Abstract
Background
Allergic bronchopulmonary aspergillosis (ABPA) is a condition characterized by a Th2 response, serum eosinophilia, and increased total serum IgE to Aspergillus fumigatus. ABPA occurs in cystic fibrosis (CF) and asthma. Omalizumab is a humanized recombinant monoclonal antibody against IgE. Previous studies reported borderline results when treating ABPA with omalizumab.
Methods
A retrospective study to investigate the efficacy of omalizumab in the treatment of ABPA in CF patients was conducted at 3 CF centers in Israel and Belgium. Data were obtained from the digital archive. We measured 4 outcome parameters: forced expiratory volume in 1 second, body mass index, pulmonary exacerbations, and steroid sparing.
Results
The database was composed on the records of 9 patients. None of the outcome parameters showed any improvement. A favorable outcome was observed in patients with higher levels of posttreatment total IgE than those with lower levels. CF-related diabetes and male gender showed trends for poorer outcomes.
Conclusion
No benefits were detected on treating ABPA in CF with omlaizumb. Monitoring the total IgE was not helpful. A prospective randomized double-blind study is needed.
Keywords:
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is a condition that occurs when the pulmonary immune system reacts to Aspergillus fumigatus (AF), a ubiquitous thermophilic fungus that can colonize the bronchial mucosa of cystic fibrosis (CF) patients.Citation1 The reaction is characterized by a Th2 response, serum eosinophilia, and increased total serum IgE to AF.Citation2 The prevalence of airway colonization with AF is found in up to 58% of all CF populations, including both children and adults.Citation3,Citation4 Isolation of Aspergillus species from respiratory secretions has been found to be significantly more frequent in older children and young adults than in younger children with CF.Citation5 Aspergillus sensitization is defined by the presence of immediate skin test positivity to Aspergillus antigens or an elevated serum AF-specific IgE level.Citation6 Allergic bronchopulmonary aspergillosis occurs in 1%–2% of all patients with persistent asthma and 2%–25% of patients with cystic fibrosis, with a pooled prevalence of 8.9%.Citation6–Citation8
Risk factors for sensitization to AF include genetic predisposition, prolonged duration of colonization with Pseudomonas aeruginosa, and prolonged use of dornase α inhalations.Citation9 High cumulative doses of inhaled corticosteroids are also associated with AF sensitization, and their role as a risk factor needs to be clarified.Citation10 As with AF isolation, the prevalence of ABPA was significantly higher in adults than in children (10.1% vs 8.9%, P<0.0001).Citation11
The diagnosis of ABPA is difficult in patients with CF because the clinical, imaging, and functional signs required for the diagnosis of ABPA are usually symptoms of CF.Citation9 Failure to diagnose ABPA may result in earlier deterioration of pulmonary function and more rapid progression of CF lung disease.Citation12 The diagnostic criteria for ABPA in patients with asthma or CF are discussed elsewhere.Citation13 The cornerstone of the treatment of ABPA is systemic corticosteroids, given orally in the long term or as pulse therapy.Citation1 The duration of both protocols (oral and intravenous) is controversial. In patients who do not respond to corticosteroids, a therapy with antifungal medication might be administrated.
Omalizumab is a humanized recombinant monoclonal antibody targeting the high affinity Fc receptor of IgE and prevents binding of IgE to the immune effector cells mediating inflammation and the hypersensitive response. A downregulation of IgE receptors is an important consequence of omalizumab treatment, thus reducing inflammatory activity.Citation14 Omalizumab reduces airway eosinophilia and decreases IL-4+ cells in allergic asthma.Citation15,Citation16 Omalizumab was proven as being safe and effective in ABPA in asthma.Citation17 While the pathological process in ABPA complicating CF is similar to that in asthma, omalizumab is thought to be an alternative therapy for ABPA in CF patients who fail to respond to systemic corticosteroids or have serious adverse effects during treatment with corticosteroids.Citation15 Omalizumab has been approved as a steroid-sparing agent for severe allergic asthma,Citation18 but there are conflicting reports in the literature regarding ABPA in CF based on case reports starting from 2007,Citation19 and no prospective trial has been completed. One double-blind randomized study was conducted, but was terminated earlier than planned due to adverse events.Citation20 A recent case series with 32 patients concluded that omalizumab might have a steroid-sparing effect but failed to improve other outcomes such as forced expiratory volume in 1 second (FEV1) or pulmonary exacerbations in patients with ABPA.Citation21 The latest review article that summarized 102 patients (most of them with asthma) with ABPA treated with omalizumab concluded that the treatment failed to improve their lung function but did lead to a reduction in exacerbation rate. The authors concluded that omalizumab might be an alternative treatment for ABPA although the duration of therapy remained ambiguous.Citation22
In Israel, there is a unique genetic CFTR abnormality seen in Ashkenazi Jews and in the genetically heterogenous group of Sephardic Jews and non-Jewish population (mainly Arabs, Muslim, or Christian). As a consequence, the disease-causing mutation W1282X (class I) is very prevalent, only in those of Ashkenazi descent. Whereas in the world CF population, this allele prevalence is estimated as 0.5%, in the Israel CF population the prevalence is 36%, almost the same prevalence as f508del, the world’s most prevalent mutation for CF.
The current study was conducted to better assess the effi-cacy of omalizumab in the treatment of ABPA in CF patients.
Methods
A retrospective study evaluating all subjects with CF treated with omalizumab in 3 CF centers was conducted in Leuven (Belgium), Rambam, and Sheba (Israel). The data were obtained from patients’ records in the years 2011–2015 and included age, sex, CFTR mutations, presence of CFRD, duration of therapy with omalizumab, number of pulmonary exacerbations during therapy, and steroid dosage before, during, and after therapy with omalizumab. In addition, FEV1, IgE, and body mass index (BMI) values were obtained pre- and posttreatment.
Patients with CF, 2 CFTR mutations, and with a diagnosis of ABPA were included if they met the following criteria: patients had clinical deterioration, IgE levels were above 1,000 or 500 ng/mL (new diagnosis or recurrent ABPA, respectively), showed a positive immediate skin reaction or if elevated specific antibodies (IgE or IgG) against AF were positive, and a central bronchiectasis shown on high-resolution computed tomography. The dosage of Xolair® (omalizumab, Novartis, CH, Basel, Switzerland) was individually adjusted to the pretreatment level of IgE and body weight, as recommended by the US Food and Drug Administration’s guidelines for allergic asthma (some patients had very high levels of IgE, which is contraindicated for treatment of asthma). No patient was treated with a 2-week dosage, given the lack of guidelines for ABPA. The indications for omalizumab in most of the patients were mainly because of adverse effects of prolonged systemic therapy with corticosteroids and failure of antifungal therapy. Five patients had a contraindication for corticosteroids; all of them were steroid-naïve and experienced a failure of treatment with antifungal therapy. Out of the 4 patients who were treated with corticosteroids, none had reduced exacerbations on pulse or oral regime ().
Table 1 Clinical outcomes
Because some of the patients were very young, BMI values were expressed in Z scores. The main outcome parameters examined were FEV1, BMI, number of pulmonary exacerbations, and reduced dosage of steroids in patients who were still on steroidal treatment.
FEV1 and BMI were considered stable if the changes were below 10%. A larger deviation was considered as deterioration or improvement.
Statistical analysis was performed by calculating the odds ratio (OR) and the confidence interval (CI). A paired t-test was performed. The level of confidence for the study was 95%.
Declarations
Approval of the local ethics committee of every hospital was given. Each hospital has its own ethics committee (Sheba, Rambam, Leuven). The approvals at Rambam and Leuven were made after the approval was obtained at Sheba. No informed consent was required to review the patients’ medical records as it was a retrospective study and patients’ names and identifying numbers were kept confidential. According to the decision of Sheba’s ethics committee, patient’s data were extracted from medical records, each patient was given a number, and then all data were saved and analyzed according to this number; the list of patients’ names and the corresponding number was kept in a notebook of the primary investigator. The notebook will be saved for 7 years.
Results
This report describes 9 patients (6 males, average age 23±9 years) who were treated in these centers (). The mean duration of treatment with omalizumab was 13.9±8.6 months. One patient was treated for only 2 months because of an allergic reaction to omalizumab. Eight patients had 2 CFTR mutations of class I or II, 4 patients with the typical Ashkenazi Jewish mutation, W1282X, 1 patient with 1 severe mutation and 1 mutation from class IV (D1152H). Seven (78%) patients had CF-related diabetes (CFRD). Four patients had sputum cultures that were positive for Pseudomonas aeruginosa, either mucoid or planktonic. All patients had been treated with dornase α prior to diagnosis; the average treatment time with dornase α was 4.5±3.2 years. The maintenance daily treatment for all patients was according to the international guidelines, (inhalations of hypertonic saline, respiratory physiotherapy, pancreatic enzyme replacement therapy, vitamins, inhaled antibiotics, and insulin). Treatment remained unchanged during the study period, with the exception of therapy enhancement during exacerbations. Three patients were treated with systemic corticosteroids at the start of the treatment (range of daily dosage 0.1–0.63 mg/kg). Five patients had a contraindication for systemic corticosteroid treatment (Mycobacterium abscessus in the sputum, psychiatric condition).
Table 2 Patient data
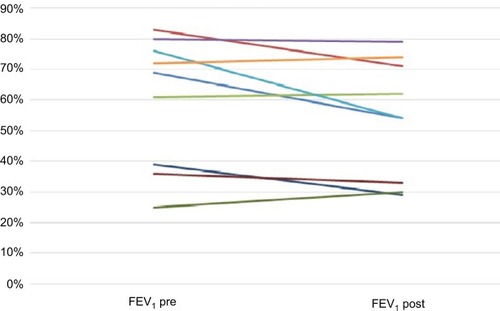
When comparing FEV1 pre- and posttreatment with omalizumab (), 4 patients had stable lung function, another 4 deteriorated, and only 1 improved (). The 2 patients without the CFRD did not deteriorate (OR =0.29, 95% CI: 0.02–4.00, not significant (NS) P=0.36), nor did female patients (OR =0.19, 95% CI: 0.014–2.75, NS P=0.22). Moreover, females had a lower pulmonary exacerbation rate than males: 2 per females and 5 per males for the whole study period.
Figure 1 FEV1 before and after treatment with omalizumab.
Abbreviation: FEV1, forced expiratory volume in 1 second.
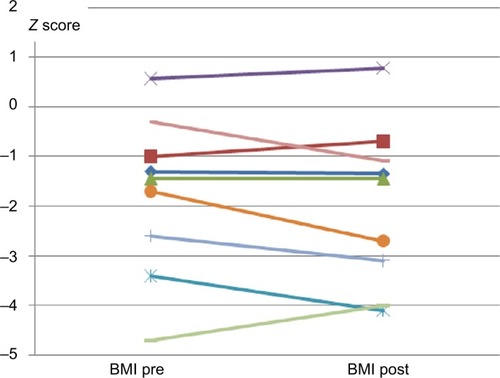
The posttreatment BMI decreased in 4 patients, was stable in 3, and improved in 2 patients ()
Figure 2 BMI before and after treatment with omalizumab.
Abbreviation: BMI, body mass index.
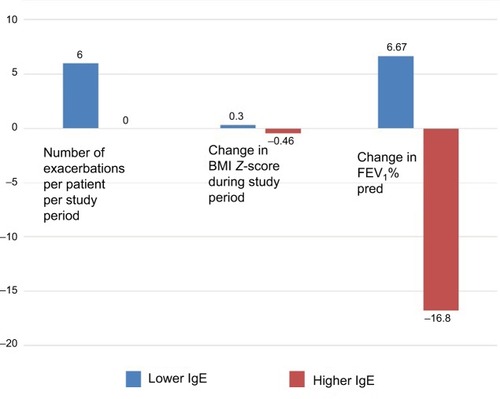
On average, patients experienced 4 exacerbations or 0.288 exacerbations per month of treatment (). Three patients had no exacerbation (1 improved his lung function and another 2 maintained stable lung function). No data were available for the pulmonary exacerbation rate pretreatment. Only 4 patients out of 9 were treated with steroids before and after treatment, and none had a reduced steroid dosage following the treatment with omalizumab.
Posttreatment IgE levels were lower than pretreatment levels in 6 patients (). None of them showed improvement in any of the outcome parameters. By contrast, an improved outcome was observed in the 3 patients with higher posttreatment IgE, (), namely, no pulmonary exacerbation, and stable or improved BMI and FEV1.
Discussion
We described a series of 9 CF patients diagnosed with ABPA and treated with omalizumab. To the best of our knowledge, this is one of the largest single studies published to date and the first series to include a specific Jewish (4 patients) subset of CF patients who carry the W1282X mutation. Larger series on ABPA have been published, however not on CF patients.
The mechanism of action of omalizumab is not clear. However, downregulation of IgE receptors, reduction in airway eosinophilia, and a decrease in IL-4+ cells in allergic disease may reduce the immune response in ABPA. This mechanism underlies the hypothesis that omalizumb might be effective in patients with CF and ABPA as proved previously in ABPA complicating asthma.Citation17 While the pathophysiology of ABPA is also not well understood, the effectiveness of omalizumab in ABPA in CF is doubtful. The literature regarding omalizumab in ABPA in CF is scarce, and the few series that have been published deal with a very small number of patients (not exceeding 50 in total). The findings suggest that this treatment has limited benefits, with the exception of some steroid-sparing effects. We hypothesize that there may be a publication bias, such that negative results were not reported.
In our case series, treatment with omalizumab did not improve the outcome in any of the parameters studied, (the steroid-sparing outcome could only be observed in 4 patients). One possible reason for these results could be that the inhibition of IgE was not sufficient to treat ABPA. In other words, in order to treat ABPA, another target cell or cytokine should be selected. Alternatively, the dosage of omalizumab may not have been sufficient and higher doses should be considered. The lack of official guidelines and the dosage warning by the drug producer regarding sedimentation of IgE complexes in patients with high levels of IgE make it challenging to examine whether higher doses of omalizumab would be effective.
It has been suggested that prolonged treatment with dornase alpha via inhalation is a risk factor for the development of ABPA.Citation9 In fact in our study, all the patients had been treated with dornase α. This is consistent with published data. We assume that chronic mucosal irritation due to prolonged use of dornase α might play a role in the colonization of CF airways with AF. Alternatively, since the majority of subjects with CF use dornase α, the association between dornase α use and ABPA may be a chance finding.
Females and patients without CFRD tended to have better outcomes. CF patients with CFRD have a poorer clinical CF course, so the improved outcome in CF patients without CFRD was expected.
Females in our study had better outcomes, but again, as with CFRD, the power of the study was not sufficient to draw firm conclusions. Females with CF tend to have a poorer disease course and shorter life expectancy. Hence, the better outcome in females might be explained by some other protective factor (eg, estrogen) that is found in females in higher levels than in males and has well established anti-inflammatory properties like inhibiting proinflammatory cytokines as IL-1, IL-6, and TNFα.Citation23
Unexpectedly, the 3 patients with increased levels of IgE at the end of the treatment had the most favorable outcomes compared to the 6 patients with decreased IgE at the end of treatment. These data suggest that monitoring the level of IgE following omalizumab treatment might not be informative at all and cannot replace the tight clinical control of those patients. This appears to be due to the fact that the total IgE measured includes the free IgE blocked by omalizumab.
This study also has limitations. The first is the small number of patients with CF and ABPA treated with omalizumab; as a result, the statistical power was not sufficient to obtain statistically significant differences, so the conclusions are limited. Nevertheless, considering the fact that ABPA in CF is not a prevalent condition, this series of 9 patients is one of the largest reported. We did not measure specific IgE, free IgE, or report on eosinophil numbers. One patient was treated for only 2 months, but we considered it important to include him as well.
Conclusion
In patients with CF, we found no advantage in administering omalizumab for the treatment of ABPA. This study contributes to the clinical equipoise surrounding omalizumab treatment in subjects with CF and ABPA that can only be answered objectively by a prospective randomized trial.
Author contributions
MA participated in study design, data collection, statistical analysis, data interpretation, and writing the paper, and is a guarantor of the paper. SS was involved in data collection. IS, BB, AD, and YB played a role in study design and data interpretation. LB helped with the data collection, data interpretation, and paper revision. KDB played a role in data collection, data interpretation, and paper revision. OE assisted with the study design, data interpretation, and paper revision. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- GreenbergerPAChapter 18: Allergic bronchopulmonary aspergillosisAllergy Asthma Proc201233Suppl 1S61S63
- KnutsenAPImmunopathology and immunogenetics of allergic bronchopulmonary aspergillosisJ Allergy (Cairo)2011201178598321966295
- SkovMMcKayKKochCCooperPJPrevalence of allergic bronchopulmonary aspergillosis in cystic fibrosis in an area with a high frequency of atopyRespir Med200599788789315939251
- de VrankrijkerAMvan der EntCKvan BerkhoutFTAspergillus fumigatus colonization in cystic fibrosis: implications for lung function?Clin Microbiol Infect20111791381138621087348
- MillaCEWielinskiCLRegelmannWEClinical significance of the recovery of Aspergillus species from the respiratory secretions of cystic fibrosis patientsPediatr Pulmonol19962116108776259
- MaturuVNAgarwalRPrevalence of Aspergillus sensitization and allergic bronchopulmonary aspergillosis in cystic fibrosis: systematic review and meta-analysisClin Exp Allergy201545121765177826177981
- PattersonKStrekMEAllergic bronchopulmonary aspergillosisProc Am Thorac Soc20107323724420463254
- HoganCDenningDWAllergic bronchopulmonary aspergillosis and related allergic syndromesSemin Respir Crit Care Med201132668269222167396
- JubinVRanqueSStremler Le BelNSarlesJDubusJCRisk factors for Aspergillus colonization and allergic bronchopulmonary Aspergillosis in children with cystic fibrosisPediatr Pulmonol201045876477120597074
- RitzNAmmannRACasaulta AebischerCSchoeni-AffolterFSchoeniMHRisk factors for allergic bronchopulmonary aspergillosis and sensitisation to Aspergillus fumigatus in patients with cystic fibrosisEur J Pediatr2005164957758215926067
- MastellaGRainisioMHarmsHKAllergic bronchopulmonary aspergillosis in cystic fibrosis. A European epidemiological study. Epidemiologic Registry of Cystic FibrosisEur Respir J200016346447111028661
- KraemerRDeloseaNBallinariPGallatiSCrameriREffect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosisAm J Respir Crit Care Med2006174111211122016959918
- GreenbergerPABushRKDemainJGLuongASlavinRGKnutsenAPAllergic bronchopulmonary aspergillosisJ Allergy Clin Immunol Pract20142670370825439360
- LinHBoeselKMGriffithDTOmalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophilsJ Allergy Clin Immunol2004113229730214767445
- TanouKZintzarasEKaditisAGOmalizumab therapy for allergic bronchopulmonary aspergillosis in children with cystic fibrosis: a synthesis of published evidencePediatr Pulmonol201449550350724167019
- LehmannSPfannenstielCFriedrichsFKrögerKWagnerNTenbrockKOmalizumab: a new treatment option for allergic bronchopulmonary aspergillosis in patients with cystic fibrosisTher Adv Respir Dis20148514114925150265
- VoskampALGillmanASymonsKClinical efficacy and immunologic effects of omalizumab in allergic bronchopulmonary aspergillosisJ Allergy Clin Immunol Pract20153219219925640470
- WongRWongMRobinsonPDFitzgeraldDAOmalizumab in the management of steroid dependent Allergic Bronchopulmonary Aspergillosis (ABPA) complicating cystic fibrosisPaediatr Respir Rev20131412224
- van der EntCKHoekstraHRijkersGTSuccessful treatment of allergic bronchopulmonary aspergillosis with recombinant anti-IgE antibodyThorax200762327627717329558
- JatKRWaliaDKKhairwaAAnti-IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosisCochrane Database Syst Rev201511CD010288
- Nové-JosserandRGrardSAuzouLCase series of omalizumab for allergic bronchopulmonary aspergillosis in cystic fibrosis patientsPediatr Pulmonol201752219019727717223
- LiJXFanLCLiMHCaoWJXuJFBeneficial effects of omalizumab therapy in allergic bronchopulmonary aspergillosis: a synthesis review of published literatureRespir Med2017122334227993289
- VegetoECianaPMaggiAEstrogen and inflammation: hormone generous action spreads to the brainMol Psychiatry20027323623811920150