inducible NOS is expressed in high quantity in epithelial cells lining the airway wall and alveoli. This increase in expression of inducible NOS contributes to increase in production of NO under the influence of IL4 and IL13.Citation20 Several analyzers are available commercially for the measurement of NO concentration in the lungs. These include NiOx Mino, NiOx Vero (Circassia, Oxford, UK), and NO Breath (Bedfont Scientific, Maidstone, UK).
The American Thoracic Society has proposed FeNO guidelines for clinical use, with FeNO <25 ppb in adults and <20 ppb in children proposed as normal.Citation30 Adults with FeNO >50 ppb are more responsive to ICSs, with a decrease in FeNO within 1 week of therapy.Citation31 If FeNO remains >50 ppb in adults (>35 ppb in children) despite ICS use, then one should consider noncompliance or decreased CS responsiveness.Citation21,Citation30 In a Cochrane review of all randomized controlled trials in both children and adults comparing adjustment of asthma medications based on FeNO levels to those not using FeNO, a decrease in FeNO was associated with a reduction in the number of asthma exacerbations, but not associated with improvement in day-to-day symptoms or reduction in total ICS used.Citation32,Citation33 Mansur et al and Hanania et al independently demonstrated that patients with severe asthma and higher baseline FeNO had a greater reduction in exacerbations with omalizumab compared to placebos.Citation20,Citation25 An FeNO of greater than 49 ppb in children within 4 weeks of ICS discontinuation was associated with an increase in asthma exacerbations.Citation34 Similarly, an increase in FeNO after discontinuation of long-term omalizumab predicted exacerbation.Citation16 It is interesting that anti-IL5 biologics decrease serum and sputum eosinophils, but do not have much effect on FeNO.Citation35
Clinically, it is important to note that FeNO can be impacted by many host and environmental factors. In particular, exercise and spirometry prior to measuring FeNO can cause a transient decrease in readings. ICSs, systemic CSs, leukotriene-receptor antagonists, and smoking have been associated with reduction in FeNO. FeNO is higher in males than females. In children, FeNO increases at a rate of 5% per year, likely attributable to increased height. High-nitrate foods have also been associated with an increase in FeNO. However, the relative ease of measurement of FeNO at the bedside allow it to serve as a biomarker for T2 high inflammation predicting response to omalizumab.
Periostin
Blood periostin is another biomarker of T2 high inflammation. It is secreted by airway epithelial cells and fibroblasts in response to IL13.Citation21 Gene expression is increased in asthma. Subjects with higher baseline periostin have a greater improvement in FEV1 than those with low periostin while being treated with an IL13 antagonist.Citation36 In the EXTRA study, the high-periostin group (>50 ng/mL at baseline) had 30% reduction in asthma exacerbations after treatment with omalizumab compared to 3% reduction in the low-periostin group (<50 ng/mL at baseline).Citation20 A prospective observational study assessed the utility of serum periostin and total IgE to predict response to omalizumab, as determined by the absence of asthma exacerbations during the first year of treatment. The high-periostin group (>60 ng/mL) experienced less frequent asthma exacerbations and achieved a clinically significant change in Asthma Quality of life Questionnaire (AQLQ) scores at 16 weeks compared to the low-periostin group (<60 ng/mL).Citation37 Unfortunately, while serum periostin may be predictive of response to omalizumab, the assay is not commercially available.
Total serum IgE and antigen-specific IgE
Total serum IgE and specific IgE are the most readily recognized risk factors for allergic asthma. In a cluster analysis done by investigators of the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program pediatric cohort, children with severe asthma had higher serum IgE and increased aeroallergen sensitization.Citation10,Citation38 As already described, IgE is a key factor in T2 high inflammation. Attachment of allergens to FcεRI-bound IgE and cross-linking of IgE receptors initiate a signaling cascade that leads to release of histamine, leukotrienes, and other inflammatory factors by human mast cells. Dendritic cells uptake and process the allergen, subsequently presenting it to naïve T cells and shifting them toward a TH2 pathway. Dendritic and B-cell antigen presentation may be facilitated through binding of allergen and specific IgE attached to the high-affinity receptor on the cell surface. This TH2 shift contributes to the perpetuation of allergen-specific IgE production. IgE also activates eosinophils and macrophages through the FcεRI receptor to produce proinflammatory cytokines that are involved in tissue remodeling. Airway smooth muscles also express FcεRI, and via IgE activation type I and III collagen production is increased, as seen in asthma remodeling.Citation39
To date, omalizumab is the only available IgE-targeted therapy approved for poorly controlled moderate–severe persistent asthma. The presence of perennial sensitization and total serum IgE within range with specified body weight (0.016 mg/kg of body weight per international unit of IgE/mL not to exceed 750 mg per month) is the approved dosing for omalizumab. Total IgE is not a reliable indicator of response to omalizumab, although the presence of an increased quantity of IgE specific to relevant allergens may predict response. Clinical trials have demonstrated a rapid decrease in serum free IgE after initiation. The reduction of serum free IgE to a concentration of ≤20.8 IU/mL in response to omalizumab is likely required for a consistent therapeutic response.Citation40–Citation42 In a pediatric population, the reduction in free serum IgE levels after omalizumab treatment is associated with a decrease in seasonal exacerbations secondary to rhinovirus.Citation43 Therefore, modification of allergy influences nonallergic asthma triggers. Unfortunately, commercially available assays are not able to distinguish between the omalizumab–IgE complex and free IgE. Once on therapy, the measurement of serum IgE reflects omalizumab-IgE complexes, and thus serum IgE during treatment is not an indicator of free IgE nor a predictor of therapeutic response to omalizumab. In conclusion, the concentration of total serum IgE and presence of specific IgE are useful biomarkers for identification of allergic asthma and potential candidates for omalizumab therapy.
Expiratory lung-function measurements
FEV1 is an essential component of pulmonary function testing used to diagnose and monitor asthma. Low FEV1 is a clinical sign suggestive of increased asthma severity. Casale et al illustrated that clinical signs of asthma severity were predictive of omalizumab response, and patients with FEV1 <65% predicated had a greater reduction in exacerbation rates following omalizumab therapy.Citation26 In a multicenter retrospective analysis of patients with severe asthma treated with omalizumab, the authors evaluated the role of lung-function studies in responders to omalizumab. Patients were separated into responders and nonresponders to treatment based on physician assessment and the use of global evaluation of treatment effectiveness. Lung-function parameters were measured at baseline and 6, 12, and 24 months. Patients in the responder group had 5.7% improvement in FEV1 after 6 months of therapy. Forced expiratory flow 25%–75% also improved within the first 6 months. The authors also noted a greater improvement in residual volume in those patients with severe obstructive airway (FEV1 <50% predicted), suggesting the possible impact of omalizumab on small airways.Citation44 Currently in Europe, FEV1 <80% is required for the approval of omalizumab.Citation44 In summary, FEV1 is a readily available clinical marker to predict severity of asthma and hence response to omalizumab.
From bench side to bedside
Asthma populations are heterogeneous, and the challenge remains to treat patients effectively and be prudent with health care resources. Several “real-life” studies published since 2008 have attempted to predict effectiveness of omalizumab based on clinical history and presentation. In a review of 24 real-life effectiveness studies of omalizumab in the treatment of severe allergic asthma, 4,117 unique cases from 32 countries were reviewed. The authors described the profile of patients more likely to have greater therapeutic benefit from omalizumab. Impaired lung function (<40%–45% of predicted FEV1), increased daytime and nighttime symptoms, prior history of exacerbation, and significantly impaired quality of life were associated with greater therapeutic benefit from omalizumab.Citation45 A small retrospective study of 41 patients with severe allergic asthma treated with omalizumab used FeNO, peripheral eosinophils, periostin, total serum IgE, and FEV1 in combination with the clinical symptoms of quality of life scores and prior history of exacerbation. At 16 weeks, of the 41 patients treated, the authors defined 28 individuals as responders to omalizumab by assessing improvement in night and day symptoms, reduced usage of rescue medication, reduction in exacerbation, and improvement in morning peak-flow rate by >15%.Citation46 Consistent with other studies, responders benefited within 16 weeks from initiation of therapy. These authors also reported that FEV1 ≤69% of predicated at baseline was a good predictor for response to omalizumab.Citation46 Responders to omalizumab experienced an improvement in AQLQ and decreased exacerbations within 16–32 weeks. Most clinicians recommend a trial of at least 6 months to assess response to treatment.
Currently there is no consensus on duration of omalizumab therapy in those who benefit. In real-life studies at 4 and 9 years of use of omalizumab, patients showed persistent, significant improvement in AQLQ, decreased exacerbations, and slight improvement in FEV1 compared to baseline.Citation47
Conclusion
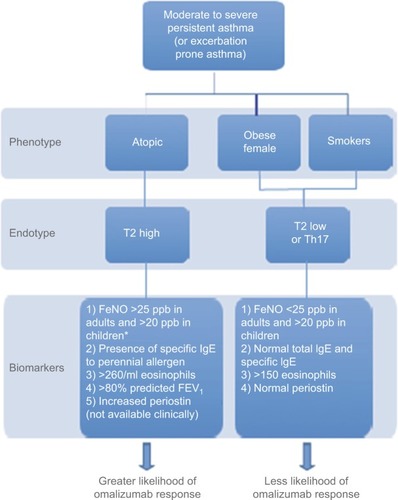
Many asthma studies provide evidence of omalizumab efficacy with reduced exacerbations and improved symptoms and quality of life in adults and children. However, some patients do not benefit. Allergic asthma is a phenotype that has been consistently used as a criterion for initiation of omalizumab. Nonallergic asthma has been treated successfully with omalizumab, but the mechanism of action in this situation is not understood.Citation48 Better clarification of asthma endotypes will likely improve the accuracy of predicting response. Currently available biomarkers are limited in number and precision, but are of value. The combination of a history of frequent exacerbations, decreased FEV1, presence of total serum IgE within dosing range (30–700 IU/mL in adolescents and adults and 30–1,300 IU/mL in children aged 6–12 years), allergen-specific IgE, increased FeNO, and increased blood eosinophils are associated with greater likelihood of response to omalizumab (). Persistent increase of eosinophils during therapy or an increase in FeNO following discontinuation may predict the need for continued therapy. Endotype recognition and associated markers will advance personalized asthma care to move beyond the one-size-fits-all or trial-and-error approach.
Figure 3 A way to categorize patients with moderate–severe asthma in clinical settings using available biomarkers.
Abbreviation: FeNO, fractional exhaled nitric oxide.
Disclosure
DKL has received research support (paid to University) from Genentech/Roche and AstraZeneca, consultant fees from AstraZeneca, speakers’ bureau fees from AstraZeneca, Genentech/Roche, Meda, Novartis, and Teva, and fees for legal opinions on drug allergy, metal allergy, radiocontrast reaction, and asthma death. The authors report no other conflicts of interest in this work.
References
- FajtMLWenzelSEDevelopment of new therapies for severe asthmaAllergy Asthma Immunol Res201791314
- BarnesPJJonssonBKlimJBThe costs of asthmaEur Respir J19969463642
- ChungKFManaging severe asthma in adults: lessons from the ERS/ATS guidelinesCurr Opin Pulm Med2015211815
- MooreWCMeyersDAWenzelSEIdentification of asthma phenotypes using cluster analysis in the Severe Asthma Research ProgramAm J Respir Crit Care Med20101814315323
- HaldarPPavordIDShawDECluster analysis and clinical asthma phenotypesAm J Respir Crit Care Med20081783218224
- BourdinAMolinariNVachierIPrognostic value of cluster analysis of severe asthma phenotypesJ Allergy Clin Immunol2014134510431050
- Xolair 2017 [updated 2017] Available from: http://www.xolair.com/allergic-asthma/hcp
- HalwaniRSultanaAVazquez-TelloAJamhawiAAl-MasriAAAl-MuhsenSTh-17 regulatory cytokines IL-21, IL-23, and IL-6 enhance neutrophil production of IL-17 cytokines during asthmaJ Asthma Epub201732
- FahyJVType 2 inflammation in asthma: present in most, absent in manyNat Rev Immunol20151515765
- ChippsBELanierBMilgromHOmalizumab in children with uncontrolled allergic asthma: review of clinical trial and real-world experienceJ Allergy Clin Immunol2017139514311444
- SamitasKDelimpouraVZervasEGagaMAnti-IgE treatment, airway inflammation and remodelling in severe allergic asthma: current knowledge and future perspectivesEur Respir Rev201524138594601
- HolowkaDSilDTorigoeCBairdBInsights into immunoglobulin E receptor signaling from structurally defined ligandsImmunol Rev200721726927917498065
- GalliSJTsaiMIgE and mast cells in allergic diseaseNat Med2012185693704
- MacGlashanDWJrBochnerBSAdelmanDCDown-regulation of FcεRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibodyJ Immunol1997158314381445
- MitchellPDEl-GammalAIO’ByrnePMAnti-IgE and biologic approaches for the treatment of asthmaHandb Exp Pharmacol201723713115227864676
- LedfordDBusseWTrzaskomaBA randomized multicenter study evaluating Xolair persistence of response after long-term therapyJ Allergy Clin Immunol2017140162.e2169.e227826098
- ChanMAGigliottiNMDotsonALRosenwasserLJOmalizumab may decrease IgE synthesis by targeting membrane IgE+ human B cellsClin Transl Allergy201332924004581
- BousquetJCabreraPBerkmanNThe effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthmaAllergy2005603302308
- HumbertMBusseWHananiaNAOmalizumab in asthma: an update on recent developmentsJ Allergy Clin Immunol Pract201425525.e1536.e1
- HananiaNAWenzelSRosénKExploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA studyAm J Respir Crit Care Med20131878804811
- BerryABusseWWBiomarkers in asthmatic patients: has their time come to direct treatment?J Allergy Clin Immunol2016137513171324
- PavordIDKornSHowarthPMepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trialLancet201238065165922901886
- PizzichiniEPizzichiniMMEfthimiadisADolovichJHargreaveFEMeasuring airway inflammation in asthma: eosinophils and eosinophilic cationic protein in induced sputum compared with peripheral bloodJ Allergy Clin Immunol1997994539544
- PriceDBRigazioACampbellJDBlood eosinophil count and prospective annual asthma disease burden: a UK cohort studyLancet Respir Med2015311849858
- MansurAHSrivastavaSMitchellVSullivanJKasujeeILongterm clinical outcomes of omalizumab therapy in severe allergic asthma: study of efficacy and safetyRespir Med2017124364328284319
- CasaleTBChippsBERosénKResponse to omalizumab using patient enrichment criteria from trials of novel biologics in asthmaAllergy Epub2017831
- BusseWSpectorSRosénKWangYAlpanOHigh eosinophil count: a potential biomarker for assessing successful omalizumab treatment effectsJ Allergy Clin Immunol20131322485.e11486.e11
- RankMAOchkurSILewisJCNasal and pharyngeal eosinophil peroxidase levels in adults with poorly controlled asthma correlate with sputum eosinophiliaAllergy2016714567570
- WrightKBioactive paper will revolutionize point-of-care diagnostics2013 Available from: http://dailynews.mcmaster.ca/article/bioactive-paper-will-revolutionize-point-of-care-diagnosticsAccessed October 28, 2017
- DweikRABoggsPBErzurumSCAn official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels FENO for clinical applicationsAm J Respir Crit Care Med20111845602615
- MehtaVStokesJRBerroARomeroFACasaleTBTime-dependent effects of inhaled corticosteroids on lung function, bronchial hyperresponsiveness, and airway inflammation in asthmaAnn Allergy Asthma Immunol200910313137
- PetskyHLKewKMChangABExhaled nitric oxide levels to guide treatment for children with asthmaCochrane Database Syst Rev201611CD01143927825189
- PetskyHLKewKMTurnerCChangABExhaled nitric oxide levels to guide treatment for adults with asthmaCochrane Database Syst Rev20169CD01144027580628
- PijnenburgMWBakkerEMLeverSHopWCDe JongsteJCHigh fractional concentration of nitric oxide in exhaled air despite steroid treatment in asthmatic childrenClin Exp Allergy2005357920925
- HaldarPBrightlingCEHargadonBMepolizumab and exacerbations of refractory eosinophilic asthmaN Engl J Med200936097398419264686
- CorrenJWoodRAPatelDEffects of omalizumab on changes in pulmonary function induced by controlled cat room challengeJ Allergy Clin Immunol20111272398405
- TajiriTMatsumotoHGonYUtility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthmaAllergy2016711014721479
- FitzpatrickAMTeagueWGMeyersDAHeterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research ProgramJ Allergy Clin Immunol20111272382389.e1e13
- PelaiaGCanonicaGWMatucciAPaoliniRTriggianiMPaggiaroPTargeted therapy in severe asthma today: focus on immunoglobulin EDrug Des Devel Ther201711197987
- BergerWGuptaNMcAlaryMFowler-TaylorAEvaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthmaAnn Allergy Asthma Immunol2003912182188
- MilgromHBergerWNayakATreatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab)Pediatrics2001108E3611483846
- KornSHaaslerIFliednerFMonitoring free serum IgE in severe asthma patients treated with omalizumabRespir Med20121061494150022884459
- TeachSJGillMATogiasAPreseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbationsJ Allergy Clin Immunol20151361476148526518090
- PaganinFMangiapanGProustALung function parameters in omalizumab responder patients: an interesting tool?Allergy Epub2017518
- AbrahamIAlhossanALeeCSKutbiHMacDonaldK‘Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic reviewAllergy20167159361026644231
- KallieriMPapaioannouAIPapathanasiouENtontsiPPapirisSLoukidesSPredictors of response to therapy with omalizumab in patients with severe allergic asthma: a real life studyPostgrad Med201712959860428427296
- MenzellaFGaleoneCFormisanoDReal-life efficacy of omalizumab after 9 years of follow-upAllergy Asthma Immunol Res201794368372
- GarciaGMagnanAChironRA proof-of-concept, randomized, controlled trial of omalizumab in patients with severe, difficult-to-control, nonatopic asthmaChest201314441141923579324
- LambrechtBNHammadHThe immunology of asthmaNat Immunol20151614556
- Kupryś-LipińskaIMolińskaKKunaPThe effect of omalizumab on eosinophilic inflammation of the respiratory tract in patients with allergic asthmaPneumonol Alergol Pol2016844232243
