Abstract
Purpose
Allergic rhinitis (AR) affects ~20% of the population worldwide. The objectives of this study were to evaluate the safety and efficacy of iodixanol nasal solution (Nasapaque) for AR treatment, using the Allergen BioCube® (ABC®), an environmental exposure unit. Iodixanol is a commonly used contrast media agent that shows efficacy on the signs and symptoms of AR.
Patients and methods
Seventy-three adult subjects with AR were randomized to iodixanol or placebo treatment in a double-masked efficacy and safety study conducted outside of ragweed pollen season. In-office treatment was administered after BioCube® ragweed pollen exposure, and again 8 days later prior to ragweed exposure. Nasal and ocular efficacy and safety assessments were conducted before and after treatment.
Results
Iodixanol treatment resulted in statistically significantly lower total nasal symptom scores as compared to placebo at several time points post-treatment and ABC exposure. Individual nasal and ocular symptoms, notably nasal itching and ocular itching, showed evidence of lower scores in the iodixanol group. Peak nasal inspiratory flow (PNIF) improved (9%–16%) with iodixanol from baseline as compared to PNIF in the placebo group which ranged from 3% worsening to improvement of 2%. Few (9) adverse events occurred.
Conclusion
Iodixanol nasal solution demonstrated efficacy for relief of several nasal and ocular allergic rhinoconjunctivitis signs and symptoms, and was safe and well tolerated in this early Phase II exploratory trial. Further studies with iodixanol are warranted. Allergy challenge models such as the ABC provide valuable assessments of allergen exposures and drug efficacies.
Study Identification Number
NCT02377895
Introduction
Allergic rhinitis (AR) affects about 10%–30% of Americans and up to 40% of the global population.Citation1–Citation4 Although formerly regarded as a nuisance disease, AR has a considerable effect on quality of life and can have significant consequences if left untreated.
An immunologic response modulated by IgE, AR is triggered by indoor and/or outdoor allergens such as dust mites, molds, pet dander, and ragweed, grass, or tree pollens. The AR response proceeds through both an early/acute phase and a late phase response. The acute phase, developing within minutes of exposure, is activated when an allergen crosslinks with an IgE antibody bound to mucosal mast cells, triggering a release of inflammatory mediators (histamine, prostaglandins, and leukotrienes). This results in a range of AR symptoms including sneezing, nasal pruritus, congestion, rhinorrhea, and itchy, watery eyes. The late phase response initiates 6–12 hours post-exposure, peaking at 12–24 hours. During the late phase response, inflammatory mediators attract, recruit, and activate additional inflammatory cells into the nasal mucosa, leading to the release of more inflammatory mediators.
Current AR therapies include H1 antihistamines, intra-nasal corticosteroids, nasal decongestants, and oral decongestants. Here we present a study investigating iodixanol nasal solution as a potential treatment for AR. Iodixanol is an iodine-containing nonionic radio contrast agent typically used in coronary angiography. Concentrated contrast media has been shown to supress mast cell activation and is proposed to act through direct interaction with IgE to diminish IgE receptor activation.Citation5,Citation6
Clinical studies of AR traditionally have been conducted during the pollen season under natural environment conditions, often using field studies in which subjects are exposed to allergens in an outdoor setting. These studies have important limitations, including variability in allergen concentration and types, and are weather and season dependent. Environmental exposure units (EEUs), first developed in the 1980s, allow for allergen specificity, control over antigen exposure levels, temperature, and air quality, and provide an accurate way of tracking self-reported and physician-observed symptoms for determining response to medication. The Allergen BioCube® (ABC®) is an EEU that has been technically and clinically validated for both Timothy-grassCitation7 and ragweedCitation8 exposures.
Following preclinical and pilot clinical studies, a clinical trial was conducted with iodixanol nasal solution (Nasapaque, 3E Therapeutics Corporation, La Jolla, CA, USA) in the ABC (Ora, Inc., Andover, MA, USA) to expose allergic subjects to ragweed pollen and evaluate treatment efficacy and safety compared to placebo.
Patients and methods
Study design
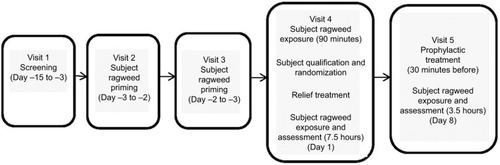
This was a single-center, randomized, double-blind, parallel-group, placebo-controlled, Phase I/II efficacy and safety study and was approved by an institutional review board (Alpha IRB). The study was conducted from March to July 2015 outside of ragweed pollen season in the ABC; follow-up occurred through August 2015 (Clinicaltrials.gov NCT02377895). The study comprised five visits over ~3 weeks. Subjects provided written informed consent prior to study initiation. The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Tripartite Guideline for Good Clinical Practice, and all relevant regulatory requirements. The study design is summarized in .
Figure 1 Study design.
Abbreviations: ABC, Allergen BioCube®; TNSS, total nasal symptom score.
Adult subjects (≥18 years) with a history of AR, positive skin test for ragweed pollen (reaction ≥3 mm larger than the negative control), and a positive BioCube challenge response to ragweed pollen at visit 4 (total nasal symptom score [TNSS] ≥6) were eligible for enrollment. Subjects were excluded if they had upper respiratory tract infection within prior 2 weeks, current diagnosis of specified nasal conditions, history of anaphylaxis, or poor tolerability of previously administered allergen, abnormal blood pressure (≤90 or ≥160 [systolic], ≤60 or ≥100 [diastolic]), or use of disallowed medications, including immunotherapy. Inclusion/ exclusion criteria were assessed at every visit.
AR symptoms assessed by subjects during the visits included nasal itching, sneezing, rhinorrhea, and nasal congestion; the sum of these scores was calculated by the investigator as TNSS, the primary end point. Ocular symptoms of itching, tearing, and redness were also assessed by subjects, with the sum calculated as total ocular symptom score (TOSS) as exploratory end points. Individual nasal and ocular symptoms were assessed using a 4-point scale (0= no symptoms; 1= mild [symptom clearly present but minimal awareness, easily tolerated]; 2= moderate [definite awareness of symptom that is bothersome but tolerable]; 3= severe [symptom that is hard to tolerate; causes interference with activities of daily living and/or sleeping]).
AR signs, evaluated by the investigator as secondary end points, included peak nasal inspiratory flow (PNIF), measured every 60 minutes during ragweed pollen exposure in the BioCube. Due to PNIF differences between the treatment groups throughout the study, a post-hoc PNIF percent change from baseline (CFB) analysis was conducted.Citation7 PNIF measurements were performed in a masked manner, with the investigator not aware of which study medication (ie, drug or placebo) each subject received. Post-BioCube exposure nasal digital imaging was also performed.
After screening (visit 1: day –15 to –3), subjects were primed for ragweed pollen exposure in the ABC (visit 2: day –3 to –2; visit 3: day –2 to –1) for up to 3 hours with assessment of nasal and other allergic symptoms evaluated every 15 minutes during exposure. PNIF was collected every 60 minutes. The purpose of priming was to increase sensitivity to the specific allergen, inducing mucosal inflammation and facilitating the development of adequate symptoms for participant inclusion. The rate and degree of symptom development can vary among individuals. The inclusion of the priming phase in EEU studies, in general, has been shown to standardize predose symptoms and greatly decrease symptom variability in the treatment phase or during baseline assessments.Citation9 All subjects attended both priming visits regardless of priming response.
At visit 4 (day 1), nasal and ocular symptoms were assessed before any ragweed exposure by subject of staff personal as appropriate, then study subjects were exposed to ragweed pollen priming in the ABC for up to 90 minutes prior to enrollment qualification review. Subjects were enrolled and randomized if they met the inclusion/exclusion criteria and had a positive challenge response at the 90 minutes time point of ABC exposure, defined as TNSS ≥6. A computer-generated randomization schedule was used to assign subjects (1:1 ratio) to the two treatment arms. Relief study treatment (250 µL/nostril, odorless to maintain mask with placebo) or placebo was then administered at the same visit, followed by 7.5 hours of BioCube allergen exposure and assessment. Symptoms were evaluated by subjects every 15 minutes during priming; PNIF and peak expiratory flow rate (PEFR) were assessed by staff at the 60 minutes time point. Post-treatment, nasal and ocular symptoms were evaluated every 5 minutes for the first 30 minutes, then every 15 minutes thereafter during BioCube exposure. PNIF and PEFR measurements were collected every 60 minutes during exposure.
Visit 5 (day 8+3) included prophylactic study treatment 30 minutes prior to 3 hours of BioCube allergen exposure and assessment. Symptoms and measurements were recorded similarly to visit 4.
Safety measures included adverse event (AE) reporting, PEFR, physical exams, nasal exams, vital signs, and urine pregnancy tests. Observed PEFR values and a drop of ≥15% from baseline were reported at all visits. Subjects with ≥15% PEFR decrease were excluded from the study for possible compromised lung function. Physical exams included general health and head, eyes, ears, nose, and throat evaluations. Nasal exams identified any edema, crusting, bleeding, or secretions and were reported as either clinically significant or not clinically significant. Vital sign evaluations included pulse and systolic/diastolic blood pressure. Treatment-emergent adverse events (TEAEs) were reported for the entire study period.
Allergen BioCube®
The allergen/air mixture was introduced to subjects through a ceiling duct in the ABC. A ragweed pollen concentration of 4,000±400 grains/m3 (Ambrosia artemesiifolia) was used, similar to that used in other EEUs and peak environmental levels, and is capable of producing AR symptoms that are high enough for assessment of therapeutic treatment efficacy.Citation9–Citation12 Spatial and temporal uniformity of allergen concentrations was verified with continuous laser particle counter measurements every 10 minutes whenever the unit was in use. Prior to this study, technical validation of the BioCube was performed using Rotorod collection and laser particle counts to ensure reproducibility of consistent and uniform ragweed pollen concentration in the BioCube.Citation8 Prior clinical validation of the BioCube for ragweed pollen was also conducted; mean TNSS increased by 4.96 units from pre- to post-BioCube ragweed pollen exposure.Citation8 Similarly, in the current iodixanol study, mean TNSS increased 5.05 units from pre- to post-BioCube exposure, further validating the reproducibility of the BioCube. Technical and/or clinical validation studies of the BioCube were also performed prior to this study for Timothy-grassCitation7 and induction of allergic signs and symptoms similar to pollen exposure in the environment (ORA, Inc).Citation13
Circulated and fresh air was passed through a high-efficiency particulate air filter to ensure that only fresh ragweed pollen entered the unit; the recirculated air did not alter ragweed pollen concentrations in the unit, which consistently remained within the target range. Any portion of the ragweed pollen that did not exit through the air return settled on the floor, where it was captured via a sticky surface so that it did not reaerosolize. An air shower at the BioCube entrance served as a cleaning unit and created buffer pressure to maintain air flow dynamics whenever subjects entered or left the room.
Statistical methods
The primary efficacy analysis was performed on the intent-to-treat (ITT) population using multiple imputation methodology following the Markov chain Monte Carlo method assuming normality. Two-sample one-sided t-tests were used to compare the mean CFB between the iodixanol and placebo groups at each time point at α=0.05 significance level. Thirty-four subjects per arm were required for 96% power to show a statistically significant difference (at α=0.05) at each time point, assuming a mean difference of 2.0 with a common SD of 2.4. No multiple testing corrections were utilized in this early-phase, proof-of-concept study.
Results
Subject demographics
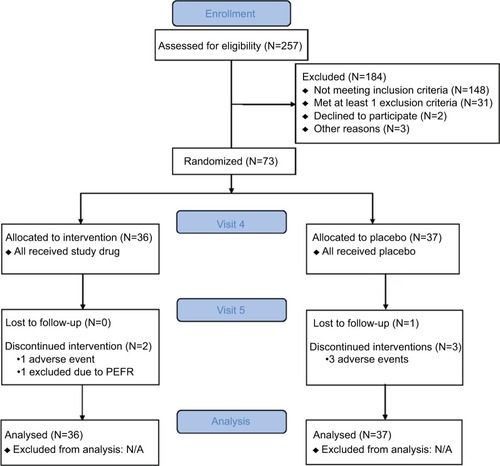
Seventy-three adult subjects were enrolled and randomized (1:1 ratio) to the iodixanol nasal solution group (N=36) or the placebo saline nasal solution group (N=37). As summarized in , all 73 enrolled subjects were included in the primary efficacy and the safety analyses; 67 subjects completed the study. Two subjects discontinued the study in the iodixanol group, one subject for AEs, and one subject who did not meet PEFR criteria at visit 5. Four subjects discontinued the study in the placebo group, three subjects for AEs, and one subject for administrative reasons. Baseline demographic and clinical characteristics of subjects were similar () except for PNIF and PEFR, as discussed later in this article.
Table 1 Baseline subject demographic and clinical characteristics
AR nasal symptoms
Ragweed pollen exposure in the BioCube prior to study treatment induced nasal and allergy responses in subjects at all time points compared to pre-BioCube levels (). Post-treatment at visit 4 during ABC exposure, subjects self-assessed nasal symptoms (nasal itching, sneezing, rhinorrhea, and nasal congestion) using a 4-point scale from 0 to 3, and TNSS, defined as the composite of the four nasal symptom scores, was determined, with a possible range from 0 to 12. Symptoms were assessed every 5 minutes post-treatment for the first 30 minutes and every 15 minutes thereafter for 7.5 hours in the ABC. Lower TNSS indicated less severe nasal symptoms and AR.
Table 2 Nasal allergy responses to BioCube ragweed pollen exposure prior to study treatment, visit 4
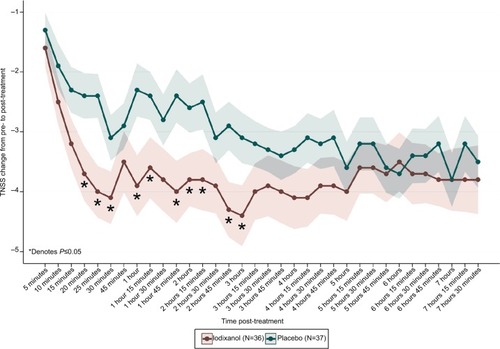
Iodixanol (Nasapaque) mean CFB scores for TNSS, ie, the primary end point, were statistically significantly improved over placebo as early as 20 minutes after treatment, and as late as 3 hours post-treatment at 10 of 16 time points (). CFB scores at 20 minutes were iodixanol =−3.7±0.46 and placebo =−2.4±0.38 (P=0.01). CBF scores at 3 hours were iodixanol =−4.4±0.51 and placebo =−3.1±0.42 (P=0.03). Peak TNSS CFB differences occurred at 25 minutes, 1 hour, and 1 hour 45 minutes (treatment differences 1.6 units at each of these time points).
Figure 3 TNSS change from pre- to post-treatment (visit 4, day 1).
Abbreviation: TNSS, total nasal symptom score.
Each individual nasal symptom score (nasal itching, sneezing, rhinorrhea, and nasal congestion) was also evaluated separately. For nasal itching, mean CFB scores were statistically significant for iodixanol compared to placebo as early as 15 minutes post-treatment (iodixanol =−0.8±0.13, placebo =−0.4±0.14, P=0.03), and as late as 4 hours 30 minutes (iodixanol =−1.2±0.16, placebo =−0.8±0.14, P=0.04) at 11 of 22 time points (). Peak differences occurred at the 4 hours 30 minutes time point. For sneezing, mean CFB scores were statistically significant for iodixanol compared to placebo as early as 10 minutes post-treatment (iodixanol =−0.8±0.16, placebo =−0.4±0.13, P=0.03), and as late as 4 hours 15 minutes (iodixanol =−0.9±0.14, placebo =−0.5±0.14, P=0.04) at 11 of 21 time points. Peak CFB differences occurred at 1 hour 45 minutes (iodixanol =−1.0±0.18, placebo =−0.4±0.15, P=0.009). For rhinorrhea, statistically significant mean CFB for iodixanol scores compared to placebo occurred at two time points (25 minutes and 1 hour, P<0.05). Peak CFB differences occurred at 1 hour (iodixanol =−1.0±0.14, placebo =−0.6±0.15, P=0.03).
Figure 4 Change in nasal itching from pre- to post-treatment (visit 4, day 1).
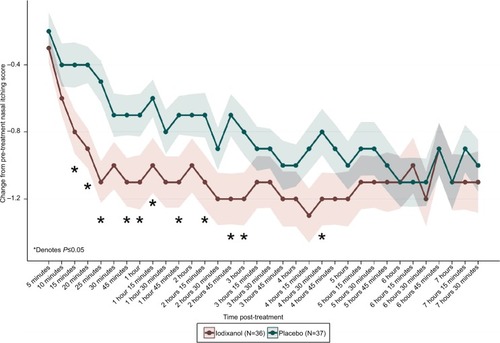
There were no significant differences in mean CFB scores between the iodixanol and placebo groups for nasal congestion at P≤0.05.
AR ocular symptoms
Ragweed pollen exposure in the BioCube prior to study treatment induced ocular allergy responses in subjects at all time points compared to pre-BioCube levels (). Post-treatment at visit 4 during ABC exposure, subjects self-assessed ocular symptoms (ocular itching, ocular tearing, and redness) using a 4-point scale from 0 to 3, and TOSS, defined as the composite of the three ocular symptom scores, was determined, with a possible range from 0 to 9. Symptoms were assessed every 5 minutes post-treatment for the first 30 minutes and every 15 minutes thereafter for 7.5 hours in the ABC. Lower TOSS indicated less severe ocular symptoms (ocular symptoms are a comorbid condition of AR). A statistically significant mean CFB treatment difference between iodixanol and placebo groups for TOSS was observed at the 25 minutes time point only (iodixanol =−1.9±0.28, placebo =−1.1±0.31, P=0.04).
Table 3 Ocular response (TOSS) to BioCube allergen exposure prior to treatment, visit 4
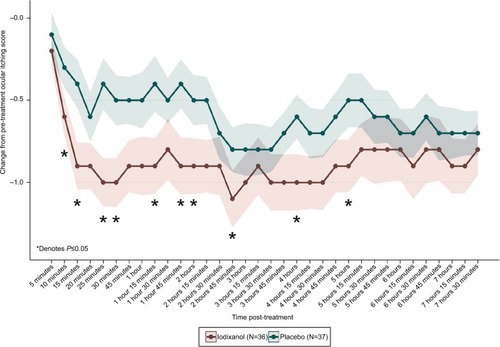
Each individual ocular symptom score (ocular itching, ocular tearing, and redness) was also evaluated separately. For ocular itching, statistically significant mean CFB iodixanol scores occurred as early as 10 minutes (iodixanol =−0.6±0.12, placebo =−0.3±0.12, P=0.05), and as late as 5 hours (iodixanol =−0.9±0.16, placebo =−0.5±0.16, P=0.04) at 10 of 24 time points (). Peak CFB differences occurred at 25 minutes (Nasapaque =−1.0±0.15, placebo =−0.4±0.16, P=0.006). For ocular tearing and ocular redness, mean differences in CFB scores for iodixanol and placebo were not statistically significant.
AR signs
PNIF was measured every 60 minutes (±15 minute) during ragweed exposure in the ABC at visits 4 and 5 and evaluated as a secondary efficacy end point using the ITT population.
Higher scores indicated improvement in inspiratory air flow. Statistically significant percent CFB treatment differences in PNIF occurred with iodixanol compared to placebo at two time points at visit 4 at 2 hours (iodixanol =9.4±3.83, placebo =−3.3±4.54, P=0.02) and 4 hours (iodixanol =15.7±6.48, placebo =1.9±4.42, P=0.04) post-treatment. PNIF improved 9%–16% for iodixanol-treated subjects; for placebo-treated subjects, PNIF ranged from a worsening of 3% to an improvement of 2%.
Iodixanol as prophylactic treatment (visit 5)
The second co-primary efficacy end point was TNSS defined as the composite score of four symptoms (nasal itching, nasal congestion, rhinorrhea, and sneezing) recorded during ABC exposures at all post-treatment time points (visit 5). TNSS was scored at 5, 10, 15, 20, 25, and 30 minutes during ABC exposure and every 15 minutes thereafter for the duration of ABC exposure. Prior to ABC allergen exposure at visit 5, subjects had been exposed to study treatment at visit 4; therefore the pre-ABC time point at visit 5 was considered a post-treatment measure. No statistically significant treatment differences occurred for mean change from pre-treatment baseline at any time point at visit 5 for any nasal or ocular symptom or sign measurement.
Safety
Few (9) AEs were reported by subjects in the study and all were mild in severity (). TEAEs occurred in 11.1% of subjects in the iodixanol group and 8.1% of subjects in the placebo group. The five TEAEs that occurred in the iodixanol group included gastroenteritis viral, blood pressure diastolic increased, epistaxis, urticaria, and thrombosis. The four TEAEs that occurred in the placebo group included gastroenteritis, epistaxis, asthma, and nasal mucosal disorder. No TEAEs in the iodixanol group were considered related to study treatment. One TEAE in the placebo group, nasal mucosal disorder, was considered related to treatment. Four subjects discontinued from the study for TEAEs; one of these subjects was in the iodixanol group and experienced two TEAEs, thrombosis (blood clots secondary to blowing nose) and epistaxis. The other three discontinued subjects were in the placebo group. No notable abnormal findings or significant safety issues were identified for other safety parameters.
Table 4 Adverse events
Discussion
Iodixanol has been used since the 1950s as a contrast medium at a concentration of 550 and 652 mg/mL. Typically used for intravascular injection, iodixanol opacifies vessels in the path of flow of the contrast agent permitting radiographic visualization of the internal structures until significant dilution and elimination occurs. The amount of contrast media used for this purpose ranges from 3 to 90 mL, with a maximum of 150 mL (equivalent to 97,800 mg of iodixanol). Some of the safety concerns associated with injection of contrast agents include a risk of anaphylaxis, anaphylactoid reactions, or nephropathy.Citation14 Severe life-threatening reactions and fatalities to this contrast media, mostly of cardiovascular origin, have occurred, but are rare (<0.01%).Citation15 These adverse reactions are not expected to occur with noninjection routes of administration. Here, we present a phase two clinical study investigating iodixanol as a nasal solution to treat AR. For this use, the concentration of iodixanol nasal solution is 320 mg/mL (250 µL per nostril, equivalent to about 160 mg of iodixanol, 600× less than the amount used in intravenous injections).
Iodixanol nasal solution showed evidence of a rapid effect and statistically significant efficacy for relief of nasal and ocular AR symptoms at numerous time points at visit 4 in this proof-of-concept study, and further study is warranted. Statistically significant differences for iodixanol compared to placebo occurred at a majority of time points for TNSS throughout the 3 hours. Results for nasal itching and ocular itching were particularly notable (eg, statistically significant CFB compared to placebo at 4.5 hours for nasal itching, and at 5 hours for ocular itching). Further, it should be noted that the placebo, saline solution, will also offer some basis for relief of AR symptoms, and iodixanol still showed statistically improved TNSSs over placebo at many time points post-treatment.
Iodixanol treatment had less of an effect on the self-reported symptom of nasal congestion than on itching and sneezing. However, statistically significant improvement did occur for PNIF in iodixanol-treated subjects. It is possible that although mast cell degranulation is being blocked by iodixanol, there is another mechanism contributing to nasal congestion. It has been shown that irritant-induced nasal congestion does not proceed through mast cell degranulation, and that subjects with AR are hypersensitive, as compared to nonallergic patients. The authors of this study hypothesized that nonmast cell–mediated mechanisms, including possible neurogenic reflexes, may be operative in the nasal congestive response.Citation16
A proof-of-concept Phase II/III randomized clinical study using the nasal route of administration has also shown the potential efficacy of the iodinated contrast agent Hexabrix™ for the treatment of AR. Results indicated reduced acute allergen challenge response, with increasing doses of nasal allergen challenge, including statistically significant reductions in sneezing and runny nose symptoms after prophylactic treatment with the contrast agent compared to placebo.Citation17 Therefore, visit 5 was included to investigate prophylactic treatment with iodixanol. Following one prophylactic treatment, visit 5 study results generally confirmed visit 4 findings, although were not as robust, which is not surprising since subjects received no allergen priming before visit 5 as they did prior to treatment at visit 4. Thus at visit 5 there was less opportunity for the drug to show a similar magnitude of treatment effect. Additional studies are needed to assess whether additional dosing and priming would enhance drug efficacy.
This study also evaluated ocular symptoms. Allergic eye symptoms such as itching, redness, and tearing, also referred to as allergic conjunctivitis, are extremely common in patients with AR, termed rhinoconjunctivitis. Further, it has been reported that both nasal and ocular allergy signs and symptoms were induced after nasal allergen challenge, and that nasal pre-treatment with the antihistamine azelastine reduced nasal and ocular symptoms after nasal challenge with antigen.Citation18 One of these proposed mechanisms is the existence of a nasal–ocular reflex where the nasal allergic reaction leads to an afferent reflex response, resulting in eye symptoms. It is speculated that the effect of intranasal steroids on eye symptoms in AR is related to their inhibition of the nasal–ocular reflex.Citation18 Other studies have also demonstrated that intranasal corticosteroids were more effective than placebo in reducing ocular allergy symptoms.Citation19,Citation20 In our iodixanol AR study, the significant reductions in ocular itching via nasal administration of iodixanol also suggest that this drug may inhibit the neuronal nasal–ocular reflex.
Results of the iodixanol AR study reflect subject responses to a uniform allergen concentration and single-dose instillation of the drug in a controlled environment setting, which provides “cleaner” results than a natural environment study. A limitation is that this study does not reflect chronic dosing under natural environment conditions, which may provide additional safety and long-term efficacy data. Field studies, however, may reflect possible confounding factors including fluctuating pollen levels and exposure to multiple allergens in the natural environment. Nevertheless, study results from the controlled setting can be generalized to real-world, natural environment conditions.
Iodixanol nasal solution showed evidence of efficacy for relief of nasal symptoms and ocular itching in this AR study, and was safe and well tolerated. Clinical trials with allergen challenge models such as the ABC provide valuable assessments of allergen exposures, subject responses, and drug efficacies. Additional studies of iodixanol nasal solution, eg, with larger sample sizes and chronic exposures, are warranted to continue assessment of iodixanol as a novel AR treatment.
Acknowledgments
The authors acknowledge the medical writing assistance provided by Michelle Olsen at Ora, Inc.
Disclosure
Paul J Gomes is an employee of Ora, Inc. Erik Viirre, MD, PhD, J Ernest Villafranca, PhD, and Elliott C Lasser, MD are employees of 3E Therapeutics. Allergen BioCube® (ABC®) is a registered trademark of Ora, Inc.
References
- WheatleyLMTogiasAClinical practice. Allergic rhinitisN Engl J Med2015372545646325629743
- ChurchDChurchMScaddingGAllergic rhinitis: impact, diagnosis, treatment and managementClin Pharmacist201688
- LinnebergADam PetersenKHahn-PedersenJHammerbyESerup-HansenNBoxallNBurden of allergic respiratory disease: a systematic reviewClin Mol Allergy20161411227708552
- MayJRDolenWKManagement of allergic rhinitis: a review for the community pharmacistClin Ther201739122410241929079387
- LasserECThe multipotential pseudoantigenicity of X-ray contrast media. Pseudoantigen excess may downregulate the release of hypotensive mediatorsInt Arch Allergy Immunol2000123428229011146385
- LasserECX-ray contrast media mechanisms in the release of mast cell contents: understanding these leads to a treatment for allergiesJ Allergy20112011115
- AngjeliEGomesPLaneKJSteinLAbelsonMBTechnical and clinical validation of the Allergen BioCube® for Timothy grassImmun Inflamm Dis201751788428250927
- GomesPJLaneKJAngjeliESteinLAbelsonMBTechnical and clinical validation of an environmental exposure unit for ragweedJ Asthma Allergy2016921522128008277
- EllisAKNorthMLWalkerTSteacyLMEnvironmental exposure unit: a sensitive, specific, and reproducible methodology for allergen challengeAnn Allergy Asthma Immunol2013111532332824125135
- DayJHBriscoeMPClarkRHEllisAKGervaisPOnset of action and efficacy of terfenadine, astemizole, cetirizine, and loratadine for the relief of symptoms of allergic rhinitisAnn Allergy Asthma Immunol19977921631729291422
- DayJHBriscoeMPWelshAOnset of action, efficacy, and safety of a single dose of fexofenadine hydrochloride for ragweed allergy using an environmental exposure unitAnn Allergy Asthma Immunol19977965335409433370
- BerkowitzRBWoodworthGGLutzCOnset of action, efficacy, and safety of fexofenadine 60 mg/pseudoephedrine 120 mg versus placebo in the Atlanta allergen exposure unitAnn Allergy Asthma Immunol20028913845
- NIH ClinicalTrials.gov [webpage on the Internet]ORA, IncA pilot study evaluating the signs and symptoms of seasonal allergic rhinoconjunctivitis following exposure in the allergen bio-cube Available from: https://clini-caltrials.gov/ct2/show/NCT00985075Accessed September 12, 2018
- PasternakJJWilliamsonEEClinical pharmacology, uses, and adverse reactions of iodinated contrast agents: a primer for the non-radiologistMayo Clin Proc201287439040222469351
- FDAVisapaqueTM (iodixanol) Injection package insert 2003 Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/20351slr013_visipaque_lbl.pdf
- ShustermanDBalmesJAvilaPCMurphyMAMatovinovicEChlorine inhalation produces nasal congestion in allergic rhinitics without mast cell degranulationEur Respir J200321465265712762352
- VishwanathSBaroodyFMChaabanMRTopical intranasal ioxaglate meglumine 39.3% and ioxaglate sodium 19.6% (Hexabrix) reduces the acute response to nasal challenge with allergenJ Allergy Clin Immunol2011128121922121414657
- BaroodyFMNaclerioRMNasal-ocular reflexes and their role in the management of allergic rhinoconjunctivitis with intranasal steroidsWorld Allergy Organ J201141 SupplS1S523283068
- NaclerioRIntranasal corticosteroids reduce ocular symptoms associated with allergic rhinitisOtolaryngol Head Neck Surg2008138212913918241703
- BaroodyFMLogothetisHVishwanathSBashirMDetineoMNaclerioRMEffect of intranasal fluticasone furoate and intraocular olopatadine on nasal and ocular allergen-induced symptomsAm J Rhinol Allergy20132714853