Abstract
Seafood allergy is a hypersensitive disorder with increasing prevalence worldwide. Effective and accurate diagnostic workup for seafood allergy is essential for clinicians and patients. Parvalbumin and tropomyosin are the most common fish and shellfish allergens, respectively. The diagnosis of seafood allergies is complicated by cross-reactivity among fish allergens and between shellfish allergens and other arthropods. Current clinical diagnosis of seafood allergy is a complex algorithm that includes clinical assessment, skin prick test, specific IgE measurement, and oral food challenges. Emerging diagnostic strategies, such as component-resolved diagnosis (CRD), which uses single allergenic components for assessment of epitope specific IgE, can provide critical information in predicting individualized sensitization patterns and risk of severe allergic reactions. Further understanding of the molecular identities and characteristics of seafood allergens can advance the development of CRD and lead to more precise diagnosis and improved clinical management of seafood allergies.
Introduction
IgE-mediated food allergy is a major global public health issue. A cross-sectional study in a United States cohort of 333,200 children reported a food allergy prevalence of 6.7%.Citation1 Sensitization usually occurs by exposure through ingestion, inhalation, or skin contact, and re-exposure to milligrams of allergens is sufficient to trigger life-threatening allergic responses.Citation2,Citation3 Another survey in the United States on 38,480 subjects younger than 18 years of age reported a food allergy prevalence of 8.0%, where 38.7% of the cohort experienced severe allergic reaction, and 30.4% developed multiple food allergies.Citation4
Fish and shellfish are among the most common culprits of food allergies. Fish allergy affects 0.2% of the general population.Citation5 In the USA, the lifetime prevalence rate for reported fish allergy was 0.4% while 0.2% of population experienced both fish and shellfish allergy.Citation6 In Asia, fish allergy prevalence was much higher in the Philippines (2.29%) than in Singapore (0.26%) and Thailand (0.29%).Citation7 Worldwide prevalence of shellfish allergy was found to be 0.6% with higher incidence reported in the Asia-Pacific region.Citation8 In the USA, the lifetime prevalence rate for reported shellfish allergy was 2%, with higher prevalence reported in adults (2.8%) compared to children (0.6%), and in women (3.6%) compared to men (2%).Citation6 Shellfish allergy is highly prevalent among teenagers in the Philippines (5.12%) and Singapore (5.23%) and is the leading cause of food anaphylaxis in Hong Kong and Taiwan.Citation9–Citation11 The increasing incidence of fish and shellfish allergy may be attributed to the growing consumption of seafood worldwide. The 2016 Food and Agriculture Organization of the United Nations report indicated that global fish consumption per capita has risen to over 20 kg year on year.Citation12 In 2014, the global capture fisheries production was 93.4 million tons, while crustacean and mollusks output from aquaculture amounted to 6.9 and 16.1 million tons, respectively.Citation12
Considering the pervasiveness of fish and shellfish allergies, developing precise diagnostic protocols is essential for appropriate prevention and management strategies including avoidance of unnecessary dietary restrictions. Conventional first-line diagnostic approach includes clinical assessment, oral food challenge (open or blinded), skin prick test (SPT), and serum-specific IgE (sIgE) measurement. Fish and shellfish extracts are commonly used in these in vivo and in vitro tests. However, the presence of cross-reactive allergens and the varying allergen contents among commercial extracts may lead to over- or underdiagnosis of seafood allergy. Fortunately, our increasing understanding of seafood allergens and improvements in technology to produce recombinant allergens allow for the detection of allergen-specific IgE and the development of component-resolved diagnosis (CRD) to reduce the ambiguities of conventional tests.
Fish allergens and cross-reactivity
Twenty-one allergens from 15 fish species are officially recognized by the World Health Organization/International Union of Immunological Societies at present (). Parvalbumin was first identified as the major fish allergen in 1969.Citation13 It is a 10–12 kDa protein abundant in muscle and is physiologically important for calcium binding. Bugajska-Schretter et al characterized the IgE reactive proteins in fish with serum samples from 30 fish-allergic patients and demonstrated that all tested sera were IgE positive to parvalbumin from cod extract (Gad c 1).Citation14 Interestingly, calcium depletion reduced IgE binding to parvalbumin in most patient sera.Citation14 Fish parvalbumin is thermally stableCitation15 and maintains its allergenic activity and antigenicity even under acidic conditions and after pepsinolysis.Citation13,Citation16 Short burst swimming in fish is powered by white muscles, which have a higher parvalbumin content than the dark muscles that drives continuous stroke.Citation17 It was, therefore, suggested that fish with more dark muscles such as tuna and mackerel is less allergenic than fish with more white muscle such as cod and haddock.Citation17 Based on amino acid sequences, the parvalbumin protein family can be classified into the less acidic alpha-subtype (nonallergenic) or the more acidic beta-subtype (allergenic).Citation18–Citation20 Fish contain mainly beta-subtype parvalbumins while the alpha-subtype is found in other vertebrates.Citation21
Table 1 Fish allergens with approved nomenclature by the World Health Organization and International Union of Immunological Societies (www.allergen.org)
In 2000, fish gelatin was discovered as a fish allergen, and in 2013, Kuehn et al reported the 50 kDa beta-enolase and 40 kDa fructose-bisphosphate aldolase A from cod, salmon, and tuna as important fish allergens.Citation22–Citation26 Specific IgE to enolases, adolases, and gelatin was detected in 62%, 50%, and 19.3% of fish-allergic subjects, respectively.Citation23 Fish gelatin was reported to trigger positive response in SPT in 10% of fish-allergic patients but oral food challenge with a cumulative dose of 3.61 g of gelatin did not trigger adverse reactions in any of the allergic subjects, thus raising the question if gelatin is a clinically relevant fish allergen.Citation27 Furthermore, it was reported that a minority of fish-allergic patients developed sIgE to other fish allergens such as aldehyde phosphate dehydrogenase from cod fish,Citation28,Citation29 triose-phosphate isomerase, and serum albumin from amago salmonCitation30 and creatine kinase from tuna.Citation31
Clinical cross-reactivity among various fish species is common even in fishes from taxonomically distinct orders.Citation32–Citation34 Parvalbumin from cod extract (Gad c 1) has been shown to cross-react with parvalbumin homologs from distantly related species such as wolffish or flounder.Citation14,Citation34–Citation37 Besides parvalbumin, cross-reactivity between fish muscle collagens from five fish species has also been reported.Citation25–Citation29 However, in some cases, codfish-allergic patients may ingest other fish without triggering allergic symptoms.Citation32,Citation35,Citation38 In addition to the cross-reactivity among fishes, clinical cross-reaction of parvalbumin between fish and other vertebrate meats has been described. Serologic cross-reactions have been described between fish and frog beta-parvalbumins.Citation39 Cross-reactivity among fish and chicken allergens including parvalbumins, enolases, and aldolases have been reported,Citation40 and described as “fish-chicken syndrome” phenomenon.
Shellfish allergens and cross-reactivity
Compared with that of fish allergens, the spectrum of shellfish allergens is more diverse ().Citation41,Citation42 Tropomyosin (TM) was identified as the major shrimp allergen in 1993.Citation43 TM is a protein of 38–41 kDa with coiled-coiled secondary structure and is highly conserved across invertebrates to regulate muscle contraction.Citation44 TM is a heat-stable allergen that can withstand high temperature and common food processing.Citation42 Usui et al examined the structural stability of shellfish TM and showed that the alpha-helical structure of TM collapsed easily upon heating to 80°C.Citation45 However, TM could regain its native circular dichroism pattern and retained its antigenicity after cooling to 25°C.Citation45 Furthermore, TM can be easily solubilized and can remain at high concentration even after thorough cooking, such as boiling and roasting.Citation46
Table 2 Officially recognized shellfish allergens with approved nomenclature by the World Health Organization and International Union of Immunological Societies (www.allergen.org)
Other shellfish allergens have also been well characterized. Arginine kinase (AK) was identified as a shrimp allergen with IgE reactivity that induced immediate skin manifestation in sensitized patients.Citation47 Although AK is abundant in shrimp muscle, unlike TM, AK is physiochemically and thermally unstable.Citation48,Citation49 Significant IgE reactivity to AK (Pen m 2) was reported in 27% of shrimp-allergic patients.Citation47 Myosin light chain (MLC) is a 20 kDa allergen displaying IgE reactivity in both raw and cooked shrimp extracts despite the alteration in its secondary structure under high temperature or acid treatment.Citation50,Citation51 Sarcoplasmic calcium-binding protein (SCP) is an allergen recognized by serum IgE in 38% of patients with shrimp allergy.Citation52 Similar to other allergenic components, SCP is highly conserved among crustaceans (alpha chain: 90%–94% identity, beta chain: 80% identity).Citation53 IgE reactivity to shrimp hemocyanin, troponin C, paramyosin, troponin I, triose phosphate isomerase, myosin heavy chain, alpha-actinin, smooth endoplasmic reticulum Ca2+ ATPase, and GADPH has been reported, but their clinical significance in food allergies is less understood.Citation54–Citation58 Although TM, AK, and SCP were well characterized as crab allergens, TM is the only allergen identified across multiple edible crustacean and mollusk species.Citation59,Citation60 There remains a clear need to compile a comprehensive shellfish allergen panel.
The major shellfish allergen, TM, has been suggested as a pan-allergen, whose cross-reactivity is likely because of the high homology in amino acid sequence (69%–100%) among crustaceans and mollusks.Citation61 Although crustacean and cephalopod TMs share only 63%–64% sequence identity, their cross-reactivity is probably due to their highly conserved IgE-binding epitopes.Citation62 Nevertheless, there are also reports on species-specific allergies to marine shrimp (Penaeus monodon) or fresh water shrimp (Macrobrachium rosenbergii) through oral challenge.Citation63 Apart from the cross-reactivity observed among edible shellfish, Leung et al also reported significant IgE reactivity of sera from shrimp-allergic subjects to grasshopper, cockroach, and fruit fly.Citation64 Cross-reactivity between shrimp and cockroach is also experimentally demonstrated in other studies.Citation65,Citation66 Reciprocally, subjects with house dust mite or cockroach allergy also showed substantial IgE reactivity to shrimp TM.Citation65–Citation67 The IgE cross-reactivity among TMs might be attributed to the recognition of similar epitopes within the eight IgE binding epitopes in shrimp TM (Pen a 1), of which five are identical to cockroach TM (Per a 7), and four are identical to lobster TM (Hom a 1) and dust mite TM (Der p 10 and Der f 10).Citation65 Besides TM, Gámez et al also reported that ubiquitin, alpha-actinin, and AK are responsible for mite-seafood cross-reactivity,Citation41 and a similar study by Pascal et al also suggested that AK and hemocyanin may be the markers of cross-reactivity between shellfish and other arthropods.Citation68
Cross-reactivity between fish and shellfish
To date, limited cases of cross-reactivity between fish and shellfish allergens have been reported,Citation69 with most of these studies suggesting TM as the possible cross-reactive allergen. In 2013, Liu et al demonstrated that sera from tilapia-allergic subjects (10/10) reacted to a 32 kDa protein that was later identified as TM.Citation69 Interestingly, there is 87.7% amino acid sequence homology between TM from tilapia and human but only 58.8% homology between the tilapia and northern shrimp (Pandalus borealis) TM. Further, this study also pointed out that antibodies against human TM isoform 5 could be present in patients with inflammatory bowel disease (IBD). It is intriguing that six out of ten of the tilapia-allergic subjects included in this study were diagnosed with IBD, bringing to question whether the detected reactivity was a consequence of allergy or autoimmunity.Citation22,Citation70,Citation71
On the other hand, Peixoto et al illustrated IgE reactivity among TMs from hake, codfish, shrimp, and Indian prawn in the serum of an 11-year-old boy in Spain by IgE immunoblotting and competitive-inhibition immunoblotting. As the specific IgE level to crustaceans (>100 kUA/L) was markedly higher than to fishes (0.02–2.77 kUA/L) accord ing to ImmunoCAP, the authors suggested shrimp TM as the primary sensitizer, while reaction with fish TM was a consequence of cross-reactivity in this subject. However, it was clearly reported in this study that this 11-year-old boy had previous experience of fish allergy that apparently occurred before the episode of shrimp anaphylaxis. It was also his first time consuming shrimp when he was admitted to hospital during an anaphylaxis.Citation69 Nevertheless, we cannot conclude yet whether cross-reactivity between fish and shellfish, and specifically among their TMs, exists based on these two studies.
Diagnosis of fish and shellfish allergies
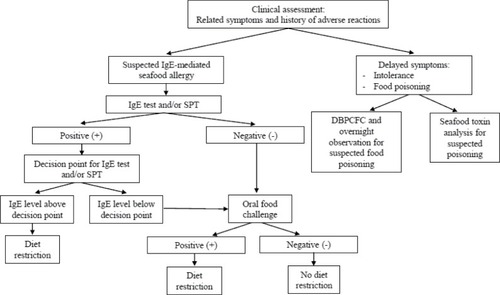
The current clinical approaches to seafood allergy diagnosis include clinical assessment, SPT, sIgE testing, and oral food challenge. A suggested algorithm is illustrated in . The emerging and promising strategy of CRD is also considered here.
Figure 1 Diagnostic algorithm and clinical management for seafood allergy.
Abbreviations: DBPCFC, double-blind placebo-controlled food challenge; IgE, immunoglobulin E; SPT, skin prick test.
Clinical assessment
Clinical assessment is the first step of allergy diagnosis. Clinical symptoms and medical history underpin the likelihood of seafood allergy. For accurate diagnosis, information is collected on allergic episodes, including the type and quantity of suspected seafood ingested, the time to onset of symptoms, whether previous exposure to suspected culprits elicited similar allergic responses, and when the last reaction to food occurred.Citation72 As shellfish is recognized as one of the major food groups to induce food-dependent exercise-induced anaphylaxis,Citation73 whether exercise was performed before an allergic episode should also be considered. Family history of food allergy is often considered during clinical assessment. However, the link between genetics and seafood allergy has not been thoroughly established, with only one report of an estimated heritability of 0.54 in a twin study on shellfish allergy.Citation74
One major drawback with clinical assessments is that patients often cannot provide precise and detailed medical histories and may fail to identify the suspected food that trigger their allergic symptoms.Citation75 It is also crucial that the history is assessed by an allergist capable of differentiating other disorders with similar clinical presentations that might be misconstrued as food allergy. Additional tests, such as SPT, sIgE levels, and/or results of oral food challenge, are usually interpreted in conjunction before reaching a diagnosis.
SPT
SPT is a common in vivo screening procedure for IgE-mediated food hypersensitivity by examining skin reactivity to food extracts. It is a reliable method for patients to rapidly determine their sensitization results and can be tested with uncommon allergens that are not available as commercial extracts. The cross-linking of specific IgE with allergens introduced into the skin triggers an immunologic milieu, leading to the release of various mediators including histamine, which is responsible for localized swelling around the prick area.
The procedure for SPT involves applying a drop of allergen test solutions to the forearm or back along with positive (histamine 1–10 mg/mL) and negative (50% glycerol saline) control drops. Modified methods may apply a lancet aid allergen penetration. Localized wheals are quantified by measuring the mean of the longest diameter and the length of the perpendicular line through its middle after 15–30 minutes of skin pricking. According to European standards, positive reactions are defined by wheal sizes from test drops that are more than 3 mm greater in diameter than those from the negative control.Citation76,Citation77 SPT requires normal healthy skin with good patient cooperation. Drugs that may interfere with skin reactivity (eg, antihistamine, phenothiazines, and antidepressants) must be avoided before SPT. However, the safety of SPT for seafood allergy has not been fully evaluated. Cases of SPT-induced anaphylaxis and fatality have been reported after application of fish, egg, shellfish nut, and peanut allergens.Citation78,Citation79 Although the risk associated with SPT is minimal, anaphylactic precautions must be in place.
Although SPT is a more sensitive test method for fish allergy than milk, egg, and peanut allergies,Citation80 commercially available fish and shellfish extracts are limited compared to the wide variety of dietary fish and shellfish. Prick-to-prick tests using fresh food could, in this regard, circumvent the obstacle.Citation81,Citation82 The reliability of SPT could be greatly ham pered by the method of SPT and measurement method, as well as the lack of allergen standardization and presence of preservatives in the extracts.Citation83–Citation86 A study by Asero et al on five commercial crustacean SPT extracts found that these commercial extracts displayed a dramatic loss of protein bands compared to fresh shrimp extract on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), which could cause heterogeneous SPT profiles. Attention should, thus, be drawn to the diagnostic sensitivity of SPT depending on the commercial extract used.
Recombinant allergens that can be standardized of its quantity and quality could be an alternative. For example, recombinant Pan b 1 was found to induce equivalent positive wheal sizes as natural Pan b 1 and commercial shrimp extract.Citation87 The drawback with these recombinant proteins, however, is that other potentially important allergens from natural extracts will be excluded. Recombinant allergens also do not address cross-reactivity when identifying a bona fide allergen. Future analyses evaluating the diagnostic utility of fish and other shellfish components in SPT are expected.
Specific IgE measurement
Serologic sIgE level is useful not only for diagnosis, but also for predicting the development of tolerance and persistence of seafood allergy, as well as monitoring allergy treatments.Citation88 Common clinically adopted sIgE measurement platforms include HYPEC-288 (Hycor-Agilent), Immunulite (Sie-mens), and the ImmunoCAP (Phadia).Citation89 In the ImmunoCAP system, 16 shellfish extracts and 28 fish extracts are readily available for routine sIgE quantification ( and ), but allergen components are scarce.
Table 3 Common crustacea and mollusca allergens included in the ImmunoCAP system (Phadia/Thermo Fisher Scientific) (http://www.phadia.com/en/Products/Allergy-testing-products/ImmunoCAP-Allergen-Information/Food-of-Animal-Origin/Shellfish/Shrimp/)
Table 4 Common fish allergens included in the ImmunoCAP system (Phadia/Thermo Fisher Scientific) (http://www.phadia.com/en/Products/Allergy-testing-products/ImmunoCAP-Allergen-Information/Food-of-Animal-Origin/Fish/Anchovy/)
It is generally believed that the levels of sIgE and the severity of allergic symptoms,Citation73 or the outcome of food challenge, are closely associated.Citation90 Mekaroonkamol et al also showed that measuring shrimp sIgE could be a useful screening test with high sensitivity (90%) and high positive predictive value (PPV; 0.86),Citation91 while individuals with serologic cod sIgE levels higher than 20kUA/L would give at least 95% certainty of positive food challenge results.Citation92,Citation93 However, the extensive IgE cross-reactivity among shrimp, cockroach, and dust mites could misrepresent the association between a positive shellfish SPT or positive sIgE results and clinical reactivity.Citation94 Children who were sensitized to cockroach through environmental exposure had higher levels of IgE to shrimp, but they did not manifest clinical symptoms of shrimp allergy, suggesting that such extract-based SPT and IgE tests indicate only IgE sensitization but not necessarily clinical allergy.Citation72,Citation94 Another study also reported that all shellfish-allergic individuals with positive SPT or IgE test results passed the food challenge,Citation95 which was consistent with previous reports.Citation96
Oral food challenge
Oral food challenge is by far the only diagnostic test reflecting clinical food allergy,Citation97 and positive food challenge usually correlates with strong SPT and IgE measurement results.Citation93 Currently, there are three types of oral food challenges: open food challenge (OFC), single-blind placebo-controlled food challenge, and double-blind placebo-controlled food challenge (DBPCFC).
In OFC, food is given in its ordinary form such that both the observer and patient recognize the tested food. The use of OFC is largely due to its convenience in clinical settings. Furthermore, OFC is also conducted when the load of food is too large to be effectively masked in a blinded challenge, for confirming a negative DBPCFC result and for children under 3 years of age.Citation98 The major flaw is obviously the high degree of bias by either the observer or the patient.
In single-blind placebo-controlled food challenge, only the patients are unaware of the kind of food administered, whereas the observer and patient are both unaware of the tested food in DBPCFC. For both types of challenges, the taste and smell of the suspected causal food are blinded by masking with other foods that are tolerated by the subjects (ie, active provocation). Placebos made with the same preparation without the allergic food are also included in both types of challenges to evaluate the allergic symptoms (both subjective and objective). The active and placebo provocations will be performed on separate days and both are given to subjects from low to increasing doses until the first incidence of observed reaction.Citation99 However, the methods for preparing blinded food for DBPCFC may vary among clinics, such as using chocolates for fish, and chocolate pudding or burgers for shrimp.Citation99
While DBPCFC represents the gold standard in food allergy diagnosis, the major drawbacks limiting its applications in clinical practice are that it is time-consuming, labor-intensive, and expensive.Citation100 Oral food challenges also pose the risk of severe anaphylactic shock,Citation101,Citation102 and thus should be optimized for safety by cautiously considering the subjects’ age, anticipated symptoms, SPT and sIgE levels, as well as the doses of the suspected allergic food used in the challenge and food contaminations before conducting a food challenge.Citation103 It is also noteworthy that the allergenicity of seafood may be altered after thermoprocessing.Citation104 For instance, some cooked shrimp and mollusk species extracts have higher allergenicity than raw preparations,Citation105,Citation106 whereas some other species were shown to induce higher IgE production when consumed raw.Citation107
Component-resolved diagnosis
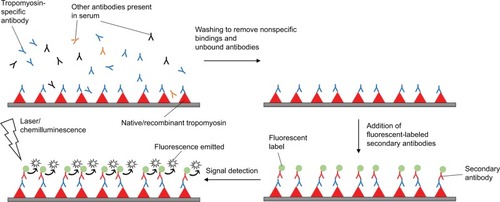
As discussed above, SPT and sIgE measurements only reflect IgE sensitization with suboptimal specificity, and do not predict the severity of allergic symptoms.Citation100 The high cost and safety issues of DBPCFC have limited its use in clinical practice. CRD has been developed recently as an emerging strategy to overcome the shortcomings of these traditional methods. CRD aims at measuring IgE antibodies to individual allergenic components in the form of proteins or peptidesCitation108 to provide more details on the sensitizing profile of patients.Citation109 The working principle of CRD is depicted in .
The utility of CRD has been well demonstrated in peanut allergy. False-positive SPT and sIgE results are common in peanut-tolerant subjectsCitation110 that could be in part due to nonspecific IgE reactivity to carbohydrate determinants and/or pollen allergens in the preparation.Citation111 It was found that subjects who failed food challenges inadvertently had higher sIgE to peanut allergens Ara h 1, 2, and 3, while positive sIgE to Ara h 8 was found in patients passing OFCs. Compared to peanut extracts that have a diagnostic specificity of only 17% and PPV of 0.67, Ara h 2 is a better diagnostic marker with a specificity of 92% and PPV of 0.94. By adding the level of Ara h 2-specific IgE as a second diagnostic step after detecting positive sIgE to peanut extract, 91.1% Ara h 2-positive subjects failed a food challenge while 78.6% of Ara h 2-negative subjects passed. This suggests that Ara h 2 is a more specific marker than peanut extract for identifying challenge-proven peanut allergy, and that sIgE level to Ara h 2 can be an indicator to reduce the need for OFC.Citation112
For fish and shellfish allergies, single-plex CRD can be performed on the ImmunoCAP system (Phadia/Thermo Fisher Scientific, Uppsala, Sweden) against fish parvalbumins rCyp c 1 and rGad c 1, as well as shrimp TM rPen a 1.Citation113–Citation115 ImmunoCAP allows quantification of sIgE level at standard unit (ie, kUA/L) but requires a larger amount of sera for analysis as each allergen component is tested individually. This might limit its application among pediatric subjects. Microarray-based ImmunoCAP ISAC system (Immuno-Solid Phase Allergen Chip; Phadia/Thermo Fisher Scientific) is also available, by which the allergen panel covers 112 inhalation and common food allergens that include cod (rGad c 1) and shrimp (nPen m 1, nPen m 2 and rPen m 4) allergens.Citation116,Citation117 Although the results between the ImmunoCAP and ISAC platforms are closely correlated, the sensitivity of ISAC is lower than that of ImmunoCAP.Citation118
Studies on the diagnostic utility of CRD in fish and shellfish allergies are lacking. Currently, in vitro assays of serologic IgE reactivity to recombinant fish parvalbumin are often used to assess for clinical cross-reactivity in fish allergy.Citation23,Citation119 A few studies, however, revealed that there was IgE binding to other allergens other than parvalbumin in patients. Monosensitivity to some fish species including swordfish and cod was observed in some patients. For example, Kelso et al found allergy specific to swordfish and not to the other nine commercial fish extracts tested.Citation120 Kuehn et al also demonstrated the correlation of fish enolases- and aldolases-specific IgE with clinical sensitivity in three cod-sensitized but parvalbumin-nonreactive patients.Citation121 Inclusion of the minor fish allergens such as enolases, aldolases, and perhaps gelatin into the testing panel will likely enhance the resolution of fish allergy diagnosis.
On the other hand, for shellfish allergy, it was reported that IgE reactivity to shrimp TM rPen a 1 was detected in 98% of shrimp-allergic patients,Citation122 and shrimp TM-specific IgE level can better predict clinical reactivity than SPT and IgE to shrimp extract at a specificity of 92.8% compared to 75% and 64.2% only, respectively.Citation123 A more comprehensive study by Pascal et al included the proteins and peptides of TM (Lit v 1), AK (Lit v 2), MLC (Lit v 3), SCP isoform alpha (Lit v 4), hemocyanin (HM), fatty-acid-binding protein, SCP isoform beta, and troponin C to study the utility of CRD for shrimp allergy diagnosis.Citation68 The sensitization profile suggested that apart from TM, which accounts for the majority of allergic symptoms after shellfish ingestion, SCP was also highly associated with allergic manifestations and MLC was a predictive marker of positive oral food challenge. AK and hemocyanin were, on the other hand, markers of cross-reactivity with a recognition frequency higher than 60% in house dust mite- and/or cockroach-allergic patients. Furthermore, the study by Asero et al reported that shrimp-allergic subjects with strong Pen m 1 hypersensitivity showed positive SPT with extracts depleted of TM and they frequently reacted to other minor allergens such as Pen m 2 and Pen m 4 on ISAC, as well as other high-molecular-weight shrimp allergens.Citation81 These two independent studies, thus, highlight the uncomprehensiveness of the current shellfish allergen panel, and perhaps fish allergy as well, which intelligibly challenge our development of CRD.
Future perspectives
Advances in the molecular and physiochemical characterization of shellfish and fish allergens have facilitated the development of CRD that can lead to more precise diagnoses and better clinical management of these allergies. However, there are still significant areas that need to be refined regarding the diagnosis of shellfish and fish allergies:
Although many shellfish and fish allergens are identified at the molecular level to date, few of these recombinant proteins are employed in diagnostic assays. Thus far, TM is the only allergen identified across different edible crustacean and mollusk species. The report by Asero et al, however, pointed out that TM may not be the only allergen for shellfish allergy diagnosis and emphasizes the values of other clinically important shellfish allergens. Identifying and characterizing seafood allergens, especially species-specific allergens, may advance the resolution of fish and shellfish allergy diagnosis.Citation81
Although CRD is an emerging method that could potentially reduce the need for oral food challenge and contribute to tailored treatment plans based on patients’ sensitization profiles, it is yet to be considered as a routine diagnostic method.Citation97 The diagnostic utility of CRD for fish and shellfish allergies is also yet to be thoroughly investigated.
Other emerging diagnostic tests are also worth investigating, including epitope binding, T-cell response, basophil activation assays, and atopy patch test. It is worth noting that sIgE measurements to extracts or allergen components indeed reflect the affinity between IgE and the allergens but not necessarily their ability to trigger subsequent degranulation in the effector cells. This issue could be addressed by using basophil activation test, which has been suggested to differentiate between subjects allergic to or tolerant to peanut, milk, and egg.Citation124–Citation126
Conclusion
With the increasing worldwide prevalence of seafood allergy, the precise diagnosis of this disorder is crucial for appropriate management strategies and unnecessary dietary avoidance. The current diagnostic methods in clinical practice for food allergy are often held back by the suboptimal specificity and safety and economic issues, especially for oral food challenges and allergen cross-reactivity. Incorporating CRD into the diagnostic workup might increase the resolution to the severity of allergic symptoms, resolve clinical cross-reactivity, and circumvent the need for oral food challenge. However, we should always appreciate that precise diagnosis should be achieved through a stepwise approach incorporating different tests to complement both sensitivity and specificity. We note that diagnosis of fish and shellfish allergy indeed becomes complicated by the extensive cross-reactivity among fish allergens and between allergens in edible shellfish and other arthropods. Large-scale studies evaluating the diagnostic accuracy and utility of the conventional tests and other emerging strategies for these allergies are also lacking. Advances in validation studies, together with the development of next-generation diagnostic strategies, are needed to improve the specificity of diagnostic workups for food allergies.
Acknowledgments
Christine YY Wai is currently funded as an AXA Postdoctoral Fellow (AXA Research Fund).
Disclosure
The authors report no conflicts of interest in this work.
References
- HillDAGrundmeierRWRamGSpergelJMThe epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort studyBMC Pediatr201616113327542726
- TaylorSLHefleSLBindslev-JensenCFactors affecting the determination of threshold doses for allergenic foods: how much is too much?J Allergy Clin Immunol20021091243011799361
- UntersmayrEVestergaardHMallingH-JIncomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergyJ Allergy Clin Immunol2007119371171717215033
- GuptaRSSpringstonEEWarrierMRThe prevalence, severity, and distribution of childhood food allergy in the United StatesPediatrics20111281e9e1721690110
- RonaRJKeilTSummersCThe prevalence of food allergy: a meta-analysisJ Allergy Clin Immunol2007120363864617628647
- SichererSHMuñoz-FurlongASampsonHAPrevalence of seafood allergy in the United States determined by a random telephone surveyJ Allergy Clin Immunol2004114115916515241360
- ConnettGJGerezICabrera-MoralesEAA population-based study of fish allergy in the Philippines, Singapore and ThailandInt Arch Allergy Immunol2012159438439022846665
- ChiangWCKidonMILiewWKGohATangJPLChayOMThe changing face of food hypersensitivity in an Asian communityClin Exp Allergy20073771055106117581199
- SmitDVCameronPARainerTHAnaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactionsJ Emerg Med200528438138815837017
- HsinYCHsinYCHuangJLYehKWClinical features of adult and pediatric anaphylaxis in TaiwanAsian Pacific J Allergy Immunol2011294307312
- KatzYGoldbergMRRajuanNCohenALeshnoMThe prevalence and natural course of food protein–induced enterocolitis syndrome to cow’s milk: a large-scale, prospective population-based studyJ Allergy Clin Immunol2011127364765321377033
- Food and Agriculture Organization of the United NationsFAO Fisheries and Aquaculture Department summary tables of fishery statistics Available from: http://www.fao.org/fishery/docs/STAT/summary/default.htmAccessed January 31, 2018
- AasKElsayedSMCharacterization of a major allergen (cod). Effect of enzymic hydrolysis on the allergenic activityJ Allergy19694463333434983319
- Bugajska-SchretterAElfmanLFuchsTParvalbumin, a cross-reactive fish allergen, contains IgE-binding epitopes sensitive to periodate treatment and Ca2+ depletionJ Allergy Clin Immunol1998101167749449503
- GriesmeierUBublinMRadauerCPhysicochemical properties and thermal stability of Lep w 1, the major allergen of whiffMol Nutr Food Res2009546861869
- ElsayedSAasKCharacterization of a major allergen (cod). Observations on effect of denaturation on the allergenic activityJ Allergy19714752832915280452
- KobayashiATanakaHHamadaYIshizakiSNagashimaYShiomiKComparison of allergenicity and allergens between fish white and dark musclesAllergy200661335736316436146
- GoodmanMPechéreJ-FThe evolution of muscular parvalbumins investigated by the maximum parsimony methodJ Mol Evol197792131158864720
- GoodmanMPechéreJ-FHaiechJDemailleJGEvolutionary diversification of structure and function in the family of intracellular calcium-binding proteinsJ Mol Evol1979134331352390164
- RadauerCBublinMWagnerSMariABreitenederHAllergens are distributed into few protein families and possess a restricted number of biochemical functionsJ Allergy Clin Immunol2008121484785218395549
- KuehnASwobodaIArumugamKHilgerCHentgesFranã§oisHentgesFFish allergens at a glance: variable allergenicity of parvalbumins, the major fish allergensFront Immunol2014517924795722
- LiuRHolckALYangELiuCXueWTropomyosin from tilapia (Oreochromis mossambicus) as an allergenClin Exp Allergy201343336537723414545
- KuehnAHilgerCLehners-WeberCIdentification of enolases and aldolases as important fish allergens in cod, salmon and tuna: component resolved diagnosis using parvalbumin and the new allergensClin Exp Allergy201343781182223786287
- TommJMvan DoTJendeCSimonJCTreudlerRvon BergenMAverbeckMIdentification of new potential allergens from Nile perch (Lates niloticus) and cod (Gadus morhua)J Investig Allergol Clin Immunol2013233159167
- SakaguchiMTodaMEbiharaTIgE antibody to fish gelatin (type I collagen) in patients with fish allergyJ Allergy Clin Immunol2000106357958410984381
- HamadaYNagashimaYShiomiKIdentification of collagen as a new fish allergenBiosci Biotechnol Biochem200165228529111302160
- HansenTKPoulsenLKStahl SkovPA randomized, double-blinded, placebo-controlled oral challenge study to evaluate the aller-genicity of commercial, food-grade fish gelatinFood Chem Toxicol200442122037204415500940
- das DoresSChopinCRomanoAIgE-binding and cross-reactivity of a new 41 kDa allergen of codfishAllergy200257s72848712144562
- HamadaYNagashimaYShiomiKReactivity of IgE in fish-allergic patients to fish muscle collagenAllergol Int2003523139147
- NakamuraRSatohRNakajimaYComparative study of GH-transgenic and non-transgenic amago salmon (Oncorhynchus masou ishikawae) allergenicity and proteomic analysis of amago salmon allergensRegul Toxicol Pharmacol200955330030819679156
- RosmilahMShahnazMMeinirJMasitaANoormalinAJamaluddinMIdentification of parvalbumin and two new thermolabile major allergens of Thunnus tonggol using a proteomics approachInt Arch Allergy Immunol2013162429930924193115
- de MartinoMNovembreEGalliLAllergy to different fish species in cod-allergic children: in vivo and in vitro studiesJ Allergy Clin Immunol19908669099142262645
- StenEHansenTKStahl SkovPCross-reactivity to eel, eelpout and ocean pout in codfish-allergic patientsAllergy200459111173118015461598
- VandoTElsayedSFlorvaagEHordvikIEndresenCAllergy to fish parvalbumins: studies on the cross-reactivity of allergens from 9 commonly consumed fishJ Allergy Clin Immunol200511661314132016337465
- Bernhisel-BroadbentJScanlonSMSampsonHAFish hypersensitivity. I. In vitro and oral challenge results in fish-allergic patientsJ Allergy Clin Immunol19928937307371545094
- HansenTKBindslev-JensenCSkovPSPoulsenLKCodfish allergy in adults: IgE cross-reactivity among fish speciesAnn Allergy Asthma Immunol19977821871949048527
- Dl-CLNeoKHFcYParvalbumin – the major tropical fish allergenPediatr Allergy Immunol200819539940718221468
- HelblingAHaydelRMccantsMLMusmandJJEl-DahrJLehrerSBFish allergy: is cross-reactivity among fish species relevant? Double-blind placebo-controlled food challenge studies of fish allergic adultsAnn Allergy Asthma Immunol199983651752310619342
- HilgerCThillLGrigioniFIgE antibodies of fish allergic patients cross-react with frog parvalbuminAllergy200459665366015147451
- KuehnACodreanu-MorelFLehners-WeberCCross-reactivity to fish and chicken meat - a new clinical syndromeAllergy201671121772178127344988
- GámezCZafraMPBoqueteMNew shrimp IgE-binding proteins involved in mite-seafood cross-reactivityMol Nutr Food Res20145891915192524978201
- PedrosaMBoyano-MartínezTGarcía-AraCQuirceSShellfish allergy: a comprehensive reviewClin Rev Allergy Immunol201549220321624870065
- ShantiKNMartinBMNagpalSMetcalfeDDRaoPVIdentification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopesJ Immunol199315110535453637693809
- Yong JeongKHongC-SYongT-SAllergenic tropomyosins and their cross-reactivitiesProtein Pept Lett200613883584517073731
- UsuiMHaradaAIshimaruTContribution of structural reversibility to the heat stability of the tropomyosin shrimp allergenBiosci Biotechnol Biochem201377594895323649255
- UsuiMHaradaAYasumotoSRelationship between the risk for a shrimp allergy and freshness or cookingBiosci Biotechnol Biochem201579101698170125966963
- CjYLinYFChiangBLChowLPProteomics and immunological analysis of a novel shrimp allergen, Pen m 2J Immunol2003170144545312496430
- HlYRuanWWCaoMJCaiQFShenHWLiuGMIdentification of physicochemical properties of Scylla paramamosain allergen, arginin kinaseJ Sci Food Agric201393224525322674459
- RosmilahMShahnazMZailatulHMNoormalinANormilahIIdentification of tropomyosin and arginine kinase as major allergens of Portunus pelagicus (blue swimming crab)Trop Biomed201229346747823018510
- AyusoRGrishinaGBardinaLMyosin light chain is a novel shrimp allergen, Lit v 3J Allergy Clin Immunol2008122479580218760458
- ZhangY-XChenH-LMalekiSJCaoMJZhangLJSuWJLiuGMPurification, characterization, and analysis of the allergenic properties of myosin light chain in Procambarus clarkiiJ Agric Food Chem201563276271628226083097
- AyusoRGrishinaGIbáñezMDSarcoplasmic calcium-binding protein is an EF-hand–type protein identified as a new shrimp allergenJ Allergy Clin Immunol2009124111412019523674
- MitaHKoketsuAIshizakiSShiomiKMolecular cloning and functional expression of allergenic sarcoplasmic calcium-binding proteins from Penaeus shrimpsJ Sci Food Agric20139371737174223180551
- Abdel RahmanAMKamathSDLopataALRobinsonJJHelleurRJBiomolecular characterization of allergenic proteins in snow crab (Chionoecetes opilio) and de novo sequencing of the second allergen arginine kinase using tandem mass spectrometryJ Proteomics201174223124121059421
- BauermeisterKWangorschAGaroffoLPGeneration of a comprehensive panel of crustacean allergens from the North Sea Shrimp Crangon crangonMol Immunol20114815–161983199221784530
- PiboonpocanunSJirapongsananurukOTipayanonTBoonchooSGoodmanREIdentification of hemocyanin as a novel non-cross-reactive allergen from the giant freshwater shrimp Macrobrachium rosenbergiiMol Nutr Food Res201155101492149821656669
- Abdel RahmanAMKamathSDGagnéSLopataALHelleurRComprehensive proteomics approach in characterizing and quantifying allergenic proteins from Northern shrimp: toward better occupational asthma preventionJ Proteome Res201312264765623268739
- SuzukiMKobayashiYHirakiYNakataHShiomiKParamyosin of the disc abalone Haliotis discus discus: identification as a new allergen and cross-reactivity with tropomyosinFood Chem20111243921926
- ChuKHWongSHLeungPSCTropomyosin is the major mollusk allergen: reverse transcriptase polymerase chain reaction, expression and IgE reactivityMar Biotechnol20002549950911246417
- LeungPSCChenY-ChenGershwinMEWongSHKwanHSChuKHIdentification and molecular characterization of Charybdis feriatus tropomyosin, the major crab allergenJ Allergy Clin Immunol199810258478529819304
- FaberMAPascalMEl KharbouchiOShellfish allergens: tropomyosin and beyondAllergy201772684284828027402
- MotoyamaKIshizakiSNagashimaYShiomiKCephalopod tropomyosins: identification as major allergens and molecular cloningFood Chem Toxicol200644121997200216904802
- JirapongsananurukOSripramongCPacharnPSpecific allergy to Penaeus monodon (seawater shrimp) or Macrobrachium rosenbergii (freshwater shrimp) in shrimp-allergic childrenClin Exp Allergy20083861038104718498545
- LeungPSCChowWKDuffeySKwanHSGershwinMEChuKHIgE reactivity against a cross-reactive allergen in crustacea and mollusca: evidence for tropomyosin as the common allergenJ Allergy Clin Immunol19969859549618939159
- AyusoRReeseGLeong-KeeSPlanteMLehrerSBMolecular basis of arthropod cross-reactivity: IgE-binding cross-reactive epitopes of shrimp, house dust mite and cockroach tropomyosinsInt Arch Allergy Immunol20021291384812372997
- YangZZhaoJWeiNCockroach is a major cross-reactive allergen source in shrimp-sensitized rural children in southern ChinaAllergy201873358559229072879
- FernandesJReshefAPattonLAyusoRReeseGLehrerSBImmunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox JewsClin Exp Allergy200333795696112859453
- PascalMGrishinaGYangACMolecular diagnosis of shrimp allergy: efficiency of several allergens to predict clinical reactivityJ Allergy Clin Immunol201534521529
- PeixotoSMonteiroTCarvalhoMVertebrate tropomyosin as an allergenJ Investig Allergol Clin Immunol20182815153
- González-FernándezJVeleiroBDaschnerACuéllarCAre fish tropomyosins allergens?Ann Allergy, Asthma Immunol20161161747626507710
- González-FernándezJDaschnerACuéllarCAllergenicity of vertebrate tropomyosins: challenging an immunological dogmaAllergol Immunopathol2017453297304
- SampsonHAAllergyFPart 2: diagnosis and managementJ Allergy Clin Immunol1999103698198910359874
- AbramsEMSichererSHDiagnosis and management of food allergyCan Med Assoc J2016188151087109327601605
- LiuXZhangSTsaiH-JGenetic and environmental contributions to allergen sensitization in a Chinese twin studyClin Exp Allergy200939799199819302247
- TsabouriSTrigaMMakrisMKalogeromitrosDChurchMKPriftisKNFish and shellfish allergy in children: review of a persistent food allergyPediatr Allergy Immunol201223760861522554093
- HeinzerlingLMariABergmannK-CThe skin prick test – European standardsClin Transl Allergy201331323369181
- PariyaprasertWPiboonpocanunSJirapongsananurukOVisitsunthornNStability and potency of raw and boiled shrimp extracts for skin prick testAsian Pacific J Allergy Immunol2015332136142
- BernsteinDIWannerMBorishLLissGMImmunotherapy Committee, American Academy of Allergy, Asthma and ImmunologyTwelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001J Allergy Clin Immunol200411361129113615208595
- NovembreEBernardiniRBertiniGMassaiGVierucciASkin-prick-test-induced anaphylaxisAllergy19955065115137573845
- KianifarHRPourrezaAJabbari AzadFYousefzadehHMasomiFSensitivity comparison of the skin prick test and serum and fecal radio allergosorbent test (RAST) in diagnosis of food allergy in childrenRep Biochem Mol Biol2016429810327536703
- AseroRScalaEVillaltaDShrimp allergy: analysis of commercially available extracts for in vivo diagnosisJ Investig Allergol Clin Immunol2017273175182
- KuehnAScheuermannTHilgerCHentgesFImportant variations in parvalbumin content in common fish species: a factor possibly contributing to variable allergenicityInt Arch Allergy Immunol2010153435936620559001
- RancéFJuchetABrémontFDutauGCorrelations between skin prick tests using commercial extracts and fresh foods, specific IgE, and food challengesAllergy Eur J Allergy Clin Immunol1997521010311035
- ZawodniakAKupczykMGórskiPKunaPComparison of standard and modified SPT methodAllergy Eur J Allergy Clin Immunol2003583257259
- MorellFCodinaRRodrigoMJIncreased positivity of skin test and allergenic stability of glycerinated soybean hull extractsClin Exp Allergy199929338839310202348
- EigenmannPASampsonHAInterpreting skin prick tests in the evaluation of food allergy in childrenPediatr Allergy Immunol1998941861919920216
- MyrsetHRBarlettaBdi FeliceGEgaasEDooperMMBWStructural and immunological characterization of recombinant Pan b 1, a major allergen of Northern shrimp, Pandalus borealisInt Arch Allergy Immunol2013160322123223075760
- PetersRGurrinLDharmageSKoplinJAllenKThe natural history of IgE-mediated food allergy: can skin prick tests and serum-specific IgE predict the resolution of food allergy?Int J Environ Res Public Health201310105039506124132133
- CoxLOverview of serological-specific IgE antibody testing in childrenCurr Allergy Asthma Rep201111644745321947715
- Celik-BilgiliSMehlAVerstegeAThe predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challengesClin Exp Allergy200535326827315784102
- MekaroonkamolJChatchateePNgamphaiboonJLertchanaruengrithPSuratannonNPredicting shrimp allergy using skin prick test, specific IgE to shrimp and Rpen a1 in area with high prevalence of house dust mite sensitizationJ Allergy Clin Immunol20151352AB248
- SampsonHAUtility of food-specific IgE concentrations in predicting symptomatic food allergyJ Allergy Clin Immunol2001107589189611344358
- PerryTTMatsuiECKay Conover-WalkerMWoodRAThe relationship of allergen-specific IgE levels and oral food challenge outcomeJ Allergy Clin Immunol2004114114414915241358
- WangJCalatroniAVisnessCMSampsonHACorrelation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city populationJ Allergy Clin Immunol2011128483483721872304
- FleischerDMBockSASpearsGCOral food challenges in children with a diagnosis of food allergyJ Pediatr2011158457858321030035
- SampsonHAMccaskillCCFood hypersensitivity and atopic dermatitis: evaluation of 113 patientsJ Pediatr198510756696754056964
- BoyceJAAssa’adABurksAWGuidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel reportJ Allergy Clin Immunol201012661105111821134568
- Nowak-WęgrzynAAssa’adAHBahnaSLBockSASichererSHTeuberSSWork Group report: oral food challenge testingJ Allergy Clin Immunol20091236SupplS365S38319500710
- Fernández-RivasMBarrealesLMackieARThe EuroPrevall outpatient clinic study on food allergy: background and methodologyAllergy Eur J Allergy Clin Immunol2015705576584
- KimJFNwaruBIMcclearyNStoddartASheikhAInvestigating the accuracy, risk impact, and cost-effectiveness of component-resolved diagnostic test for food allergy: a systematic review protocolNPJ Prim Care Respir Med20172711228077854
- CianferoniAKhullarKSaltzmanROral food challenge to wheat: a near-fatal anaphylaxis and review of 93 food challenges in childrenWorld Allergy Organ J2013611423965733
- SampsonHAMendelsonLRosenJPFatal and near-fatal anaphylactic reactions to food in children and adolescentsN Engl J Med199232763803841294076
- BahnaSLFood challenge procedure: optimal choices for clinical practiceAllergy Asthma Proc200728664064618201427
- ThomasKHerouet-GuicheneyCLadicsGEvaluating the effect of food processing on the potential human allergenicity of novel proteins: international workshop reportFood Chem Toxicol20074571116112217395354
- CarnésJFerrerÁngelHuertas ÁngelJAndreuCLarramendiCHFernández-CaldasEThe use of raw or boiled crustacean extracts for the diagnosis of seafood allergic individualsAnn Allergy Asthma Immunol200798434935417458431
- LopataALZinnCPotterPCCharacteristics of hypersensitivity reactions and identification of a unique 49 kd IgE-binding protein (Hal-m-1) in abalone (Haliotis midae)J Allergy Clin Immunol199710056426489389294
- YadzirZHMisnanRBakhtiarFAbdullahNMuradSTropomyosin, the major tropical oyster Crassostrea belcheri allergen and effect of cooking on its allergenicityAllergy Asthma Clin Immunol20151113026504467
- WolthersODComponent-resolved diagnosis in pediatricsISRN Pediatr2012201280692022919510
- IncorvaiaCRapettiAAlianiMFood allergy as defined by component resolved diagnosisRecent Pat Inflamm Allergy Drug Discov201481597324483212
- BeyerKEllman-GruntherLJärvinenKMWoodRAHourihaneJSampsonHAMeasurement of peptide-specific IgE as an additional tool in identifying patients with clinical reactivity to peanutsJ Allergy Clin Immunol2003112120220712847500
- NicolaouNPoorafsharMMurrayCAllergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnosticsJ Allergy Clin Immunol2010125119119720109746
- WangJSampsonHAFood allergyJ Clin Invest2011121382783521364287
- Thermo Fisher ScientificAllergen component: rCyp c 1carp Available from: http://www.phadia.com/en/Products/Allergy-testing-products/ImmunoCAP-Allergen-Information/Food-of-Animal-Origin/Allergen-Components/rCyp-c-1-Carp/Accessed May 22, 2018
- Thermo Fisher ScientificAllergen component: rGad c 1cod Available from: http://www.phadia.com/en/Products/Allergy-testing-products/ImmunoCAP-Allergen-Information/Food-of-Animal-Origin/Allergen-Components/rGad-c-1-Cod/Accessed May 22, 2018
- Thermo Fisher ScientificAllergen component: rPen a 1tropomyosin, shrimp Available from: http://www.phadia.com/en/Products/Allergy-testing-products/ImmunoCAP-Allergen-Information/Food-of-Animal-Origin/Allergen-Components/rPen-a-1-Tropomyosin-Shrimp/Accessed May 22, 2018
- LopataALKleine-TebbeJKamathSDAllergens and molecular diagnostics of shellfish allergy: Part 22 of the Series Molecular AllergologyAllergo J Int201625721021828239537
- JakobTForstenlechnerPMatricardiPKleine-TebbeJMolecular allergy diagnostics using multiplex assays: methodological and practical considerations for use in research and clinical routine: Part 21 of the Series Molecular AllergologyAllergo J Int20152432033227069843
- Huss-MarpJGutermuthJSchäffnerIComparison of molecular and extract-based allergy diagnostics with multiplex and singleplex analysisAllergo J Int201524465326709369
- SwobodaIBugajska-SchretterAVerdinoPRecombinant carp parvalbumin, the major cross-reactive fish allergen: a tool for diagnosis and therapy of fish allergyJ Immunol200216894576458411971005
- KelsoJMJonesRTYungingerJWMonospecific allergy to swordfishAnn Allergy Asthma Immunol19967732272288814049
- KuehnAFischerJHilgerCSparlaCBiedermannTHentgesFCorrelation of clinical monosensitivity to cod with specific IgE to enolase and aldolaseAnn Allergy Asthma Immunol2014113667067125304340
- GámezCSánchez-GarcíaSIbáñezMDTropomyosin IgE-positive results are a good predictor of shrimp allergyAllergy Eur J Allergy Clin Immunol2011661013751383
- YangACArrudaLKSantosABMeasurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestionJ Allergy Clin Immunol2010125487287820226506
- WanichNNowak-WegrzynASampsonHAShrefflerWGAllergen-specific basophil suppression associated with clinical tolerance in patients with milk allergyJ Allergy Clin Immunol2009123478979419348919
- ValdimarssonVSigurdardottirSSkaftadottirIClausenMLudvigssonBBasophil activation test for diagnosis of food allergyJ Allergy Clin Immunol20081212S250
- OcmantAMulierSHanssensLBasophil activation tests for the diagnosis of food allergy in childrenClin Exp Allergy20093981234124519549026
- SwobodaIBalicNKlugCA general strategy for the generation of hypoallergenic molecules for the immunotherapy of fish allergyJ Allergy Clin Immunol2013132497998123763969
- Bugajska-SchretterAGroteMVangelistaLPurification, biochemical, and immunological characterisation of a major food allergen: different immunoglobulin E recognition of the apo- and calcium-bound forms of carp parvalbuminGut200046566166910764710
- van doTHordvikIEndresenCElsayedSThe major allergen (parvalbumin) of codfish is encoded by at least two isotypic genes: cDNA cloning, expression and antibody binding of the recombinant allergensMol Immunol2003391059560212431393
- SharpMFKamathSDKoeberlMDifferential IgE binding to isoallergens from Asian seabass (Lates calcarifer) in children and adultsMol Immunol2014621778524973736
- Mäkinen-KiljunenSKiistalaRVarjonenESevere reactions from roe without concomitant fish allergyAnn Allergy Asthma Immunol200391441341614582823
- RuethersTRaithMSharpMFCharacterization of Ras k 1 a novel major allergen in Indian mackerel and identification of parvalbumin as the major fish allergen in 33 Asia-Pacific fish speciesClin Exp Allergy201848445246329193486
- LindstrømCDvan DôTHordvikIEndresenCElsayedSCloning of two distinct cDNAs encoding parvalbumin, the major allergen of Atlantic salmon (Salmo salar)Scand J Immunol19964443353448845026
- BealeJEJeebhayMFLopataALCharacterisation of purified parvalbumin from five fish species and nucleotide sequencing of this major allergen from Pacific pilchard, Sardinops sagaxMol Immunol200946152985299319616851
- GajewskiKGHsiehYHMonoclonal antibody specific to a major fish allergen: parvalbuminJ Food Prot200972481882519435232
- GriesmeierUVázquez-CortésSBublinMExpression levels of parvalbumins determine allergenicity of fish speciesAllergy Eur J Allergy Clin Immunol2010652191198
- KondoYKakamiMKoyamaHIgE cross-reactivity between fish roe (Salmon, Herring and Pollock) and chicken egg in patients anaphylactic to Salmon RoeAllergol Int2005542317323
- LeungPSChenYCMyklesDLChowWKLiCPChuKHMolecular identification of the lobster muscle protein tropomyosin as a seafood allergenMol Mar Biol Biotechnol19987112209597774
- LiuGMChengHNesbitJBSuWJCaoMJMalekiSJEffects of boiling on the IgE-binding properties of tropomyosin of shrimp (Litopenaeus vannamei)J Food Sci2010751T1T520492208
- KumjimSJirapongsananurukOPiboonpocanunSCloning and characterization of recombinant tropomyosin of giant freshwater shrimp M. rosenbergii to determine major allergens causing allergic reactions among shrimp-allergic childrenAsian Pac J Allergy Immunol201634322923527001653
- KoeberlMKamathSDSaptarshiSRAuto-induction for high yield expression of recombinant novel isoallergen tropomyosin from King prawn (Melicertus latisulcatus) for improved diagnostics and immunotherapeuticsJ Immunol Methods201441561625450004
- LeungPSChuKHChowWKCloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergenJ Allergy Clin Immunol19949458828907963157
- DaulCBSlatteryMReeseGLehrerSBIdentification of the major brown shrimp (Penaeus aztecus) allergen as the muscle protein tropomyosinInt Arch Allergy Immunol1994105149557916224
- Abdel RahmanAMRahmanAMKamathSLopataALHelleurRJAnalysis of the allergenic proteins in black tiger prawn (Penaeus monodon) and characterization of the major allergen tropomyosin using mass spectrometryRapid Commun Mass Spectrom201024162462247020658686
- García-OrozcoKDAispuro-HernándezEYepiz-PlascenciaGCalderón-de-La-BarcaAMSotelo-MundoRRMolecular characterization of arginine kinase, an allergen from the shrimp Litopenaeus vannameiInt Arch Allergy Immunol20071441232817496423
- MyklesDLHeterogeneity of myofibrillar proteins in lobster fast and slow muscles: variants of troponin, paramyosin, and myosin light chains comprise four distinct protein assemblagesJ Exp Zool1985234123323157773
- ShiomiKSatoYHamamotoSMitaHShimakuraKSarcoplasmic calcium-binding protein: identification as a new allergen of the black tiger shrimp Penaeus monodonInt Arch Allergy Immunol20081462919818204275
- KobayashiTTakagiTKonishiKCoxJAAmino acid sequence of crayfish troponin IJ Biol Chem19892643155115572912973
- ZhuLSheTZhangYIdentification and characterization of ovary development-related protein EJO1 (Eri s 2) from the ovary of Eriocheir sinensis as a new food allergenMol Nutr Food Res201660102275228727240706
- NiggemannBBeyerKDiagnosis of food allergy in children: toward a standardization of food challengeJ Pediatr Gastroenterol Nutr200745439940418030203
- LopataALAndreasLKamathSShellfish allergy diagnosis - gaps and needsCurr Allergy Clin Immunol20122526066
- Thermo Fisher ScientificAllergen testing products: fish Available from: http://www.phadia.com/en/Products/Allergy-testing-products/ImmunoCAP-Allergen-Information/Food-of-Animal-Origin/Fish/Anchovy/Accessed May 22, 2018
- LeungPSCChuKHcDNA cloning and molecular identification of the major oyster allergen from the Pacific oyster Crassostrea gigasClinical Experimental Allergy2001311287129411529900
- AsturiasJAErasoEArillaMCGómez-BayónNInácioFMartínezACloning, Isolation, and IgE-Binding Properties of Helix aspersa (Brown Garden Snail) TropomyosinInternational Archives of Allergy and Immunology2002128909612065908
- RollandJMVareseNPAbramovitchJBAnaniaJNugrahaRKamathSEffect of Heat Processing on IgE Reactivity and Cross- Reactivity of Tropomyosin and Other Allergens of Asia-Pacific Mollusc Species: Identification of Novel Sydney Rock Oyster Tropomyosin Sac g 1Molecular Nutrition & Food Research2018621800148
- MiyazawaHFukamachiHInagakiYReeseGDaulCBLehrerSBIdentification of the first major allergen of a squid (Todarodes pacificus)Journal of Allergy and Clinical Immunology1996989489538939158
- ChenH-LMaoH-YCaoM-JCaiQ-FSuW-JZhangY-XPurification, physicochemical and immunological characterization of arginine kinase, an allergen of crayfish (Procambarus clarkii)Food and Chemical Toxicology20136247548424055770
- ShenYCaoM-JCaiQ-FSuW-JYuH-LRuanW-WPurification, cloning, expression and immunological analysis of Scylla serrata arginine kinase, the crab allergenJournal of the Science of Food and Agriculture2011911326133521432856
- HuM-JLiuG-YYangYPanT-MLiuY-XSunL-CCloning, Expression, and the Effects of Processing on Sarcoplasmic-Calcium-Binding Protein: An Important Allergen in Mud CrabJournal of Agricultural and Food Chemistry2017656247625728692255
- KalyanasundaramASantiagoTCIdentification and characterization of new allergen troponin C (Pen m 6.0101) from Indian black tiger shrimp Penaeus monodonEuropean Food Research and Technology2014240509515
- YangYZhangY-XLiuMMalekiSJZhangM-LLiuQ-MTriosephosphate Isomerase and Filamin C Share Common Epitopes as Novel Allergens of Procambarus clarkiiJournal of Agricultural and Food Chemistry20176595096328072528