Abstract
Seasonal allergic rhinitis (SAR) is increasing in prevalence such that 1 in 4 persons is affected in the UK. It represents a considerable burden of disease since in a significant proportion of individuals the severity of nasal–ocular symptoms has an important effect on daily activity, performance and quality of life. Intranasal steroids (INS) form the mainstay of treatment, having been shown in meta-analyses to be superior to oral antihistamines, intranasal antihistamines and anti-leukotrienes. Fluticasone propionate is an established INS for the treatment of rhinitis, including SAR. Its favorable pharmacological profile combining high local efficacy with low systemic bioavailability has established fluticasone propionate as an effective intervention. The more recent introduction of structurally related fluticasone furoate with similar but enhanced pharmacological characteristics with a novel delivery device may confer further therapeutic advantages.
Seasonal allergic rhinitis
Allergic rhinitis is a highly prevalent chronic condition, which presents an enormous global health burden. It is estimated that at least 500 million individuals have allergic rhinitis (AR) and it is one of the most common reasons for attendance with a primary care practitioner.Citation1 The nasal manifestations of AR are congestion, rhinorrhea, itching and sneezing. Ocular symptoms occur in at around 70% of individuals with seasonal allergic rhinitis (SAR), so it is more appropriate to use the term allergic rhinoconjunctivitis. The ocular manifestations are watery eyes, itching, burning (irritability), redness and injection of the conjunctiva and sometimes periorbital edema. In Europe, 71% of patients experience both nose and eye symptoms and up to 33% of these are moderate or severe.Citation2
The relationship between nasal-ocular symptoms and seasonal allergen exposure plus demonstration of IgE-specific sensitization is diagnostic of SAR.
Seasonal allergic rhinitis (SAR) or hay fever (termed ‘spring catarrh’ back in the 19th century) has rapidly increased in prevalence in the last 50 years, particularly in the developed world.Citation3–Citation5 A recent multi-center study involving 33 centers in Europe demonstrated sensitization to grass pollen (predominantly timothy grass) with a median prevalence of 16.9%, just behind sensitization to house dust mite (Dermatophagoides pteronyssinus) at 21.7%.Citation6
Rhinitis is also defined in the ‘allergic rhinitis and its impact in asthma’ (ARIA) document in terms of the duration of symptoms (ie, intermittent versus persistent) and effects on quality of life (QOL), thus aiding treatment decisions.Citation1 However, given the spectrum of respiratory disease that can present with rhinitis symptomsCitation7 and the possible use of allergen specific immunotherapy, retention of the key term seasonality is very useful in the diagnostic algorithm and has been retained by several guidelines and standards of care documents.Citation8
Mechanisms of allergic rhinitis
The basic concepts that underlie allergic inflammation are outlined. Antigen presenting cells (APC) take up allergen, which has reached the nasal mucosa. Dendritic cells in particular present processed allergen peptides in the context of MHC Class II to naïve and antigen-specific memory T cells, leading to a Th2 polarized response in atopic individuals. Allergen specific IgE is tightly held on the surface of resident mast cells that express the high affinity (FcɛRI) IgE receptor. This is allergen sensitization. Subsequent allergen impaction on the nasal mucosal surface leads to solubilization and diffusion across to sites of mast cell (MC) residence. Cross-linking of two or more affinity IgE molecules in response to allergen binding leads to MC activation, degranulation, and release of mediators such as histamine, leukotrienes and neuropeptides, initiating an inflammatory signaling cascade. Rapid neuronal activation and vascular leakage are manifest by almost instantaneous sneezing, nasal itch and congestion. Release of pre-formed key cytokines such as interleukin (IL)-13 contribute to mucus hypersecretion and together with IL-5 and IL-4 drive further cellular recruitment and sustain the inflammatory response. Intense cellular infiltration is associated with further priming of the upper airway innate and adaptive immune responses along with structural cell activation (epithelium and submucosal fibroblasts) and enhanced local IgE production.Citation9 This leads to a primed and rapid immune response on further allergen exposure that contributes to the severity and chronicity of symptoms. Ocular symptoms can be particularly troublesome in SAR.Citation10 Whilst some of these relate to direct allergen impaction on the eye with local mast cell degranulation and initiation of immune signaling cascades, deposition of allergen in the nasal mucosa alone can activate ocular responses termed the ‘the naso-ocular reflex’.Citation11 Steroids attenuate key aspects of this allergen-induced inflammatory process. The clinical translation is effective therapeutic intervention.
Treatment of allergic rhinitis
Oral and intranasal antihistamines, mast cell stabilizers, leukotriene inhibitors, decongestants and intranasal anticholinergics, in addition to intranasal steroids, are all established evidence-based therapeutic interventions for AR.Citation1 They are not equally effectiveCitation12 and INS on meta-analyses are significantly more effective than oral or intranasal antihistamines and anti-leukotrienes and equal to the combination of anti-histamine plus anti-leukotriene.Citation13–Citation15
For mild disease either a second-generation antihistamine or topical nasal corticosteroid (INS) is recommended.Citation1,Citation16 For moderate to severe disease or when nasal congestion is predominant, INS are first line treatment.Citation17,Citation8 For the majority of patients with allergic rhinoconjunctivitis, intranasal steroids remain the most effective treatment since all of the major symptoms associated with AR are effectively attenuated.
An important aspect is the effect of upper airway inflammation on lower airway symptoms. It is often not recognized by the respiratory community that up to 80% individuals with asthma have rhinitis.Citation18 SAR is commonly associated with seasonal allergic asthma, and with demonstrable increases in airway hyper-responsiveness (AHR) that can translate into asthma exacerbation.Citation19 Intranasal steroids alone can prevent this seasonal increase in AHR and symptoms.Citation20 Intranasal steroid can be more effective in this respect than inhaled corticosteroids.Citation21,Citation22
Efficacy and compliance
For any drug to have significant impact upon a disease, it must demonstrate clinical efficacy, an excellent safety profile and must be used appropriately. Minimal dosing frequency and ease of delivery device will promote compliance with therapy. Important pharmacological characteristics for a topical steroid are a high affinity for the target tissue and steroid receptor with subsequent slow dissociation, ensuring maximal and prolonged local tissue effects. This will also decrease potential systemic effects by delayed release from target tissue. Given that more than 80% of any nasal steroid is swallowed, a molecule that is either minimally absorbed from the gastrointestinal tract and/or undergoes maximal hepatic first-pass metabolism will substantially decrease systemic bioavailability. The development of intranasal steroids is one of the best examples of molecular modification of a compound to achieve the best therapeutic index.
The emergence of intranasal steroids
The steroid compounds cortisol and cortisone were first identified from the adrenal cortex in the late 1930s.Citation23 With the realization that these were potent anti-inflammatory agents,Citation24 methods of producing large amounts of synthetic cortisone were sought. However, it became evident that both cortisone and cortisol induced clinically significant adverse effects such as severe electrolyte disturbances that limited the dose and duration of use. The development of compounds that utilized the anti-inflammatory potency of cortisone without the systemic effects was urgently required. Structure – activity relationship to absorption, distribution, metabolism and elimination of steroid compounds could be demonstrated. Functional modification of the steroid structure generated compounds with increased activity and improved safety profile.Citation25
Target-specific delivery is still the most effective strategy to minimalize systemic effects of any drug, yet it was not until the early 1970s that attempts to deliver airway-specific steroid in the form of beclomethasone dipropionate (BDP) for asthma were undertaken.Citation26 The first intranasal delivery of BDP for SAR was in 1973Citation27 and BDP remains the most clinically used steroid formulation. Seven further licensed intranasal preparations are currently available: flunisolide (since 1976),Citation28 budesonide since the early 1980s,Citation29 fluticasone propionate (FP) and triamcinolone acetonide since the early 1990s.Citation30,Citation31 Trials with ciclesonide were first published in 1999 and mometasone furoate since1996.Citation32,Citation33 Fluticasone furoate (FF) was launched in 2009. Each corticosteroid is defined by a specific pharmacokinetic and pharmacodynamic profile. The early market entry of FP with the demonstration of high clinical efficacy and negligible oral bioavailability established it as a key therapeutic intervention in AR and asthma. Fluticasone furoate (FF) is an evolution of FP and there are reports of therapeutic advantages over FP.
Structure–activity relationship
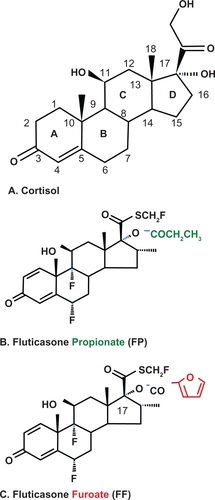
Cortisol is a natural steroid with potent glucocorticoid and mineralocorticoid action. Four carbon rings (3 rings with 6 carbons and 1 ring with only 5 carbons define the structure) (). In addition there is a 11β-hyroxyl group that is essential for the glucocorticoid and anti-inflammatory effect of the molecule. All corticosteroids maintain this core structure and the 4,5-carbon unsaturated double bond and the 3-carbon position keto-group are considered essential for bioactivity (). Manipulation of the structural attachments in the 5-carbon ring has allowed manufacture of synthetic corticosteroids with characteristic profiles. The addition of a double bond to the 1,2-carbon position of the first ring increased glucocorticoid potency and decreased metabolism (). The introduction of halogenation at specific points of the molecule increased both the glucocorticoid and mineralocorticoid effects.Citation34 FP is halogenated at both the 6α and the 9α position.Citation35 Adding a methyl group to the 16-carbon position abolished mineralocorticoid activity. Topical potency was increased by adding an esterified lipophilic group to the 5-carbon ring (17α) position.Citation36,Citation37 Such halogenation was associated with an almost 7-fold increase in binding affinity for the glucocorticoid receptor.Citation38 Lipophilicity of the compound is correlated with tissue absorption and retention.Citation37
Mechanism of action of steroids in airway inflammation
Glucocorticoids work by penetrating the plasma membrane of the cell and by binding to the cytosolic glucocorticoid receptor (GR). In humans a single GR gene transcribes two separate receptors GR-alpha (α) and GR-beta (β). GR-α is ubiquitously expressed and is considered the key GR in anti-inflammatory responses. Upon GR binding, the GC-GR complex translocates into the nucleus. Repression of inflammatory gene activation is achieved through several heterogeneous mechanisms. The GC-GR complex binds DNA at the glucocorticoid response elements (GRE) in the 5′-upstream region of the steroid responsive genes. Transcriptional activation of anti-inflammatory genes or repression of pro-inflammatory ones can now occur. Other mechanisms of regulating inflammation are via protein – protein sequestration via binding to other pro-inflammatory transcription factors such as activator protein (AP-1), leading to the inhibition of the transcription of inflammatory genes. The GC-GR complex can also act indirectly via the induction of inhibitory proteins, for example IκB that suppresses NF-κB activity.Citation39 Although GR-β expression has been demonstrated in asthma and nasal polyposis, particularly in relation to inflammatory cells, its role remains uncertain.
Fluticasone propionate
Fluticasone propionate is a topically active corticosteroid with established efficacy in seasonal and perennial AR. The excellent pharmacodynamic and pharmacokinetic properties along with safety have given FP a key position as one of the market leaders. This impressive therapeutic and safety profile is a reflection of its rapid and extensive uptake by airway tissue, marked affinity for the GR and almost undetectable systemic bioavailability.Citation40
The propionate ester side chain renders FP highly lipophilic. Such lipophilicity is a key determinant of its pharmacological profile and allows the drug to bind tissue rapidly and strongly with more prolonged retention than more hydrophilic molecules such as budesonide and hydrocortisone.Citation41 Potency and hence therapeutic efficacy is further determined by the GR binding affinity. The greater the steroid – receptor affinity the longer is the binding time and hence transcriptional regulation of genes and protein interactions. Binding studies using competition assays and in vitro binding kinetic studies have confirmed the high selectivity and affinity of FP for the GR receptor. FP demonstrates a high association rate constant and a pronounced low dissociation constant predicting an equilibrium dissociation constant (Kd) (ie, the propensity of the GC-GR complex to dissociate) of 0.49/0.51 nmol/L compared to the Kd of dexamethasone at 9.36/8.80 nmol/L. The relative receptor affinity (RRA) of FP to the human GR (compared relative to dexamethasone with an RRA of 100) is 1910/1775.Citation41,Citation42 These data are summarized in . Such a fast association, high receptor affinity and subsequent low dissociation predicts clinical efficacy and the long half-time of the FP-GR complex supports the practicality of using once-daily dosing schedules.
Table 1 Summary of glucocorticoid receptor (GR) binding kinetics of fluticasone propionate and fluticasone furoate relative to dexamethasone (Dex)Citation42
The systemic availability of intranasal FP is very low when using standard dosing regimens that the plasma concentration of FP is often below the limit of detection. Using a higher dosing schedule of up to 12 times the normal dose and a more sensitive detection procedure, the mean absolute bioavailability for FP intranasal drops and spray was 0.06% and 0.51%, respectively, of the administered dose.Citation43 Thus the data related to the pharmacokinetics of intranasal FP are limited and much of the information we have is related to studies using either oral or intravenous dosing regimes. Even with oral doses of 10 mg twice daily, the systemic bioavailability of FP was less than 1%.Citation44 Following intravenous administration at a dose range of 0.25 to 1 mg, FP demonstrated extensive tissue distribution with a mean residence time of 4.9 hours and rapid tissue clearance with the elimination half-life measured at 7.8 hours.Citation45 Oral bioavailability of FP is almost absent due to poor gut absorption and extensive hepatic first pass metabolismCitation46 such that any systemic concentrations of intranasal FP will be as a result of absorption via the nasal mucosa. Given that a significant proportion of inhaled nasal steroids will be swallowed, FP will have negligible systemic availability and thus predictably have no bio-systemic effects. FP has a rapid rate of systemic clearance with oral doses of up to 1 to 16 mg becoming undetectable in blood 6 hours after administration. Intravenous administration was associated with clearance rates that approximated hepatic blood flow consistent with extensive hepatic first pass metabolism.Citation47 During hepatic metabolism, FP undergoes de-esterification with cleavage of the fluromethylthioester group at the 17β position to the 17β-carboxylic acid.Citation46 This inactivates the glucocorticoid molecule before its release into the systemic circulation. There does not appear to be any saturation of this process even at oral doses of 4000 μg. Oral FP is excreted predominantly via the gut in the range of 87% to 100%, up to 40% as the 17β-carboxylic acid metabolite. Less than 5% is removed in the urine with around 18% as the 17β-carboxylic acid.Citation47
Therapeutic efficacy in seasonal allergic rhinitis
Several large, double-blind, placebo-controlled studies have established the clinical efficacy of FP in SAR. An initial dose-ranging study established that 25 μg, 100 μg and 400 μg given on a twice-daily regime had superior clinical efficacy compared to placebo and this was evident as early as 3 days into dosing.Citation30 Subsequent studies evaluated clinical efficacy and the further safety of FP. A study of adults with SAR in season evaluated FP aqueous nasal spray using either 200 μg daily versus 100 μg twice daily.Citation48 Both regimes led to significant improvements in total symptom reduction with no significant difference between them. However, a significant reduction in nasal obstruction only was demonstrated for 100 μg twice daily rather than 200 μg once daily in the 1-study of 4 weeks’ duration.Citation48 In the same study, only the 100 μg twice-daily regime demonstrated a statistically significant reduction in the use of rescue medication compared to placebo. A significant proportion of individuals continue to have severe SAR despite intervention with intranasal steroids. Given the encouraging safety profile from the earlier dose-ranging studies, several groups investigated whether doubling the doses of FP would confer any further impact on disease. A single-center study enrolling 90 volunteers with more severe SAR demonstrated that 200 μg twice daily versus 200 μg once daily leads to a significant improvement in the number of symptom free days without nasal itching and conjunctivitis.Citation49 Although an important trend in the overall reduction of symptom scores was demonstrated here, significant reduction of the individual symptom score was seen only for nasal itch.Citation49 In a separate study, individuals with severe SAR uncontrolled with 200 μg of FP once daily over 2 weeks were then randomized to 200 μg twice daily for a further 2 weeks.Citation50 This conferred a statistically significant advantage with increased symptom-free days in terms of decreased nasal congestion on waking and in the daytime. Although there were improvements in rhinorrhea, sneezing and nasal itch these were not statistically significant.
Response to treatment can also be assessed in terms of rescue medication use. FP at either 100 μg twice daily or 200 μg once a day confers a significant advantage over placebo in all studies in reducing rescue medication. Although 100 μg twice daily appears to confer an advantage over 200 μg once daily in at least 2 studies,Citation48,Citation49 a total daily dosage higher than 200 μg of FP does not confer any further advantage in this respect. FP 200 μg provided significant symptom relief in SAR even when used on an ‘as needed basis’.Citation51 The pooled data from studies predict the onset of therapeutic effect of FP to be within 12 hours after intranasal administration.Citation52 Once-daily dosing with FP at either 100 μg or 200 μg confers similar clinical efficacy both superior to placebo in children 4 to 12 years of age and adolescents with SAR.Citation53–Citation55 In clinical practice, FP is prescribed at 100 μg into each nostril once a day, which is an effective dose for most individuals with AR. With more severe disease, particularly nasal congestion, it is common practice to use a twice-daily regime with 200 μg until control is achieved. It is safe to use higher doses of up to 400 μg, the standard dose per unit contained with FP nasule (drop) preparations in individuals with severe disease.Citation56 The bioavailability of the intranasal drops formula is estimated to be 8 times lower than the equivalent dose of the nasal spray formulation.Citation43
Fluticasone furoate
Fluticasone furoate is a new topical corticosteroid with a licence for the treatment of both seasonal and perennial AR. It is delivered as a well-dispersed mist composed of fine droplets of particles 20 to 50 μm in diameter, probably leading to an even area of distribution over the nasal mucosa.Citation57 FF is a trifluorinated molecule that is similar in structure to FP other than the addition of a furoate ester to the 17α-OH group (). In FP, this location is esterified with propionic acid (). Given the structural homology, one would predict a similar pharmacokinetic and pharmacodynamic profile and therapeutic efficacy of FF to FP. In fact, the emerging evidence suggests that FF may have a superior product profile and enhanced clinical efficacy in AR.Citation57
As with FP, FF demonstrates a high lipophilicity with a remarkably fast association with the glucocorticoid receptor and a subsequently slow dissociation rate. The propensity of the GC-GR to dissociate, expressed as the Kd, is 0.3 nmol/L for FF. For comparision the Kd of dexamethasone was 8.8 nmol/L, Kd of FP 0.51 nmol/L and the Kd of mometasone furoate (MF) 0.41 nmol/L in this particular set of experiments ().Citation42 The relative receptor affinity (RRA) of FF to the GR has been shown to be 2989, compared to dexamethasone with an RRA at 100, and with FP and MF at 1775 and 2244 respectively.Citation42 The difference in the RRA between FF and FP reflects the potent effects of ester-furoylation at the 17α-OH location. The 17α-furoate ester substitute allows the FF molecule to fully occupy the GR 17α-pocket. Such molecular intimacy will allow additional structures of the fluticasone core to further interact with the receptor. In vitro data demonstrate enhanced activity of FF for the GR with potent activation of the glucocorticoid response element pathway along with repression of NF-κB induction of transcriptional events. Almost complete abolition of eosinophil influx into the airway in an animal model of allergic inflammation could be demonstrated with an intratracheal FF dose of only 30 μg.Citation57 Detection of plasma FF following intranasal dosing was again below the level of detection with even up to doses of 880 μg per day and the average absolute bioavailability following intranasal administration was only 0.5%.Citation58 As with FP, extensive hepatic first pass metabolism of FF via the cytochrome P450 3A4 enzyme system limits oral bioavailability.Citation46,Citation59 Thus FF presents an impressive pharmacodynamic profile compared to the other new generation glucocorticoids. Rapid high affinity binding along with prolonged tissue retention, and minimal systemic availability markets FF is an attractive option for topical therapy.
Seasonal allergic rhinitis
Four double-blind placebo-controlled studies have been conducted on FF in adults and children over 12 years of age with SAR. One was a dose ranging study to evaluate the efficacy and safety of FF at 55 μg, 110 μg, 220 μg and 440 μg once daily in the US mountain cedar pollen season.Citation60 Although all doses other than 55 μg demonstrated similar clinical efficacy that was statistically significant compared to placebo, it was the 110 μg dose that was thought to offer the optimal therapeutic ratio and was chosen for further clinical evaluation. Three phase III studies assessed the clinical efficacy and safety of once daily FF at 110 μg at the height of season for European grass pollen, US ragweed and US mountain cedar.Citation61,Citation62,Citation63 In total 1142 volunteers with SAR were randomized to either 110 μg of FF (n = 571) or placebo (n = 570) in season. Summated data from these studies confirmed the onset of efficacy of FF to be within 24 hours of the initial dosing. Each study demonstrated significant improvement in clinical parameters assessed related to nasal and ocular symptoms. As well as improvement in the reflective total nasal symptom scores (rTNSS), there was also significant improvement in the instantaneous iTNSS (iTNSS) recorded each day just prior to dose administration. This confirmed that a once-daily dosing is efficacious for the entire 24-hour period. Currently the recommended starting dose of FF in adults and children over 12 years of age is 110 μg daily per nostril, with the aim of reducing to 55 μg once daily once symptom control has been achieved.
An impressive finding from these studies was the consistent efficacy of FF treatment on reducing ocular manifestations in SAR. Symptom reduction was associated with increased positive scoring in the rhinoconjunctivitis quality of life questionnaire (RQLQ). Ocular symptoms of allergy can be particularly troublesome for the patient, are often difficult to treat and therefore represent an unmet clinical need. Although other INS demonstrate some effect on the ocular symptoms of SAR, this efficacy has not always been reproducible.Citation64 Studies with FF demonstrate consistency and reproducibility in reduction of ocular symptoms.Citation64 It is presumed the effect of intranasal steroids on eye manifestations of allergy is related to the effects of decreased inflammatory mediators that prime nasal neurogenic tissue and initiate the nasal – ocular reflex.Citation11,Citation65 The high affinity of FF for the glucocorticoid receptor and slow dissociation rate probably give FF superiority in terms of attenuation of inflammation,Citation66 which may reduce neuronal activation.Citation67
The experience of FF for SAR in children is limited at present to a single study of either 55 μg or 110 μg once daily versus placebo in 554 children aged between 2 and 11 years of age over 2 weeks.Citation68 Clinical efficacy was most evident only at the 110 μg dose. Despite these findings caution is still advised with children and the recommended starting dose of FF for children 2 to 11 years of age is 55 μg per daily dose, increasing to 110 μg daily only for non-responders or during exacerbation periods.
Safety and adverse events
The term steroid is associated with apprehension of adverse systemic effects by both clinicians and patients. In particular, concerns over effects on hypothalamic – pituitary – adrenal (HPA) suppression, bone growth and density, posterior sub-capsular cataract formation and raised ocular pressure associated with systemic administration of steroids have often been incorrectly extrapolated to locally delivered steroids, regardless of specific formulation, dosage and site of administration. This has led to poor adherence and treatment failure.Citation69 Generally, studies in children and adults have consistently failed to demonstrate any clinically significant effect on the HPA-axis,Citation70 bone growth or cataract formation/glaucoma. This is a reflection of low systemic bioavailability following intranasal administration. Clinical guidelines provide reassurance.Citation17 The high therapeutic index of both FP and FF predicted by the key pharmacological properties has been borne out by the detailed clinical safety studies of these products.
Safety of FP was established early on. In the initial dose-ranging study, even with the highest dose of 400 μg twice-daily there were no increased side effects versus placebo for FP.Citation30 Although only a 2-week dosing period was used, no HPA axis effects were demonstrated. The safety and tolerance of FP in doses of 50 μg, 200 μg or 800 μg twice daily over a 4-week period in patients with SAR con-firmed that even at the highest total dose of 1600 μg there were no increased adverse events compared to placebo.Citation71 Detailed evaluation of adrenal function in terms of early morning and ACTH-stimulated cortisol and 24-hour urinary cortisol excretion demonstrated no effect of FP. Several other studies using higher than standard doses of FP have failed to demonstrate HPA-axis effects.Citation72,Citation73 Studies assessing adverse effects on other body systems are limited. A 12-month study with FP with 200 μg daily did not demonstrate any effect on bone density or occurrence of cataract or glaucoma.Citation74 A similar open year-long study of 200 μg daily showed no ill effects on the nasal mucosa, in fact saccharin clearance was improved following therapy.Citation75 Overall there was no increased severity or frequency of adverse events with FP compared to placebo. The pediatric studies were similarly encouraging. Several studies in children 4 to 11 years of age or adolescents 12 to 17 years of age with FP at 100 or 200 μg per day over 2 to 12 weeks failed to show any HPA-axis effect or any increased adverse effect compared to placebo.Citation76,Citation77
With FF, the dosing study with up to 440 μg once daily for 2 weeks in adults with SAR failed to show any effect on HPA function.Citation60 A study of longer duration in adults and adolescents with perennial allergic rhinitis also confirmed the long term safety of FF.Citation78 Detailed evaluation of FF in children in children 2 to 11 years of age at 110 μg daily for 6 weeks,Citation79 2 weeksCitation68 and 12 weeksCitation80 all confirmed an absence of any effect on HPA function.Citation81 Effect on bone growth is of particular concern in children. FP at 110 μg per day over 2 weeks had no effect on lower leg growth in a 6- to 12-year group over a 2-week period.Citation82 Further studies of longer duration are still needed but the safety data of FF in children is encouraging. The European Medicines Agency (EMEA) has granted the licence for the use of FF (Avamys®; GlaxoSmithKline) for adults, adolescents from 12 years upward and children from 6 to 11 years only. The US Food and Drug Administration (FDA) agency, however, has improved FF (Veramyst®; GlaxoSmithKline) for children from 2 years upwards.
Tolerability and compliance
No product will have any impact on disease unless the patient group uses it correctly. Tolerability of the product and concordance with therapy is determined to some extent by the ease of delivery, the sensory attributes of the product such as taste along with the convenience of once-daily use and the absence of any adverse events.Citation83–Citation85 Ease of any delivery device is dictated by the absence of any complicated priming procedure prior to use. Delivery of a set drug dose independent of the pressure or speed applied by the patient to the device is particularly relevant to a pediatric and elderly population. Clear indication of the remaining amount of available drug in the apparatus simplifies self-management.Citation86 Comfort during administration is also important and local adverse events that arise from mechanical nasal irritation such as anterior nasal crusting and bleeding are particularly bothersome, and reported to affect up to 20% of individuals leading to cessation of therapy.Citation87 The association of systemic and dermatological topical steroids with direct connective tissue atrophy has often been incorrectly extrapolated as casual for nasal crusting and bleeding in the nasal mucosaCitation88 and has led to patients and on occasions physicians to discontinue treatment. Advice regarding administration to the outer aspect of the nasal lining using two different sites for the two actuations, in order to maximize the area of mucosal contact and avoid septal deposition, is important. A reminder not to sniff the drug back heartily, thus removing it too quickly from nose to the posterior pharynx, should also be given. The drug delivery device and vehicle are therefore essential aspects to consider when prescribing an INS.
Patient preference in regard to specific sensory attributes of a drug may be determinant of adherence to therapy. Important sensory attributes include minimal odor and irritant effect, absent taste and product moistness. It has been shown that the intensity of such sensory components is inversely correlated with preference.Citation84 Sensory attributes vary considerably between current market preparations.Citation84,Citation85,Citation89 For example triamcinolone acetonide (TAA) demonstrates less odor and taste with no local irritation and has been shown to have a sensory advantage over FP and MF in studies.Citation89,Citation90 However, the pharmacokinetic and pharmacodynamic characteristics of TAA would not favor first-line intranasal use.Citation91
The established INS are currently delivered using a basic mechanical pump system that delivers a jet or squirt of drug. However, they differ considerably in terms of exact priming procedure, nozzle size and the ability to hit the nasal mucosa without change in head posture. Much of the focus with these products has been with clinical efficacy of the drug, without too much attention to the importance of device design in relation to factors that ensure compliance and patient acceptance. Understanding the importance of the delivery device relative to features that ensure compliance with therapy has led to extensive investment in the design of the delivery device for the newest product FF. The system for FF has an innovative design that is a prime-free device, allows easy grip with a side actuation mechanism such that the user’s fingers are away from the delivery nozzle when activating the spray.Citation92 Abolishing the need for fingers on the nozzle base allow the use of a shorter delivery nozzle, making the device less invasive. Nasal pain, bleeding and crusting from mechanical irritation are important aggravating factors for patients and a less penetrative device may be better tolerated, particularly by children. Parents can more effectively administer the spray to children using a minimum actuation force. The formulation is alcohol free and hence there is less nasal burning and irritation on mucosal deposition.
Conclusion
Both FP and FF are excellent examples of how an understanding of molecular structure – function relationships can be used to achieve good clinical efficacy without compromising safety. FP is a long established intervention for AR with an excellent therapeutic index. FF represents an advance not simply in how sophisticated molecular modification can further improve pharmacological profile, but also shows the importance of focus on factors that may improve patient tolerability and hence compliance. Such focus should further advance therapeutic intervention for patients with AR.
Disclosures
GKS has lectured for GSK, and has also served on rhinitis boards and received research finding from GSK.
References
- BousquetJKhaltaevNCruzAAAllergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen)Allergy200863Suppl 86816018331513
- CanonicaGWBousquetJMullolJScaddingGKVirchowJCA survey of the burden of allergic rhinitis in EuropeAllergy200762Suppl 85172517927674
- EmanuelMBHay fever, a post industrial revolution epidemic: a history of its growth during the 19th centuryClin Allergy1988182953043293845
- StrachanDPEpidemiology of hay fever: towards a community diagnosisClin Exp Allergy1995252963037600374
- von MutiusEWeilandSKFritzschCDuhmeHKeilUIncreasing prevalence of hay fever and atopy among children in Leipzig, East GermanyLancet19983518628669525363
- BousquetPJChinnSJansonCKogevinasMBurneyPJarvisDGeographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey IAllergy20076230130917298348
- ScaddingGKKariyawasamHHAirways disease: just nosing around?Thorax20096492392519864539
- ScaddingGKDurhamSRMirakianRBSACI guidelines for the management of allergic and non – allergic rhinitisClin Exp Allergy200838194218081563
- RondonCFernandezJLopezSNasal inflammatory mediators and specific IgE production after nasal challenge with grass pollen in local allergic rhinitisJ Allergy Clin Immunol20091241005101119796796
- CiprandiGCirilloIVizzaccaroAToscaMPassalacquaGPallestriniESeasonal and perennial allergic rhinitis: is this classification adherent to real life?Allergy20056088288715932377
- NaclerioRMPintoJdeTineoMBaroodyFMElucidating the mechanism underlying the ocular symptoms associated with allergic rhinitisAllergy Asthma Proc200829242818302834
- PortnoyJMVan OsdolTWilliamsPBEvidence-based strategies for treatment of allergic rhinitisCurr Allergy Asthma Rep2004443944615462709
- WeinerJMAbramsonMJPuyRMIntranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trialsBMJ1998317162416299848901
- YanezARodrigoGJIntranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysisAnn Allergy Asthma Immunol20028947948412452206
- WilsonAMO’ByrnePMParameswaranKLeukotriene receptor antagonists for allergic rhinitis: a systematic review and meta-analysisAm J Med200411633834414984820
- DykewiczMSFinemanSSkonerDPDiagnosis and management of rhinitis: complete guidelines of the Joint Task Force on Practice Parameters in Allergy, Asthma and Immunology. American Academy of Allergy, Asthma, and ImmunologyAnn Allergy Asthma Immunol1998814785189860027
- WallaceDVDykewiczMSBernsteinDIThe diagnosis and management of rhinitis: an updated practice parameterJ Allergy Clin Immunol2008122S1S8418662584
- LeynaertBNeukirchCKonySAssociation between asthma and rhinitis according to atopic sensitization in a population-based studyJ Allergy Clin Immunol2004113869314713912
- MadoniniEBriatico-VangosaGPappacodaAMaccagniGCardaniASaporitiFSeasonal increase of bronchial reactivity in allergic rhinitisJ Allergy Clin Immunol1987793583633819219
- CorrenJAdinoffADBuchmeierADIrvinCGNasal beclomethasone prevents the seasonal increase in bronchial responsiveness in patients with allergic rhinitis and asthmaJ Allergy Clin Immunol1992902502561500629
- AubierMLevyJClericiCNeukirchFHermanDDifferent effects of nasal and bronchial glucocorticosteroid administration on bronchial hyperresponsiveness in patients with allergic rhinitisAm Rev Respir Dis19921461221261626795
- StelmachRdo PatrocinioTNRibeiroMCukierAEffect of treating allergic rhinitis with corticosteroids in patients with mild-to-moderate persistent asthmaChest20051283140314716304254
- MurrayJRThe history of corticosteroidsActa DermVenereol Suppl (Stockh)198915146
- HenchPSKendallECThe effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone; compound E) and of pituitary adrenocorticotropic hormone on rheumatoid arthritisMayo Clin Proc19492418119718118071
- KatzMGansEHTopical corticosteroids, structure-activity and the glucocorticoid receptor: discovery and development – a process of “planned serendipity”J Pharm Sci2008972936294717975808
- BrownHMStoreyGGeorgeWHBeclomethasone dipropionate: a new steroid aerosol for the treatment of allergic asthmaBr Med J197215855904335298
- MygindNLocal effect of intranasal beclomethasone dipropionate aerosol in hay feverBr Med J197344644664586044
- TurkeltaubPCNormanPSCrepeaSTreatment of ragweed hay fever with an intranasal spray containing fluinsolide, a new synthetic corticosteroidJ Allergy Clin Immunol197658597606789436
- BalleVHPedersenUEngbyBAllergic perennial and non-allergic, vasomotor rhinitis treated with budesonide nasal sprayRhinology1980181351426997974
- MeltzerEOOrgelHABronskyEAA dose-ranging study of fluticasone propionate aqueous nasal spray for seasonal allergic rhinitis assessed by symptoms, rhinomanometry, and nasal cytologyJ Allergy Clin Immunol1990862212302200821
- FindlaySHuberFGarciaJHuangLEfficacy of once-a-day intranasal administration of triamcinolone acetonide in patients with seasonal allergic rhinitisAnn Allergy1992682282321546817
- SchmidtBMTimmerWGeorgensACThe new topical steroid ciclesonide is effective in the treatment of allergic rhinitisJ Clin Pharmacol1999391062106910516941
- HebertJRNolopKLutskyBNOnce-daily mometasone furoate aqueous nasal spray (Nasonex) in seasonal allergic rhinitis: an active- and placebo-controlled studyAllergy1996515695768874661
- BikowskiJPillaiRShrootBThe position not the presence of the halogen in corticosteroids influences potency and side effectsJ Drugs Dermatol2006512513016485880
- PopperTLGentlesMJKungTTStructure-activity relationships of a series of novel topical corticosteroidsJ Steroid Biochem1987278378433695507
- PhillippsGHBaileyEJBainBMSynthesis and structure-activity relationships in a series of antiinflammatory corticosteroid analogues, halomethyl androstane-17 beta-carbothioates and -17 beta-carboselenoatesJ Med Chem199437371737297966132
- DahlbergEThalenABrattsandRCorrelation between chemical structure, receptor binding, and biological activity of some novel, highly active, 16 alpha, 17 alpha-acetal-substituted glucocorticoidsMol Pharmacol19842570786708937
- BuchwaldPGlucocorticoid receptor binding: a biphasic dependence on molecular size as revealed by the bilinear LinBiExp modelSteroids20087319320818022656
- PujolsaLMullolJPicadoCGlucocorticoid receptor in human respiratory epithelial cellsNeuroimmunomodulation20091629029919571590
- BrysonHMFauldsDIntranasal fluticasone propionate. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in allergic rhinitisDrugs1992437607751379151
- HoggerPRohdewaldPBinding kinetics of fluticasone propionate to the human glucocorticoid receptorSteroids1994595976027878687
- ValotisAHoggerPHuman receptor kinetics and lung tissue retention of the enhanced-affinity glucocorticoid fluticasone furoateRespir Res200785417650349
- Daley-YatesPTBakerRCSystemic bioavailability of fluticasone propionate administered as nasal drops and aqueous nasal spray formulationsBr J Clin Pharmacol20015110310511167672
- FalcozCOliverRMcDowallJEVentrescaPByeADaley-YatesPTBioavailability of orally administered micronised fluticasone propionateClin Pharmacokinet200039Suppl 191511140434
- MackieAEVentrescaGPFullerRWByeAPharmacokinetics of intravenous fluticasone propionate in healthy subjectsBr J Clin Pharmacol1996415395428799519
- PearceRELeederJSKearnsGLBiotransformation of fluticasone: in vitro characterizationDrug Metab Dispos2006341035104016565171
- HardingSMThe human pharmacology of fluticasone propionateRespir Med199084Suppl A25292287792
- LaForceCFDockhornRJFindlaySRFluticasone propionate: an effective alternative treatment for seasonal allergic rhinitis in adults and adolescentsJ Fam Pract1994381451528308505
- DolovichJO’ConnorMStepnerNSmithASharmaRKDouble-blind comparison of intranasal fluticasone propionate, 200 micrograms, once daily with 200 micrograms twice daily in the treatment of patients with severe seasonal allergic rhinitis to ragweedAnn Allergy1994724354408179230
- PedersenBDahlRRichardsDHOnce daily fluticasone propionate aqueous nasal spray controls symptoms of most patients with seasonal allergic rhinitisAllergy199550794998607560
- DykewiczMSKaiserHBNathanRAFluticasone propionate aqueous nasal spray improves nasal symptoms of seasonal allergic rhinitis when used as needed (prn)Ann Allergy Asthma Immunol200391444812877448
- MeltzerEORickardKAWestlundRECookCKOnset of therapeutic effect of fluticasone propionate aqueous nasal sprayAnn Allergy Asthma Immunol20018628629111289326
- BonerASetteLMartinatiLSharmaRKRichardsDHThe efficacy and tolerability of fluticasone propionate aqueous nasal spray in children with seasonal allergic rhinitisAllergy1995504985057573843
- GrossmanJBanovCBronskyEAFluticasone propionate aqueous nasal spray is safe and effective for children with seasonal allergic rhinitisPediatrics1993925945998414833
- MunkZMPearlmanDGraftDIntranasal fluticasone propionate is effective and well-tolerated in adolescents with seasonal allergic rhinitisPediatr Asthma Allergy Immunol199483946
- KeithPNieminenJHollingworthKDolovichJEfficacy and tolerability of fluticasone propionate nasal drops 400 microgram once daily compared with placebo for the treatment of bilateral polyposis in adultsClin Exp Allergy2000301460146810998024
- SalterMBiggadikeKMatthewsJLPharmacological properties of the enhanced-affinity glucocorticoid fluticasone furoate in vitro and in an in vivo model of respiratory inflammatory diseaseAm J Physiol Lung Cell Mol Physiol2007293L660L66717575011
- AllenADownGNewlandAAbsolute bioavailability of intranasal fluticasone furoate in healthy subjectsClin Ther2007291415142017825692
- HughesSCShardlowPCHollisFJMetabolism and disposition of fluticasone furoate, an enhanced-affinity glucocorticoid, in humansDrug Metab Dispos2008362337234418694910
- MartinBGRatnerPHHampelFCOptimal dose selection of fluticasone furoate nasal spray for the treatment of seasonal allergic rhinitis in adults and adolescentsAllergy Asthma Proc20072821622517479608
- FokkensWJJogiRReinartzSOnce daily fluticasone furoate nasal spray is effective in seasonal allergic rhinitis caused by grass pollenAllergy2007621078108417686111
- KaiserHBNaclerioRMGivenJTolerTNEllsworthAPhilpotEEFluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitisJ Allergy Clin Immunol20071191430143717418384
- JacobsRMartinBHampelFTolerWEllsworthAPhilpotEEffectiveness of fluticasone furoate 110 mug once daily in the treatment of nasal and ocular symptoms of seasonal allergic rhinitis in adults and adolescents sensitized to mountain cedar pollenCurr Med Res Opin2009251393140119419338
- KeithPKScaddingGKAre intranasal corticosteroids all equally consistent in managing ocular symptoms of seasonal allergic rhinitis?Curr Med Res Opin2009252021204119569975
- BaroodyFMShenaqDdeTineoMWangJNaclerioRMFluticasone furoate nasal spray reduces the nasal-ocular reflex: a mechanism for the efficacy of topical steroids in controlling allergic eye symptomsJ Allergy Clin Immunol20091231342134819428097
- BaumannDBachertCHoggerPDissolution in nasal fluid, retention and anti-inflammatory activity of fluticasone furoate in human nasal tissue ex vivoClin Exp Allergy2009391540155019538495
- RaapUBraunstahlGJThe role of neurotrophins in the pathophysiology of allergic rhinitisCurr Opin Allergy Clin Immunol20101081319935061
- MeltzerEOLeeJTripathyILimJEllsworthAPhilpotEEfficacy and safety of once-daily fluticasone furoate nasal spray in children with seasonal allergic rhinitis treated for 2 weeksPediatr Allergy Immunol20092027928618680490
- SkonerJDSchaffnerTJSchadCAKwonAYSkonerDPAddressing steroid phobia: improving the risk-benefit ratio with new agentsAllergy Asthma Proc20082935836418702881
- BruniFMDe LucaGVenturoliVBonerALIntranasal corticosteroids and adrenal suppressionNeuro immuno modulation200916353362
- van AsABronskyEGrossmanJMeltzerERatnerPReedCDose tolerance study of fluticasone propionate aqueous nasal spray in patients with seasonal allergic rhinitisAnn Allergy1991671561621867454
- VargasRDockhornRJFindlaySRKorenblatPEFieldEAKralKMEffect of fluticasone propionate aqueous nasal spray versus oral prednisone on the hypothalamic-pituitary-adrenal axisJ Allergy Clin Immunol19981021911979723660
- HayeRGomezEGA multicentre study to assess long-term use of fluticasone propionate aqueous nasal spray in comparison with beclomethasone dipropionate aqueous nasal spray in the treatment of perennial rhinitisRhinology1993311691748140383
- HowlandWCIIIFluticasone propionate: topical or systemic effects?Clin Exp Allergy199626Suppl 318228735854
- ScaddingGKLundVJHolmstromMDarbyYCClinical and physiological effects of fluticasone propionate aqueous nasal spray in the treatment of perennial rhinitisRhinol Suppl19911137431888556
- RichardsDHMiltonCMFluticasone propionate aqueous nasal spray: a well-tolerated and effective treatment for children with perennial rhinitisPediatr Allergy Immunol1996735438792382
- Treatment of seasonal allergic rhinitis with once-daily intranasal fluticasone propionate therapy in childrenFluticasone Propionate Collaborative Pediatric Working GroupJ Pediatr19941256286347931889
- RosenblutABardinPGMullerBLong-term safety of fluticasone furoate nasal spray in adults and adolescents with perennial allergic rhinitisAllergy2007621071107717686110
- TripathyILevyARatnerPClementsDWuWPhilpotEHPA axis safety of fluticasone furoate nasal spray once daily in children with perennial allergic rhinitisPediatr Allergy Immunol20092028729419175889
- MasperoJFRosenblutAFinnAJrLimJWuWPhilpotESafety and efficacy of fluticasone furoate in pediatric patients with perennial allergic rhinitisOtolaryngol Head Neck Surg2008138303718164990
- MeltzerEOTripathyIMasperoJFWuWPhilpotESafety and tolerability of fluticasone furoate nasal spray once daily in paediatric patients aged 6–11 years with allergic rhinitis: subanalysis of three randomized, double-blind, placebo-controlled, multicentre studiesClin Drug Investig2009297986
- GradmanJCaldwellMFWolthersODA 2-week, crossover study to investigate the effect of fluticasone furoate nasal spray on short-term growth in children with allergic rhinitisClin Ther2007291738174717919555
- KhannaPShahAAssessment of sensory perceptions and patient preference for intranasal corticosteroid sprays in allergic rhinitisAm J Rhinol20051931632116011141
- MahadeviaPJShahSLeibmanCKleinmanLO’DowdLPatient preferences for sensory attributes of intranasal corticosteroids and willingness to adhere to prescribed therapy for allergic rhinitis: a conjoint analysisAnn Allergy Asthma Immunol20049334535015521370
- MeltzerEOBardelasJGoldsobelAKaiserHA preference evaluation study comparing the sensory attributes of mometasone furoate and fluticasone propionate nasal sprays by patients with allergic rhinitisTreat Respir Med2005428929616086602
- BousquetJVan CauwenbergePBachertCRequirements for medications commonly used in the treatment of allergic rhinitis. European Academy of Allergy and Clinical Immunology (EAACI), Allergic Rhinitis and its Impact on Asthma (ARIA)Allergy20035819219712653792
- WaddellANPatelSKTomaAGMawARIntranasal steroid sprays in the treatment of rhinitis: is one better than another?J Laryngol Otol200311784384514670141
- BaroodyFMChengCCMoylanBAbsence of nasal mucosal atrophy with fluticasone aqueous nasal sprayArch Otolaryngol Head Neck Surg200112719319911177038
- StokesMAmorosiSLThompsonDDupclayLGarciaJGeorgesGEvaluation of patients’ preferences for triamcinolone acetonide aqueous, fluticasone propionate, and mometasone furoate nasal sprays in patients with allergic rhinitisOtolaryngol Head Neck Surg200413122523115365540
- BachertCEl AkkadTPatient preferences and sensory comparisons of three intranasal corticosteroids for the treatment of allergic rhinitisAnn Allergy Asthma Immunol20028929229712269650
- Daley-YatesPTRichardsDHRelationship between systemic corticosteroid exposure and growth velocity: development and validation of a pharmacokinetic/pharmacodynamic modelClin Ther2004261905191915639702
- BergerWEGodfreyJWSlaterALIntranasal corticosteroids: the development of a drug delivery device for fluticasone furoate as a potential step toward improved complianceExpert Opin Drug Deliv2007468970117970670