Abstract
Anaphylaxis is the most serious of all allergic reactions and can be fatal. The diagnosis is frequently delayed, and misdiagnosis often occurs with asthma or urticaria. Biomarkers such as tryptase are not routinely checked, and appropriate treatment with epinephrine is not administered in a majority of cases, increasing the risk of poor outcomes. The objective of this review is to provide a better understanding of the pathophysiology of anaphylaxis with a description of phenotypes, endotypes, and biomarkers available in both the clinical and research settings. Expanding knowledge with regard to the presentation, causes, and triggers for anaphylaxis among health care providers will improve its diagnosis and management, increase patient safety, and decrease morbidity and mortality.
Defining anaphylaxis: history and consensus
In 1839, François Magendie was the first to describe the phenomenon of anaphylaxis experimentally when he found that rabbits that were able to tolerate a single injection of egg albumin often died after the second or third injection. He assumed these reactions were “toxic” because the injected albumin in these animals acted as a poison.Citation1 In 1902, Charles Richet and Paul Portier coined the term anaphylaxis from ana (against) and phylaxia (protection) in Greek for the “property that has a poison to lower immunity rather than reinforce it.”Citation2 Current expert consensus has defined anaphylaxis as a serious allergic reaction that is rapid in onset and can be fatal.Citation3,Citation4 The diagnosis is based on three possible clinical scenarios:
Sudden onset of an illness (minutes to hours) with involvement of the skin, mucosal tissue (or both), and at least one of the following: a) respiratory compromise and b) reduced blood pressure or symptoms of end-organ dysfunction;
Two or more of the following that occur after exposure to a likely allergen or other triggers (minutes to several hours): skin/mucosal symptoms and signs, respiratory compromise, reduced blood pressure or associated symptoms, and/or gastrointestinal symptoms (crampy abdominal pain or vomiting).
Reduced blood pressure after exposure to a known allergen (minutes to hours).Citation5
Critically, anaphylaxis involves at least two organ systems or sudden changes in vital signs. Skin and mucosal changes are usually but not always present, whereas hypotension and shock features are not mandatory for the diagnosis.Citation6,Citation7
Anaphylactoid reactions are clinically indistinguishable from anaphylaxis, with no demonstrable involvement of immunoglobulin E (IgE), and the term is no longer used, mainly to avoid unnecessary delays in diagnosis and treatment.Citation3,Citation8,Citation9
Despite advances in the field of allergy, the symptoms of anaphylaxis continue to be under-recognized, diagnosis is often missed, treatment is often delayed (including the lack of epinephrine use), and the underlying causes are under-investigated worldwide.Citation5,Citation7,Citation10
In 2003, to homogenize the nomenclature of allergy worldwide, the World Allergy Organization (WAO) proposed two classifications of anaphylaxis on the basis of the pathophysiological mechanism involved in the reaction. The term allergic anaphylaxis denotes reactions mediated by an immunologic mechanism – for example, IgE-, Ig, or an immune complex complement-related (corresponding to the classic hypersensitivity reactions [HSRs] described by Gell and Coombs) pathways. The term non-allergic anaphylaxis denotes reactions mediated by other mechanisms (eg, direct activation by bradykinin or complement), which are usually triggered by agents or events that induce sudden mast cell or basophil activation.Citation11
Between 2004 and 2005, several organizations came together to update the definition and emphasize the use of epinephrine as a first-line treatment for anaphylaxis.Citation6,Citation12 Several studies were carried out to validate the criteria and to expand the scientific evidence with regard to the pathophysiology, triggers, and clinical management of anaphylaxis. The WAO provided a consensus document for the diagnosis and treatment of anaphylaxis. This report has been reviewed and updated to incorporate changes based on updated scientific evidence.Citation3,Citation4,Citation8,Citation9
Recently, a consensus document between the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology was published, which summarizes current knowledge in the field of allergy. They proposed a new approach based on precision medicine through phenotypes, which is associated with specific mechanisms that are defined as endotypes and the associated diagnostic biomarkers in food and drug allergies and anaphylaxis.Citation13 This new classification encompasses the classic HSRs described by Gell and Coombs as well as reactions outside the classification.Citation14
Phenotypes, endotypes, and biomarkers of anaphylaxis
Phenotypes are defined by clinical presentation, and endotypes refer to the cellular and molecular mechanisms of the HSRs defined by the diagnostic biomarkers (skin testing, tryptase, IgE, interleukin [IL]-6, and others).
Phenotypes of anaphylaxis are classified, by their clinical presentation, into Type I reactions, cytokine-release reactions (CRRs), mixed reactions, and, finally, bradykinin- and complement-like reactions. The corresponding endotypes underlying these phenotypes include IgE- and non–IgE-mediated mechanisms, cytokine-mediated mechanisms, mixed processes, and direct activation of immune cells by either complement or bradykininCitation14 ().
Figure 1 Pathways of anaphylaxis.
Abbreviations: IgE, immunoglobulin E; IgG, immunoglobulin G; IL, interleukin; PAF, platelet-activating factor; TNF-α, tumor necrosis factor alpha.
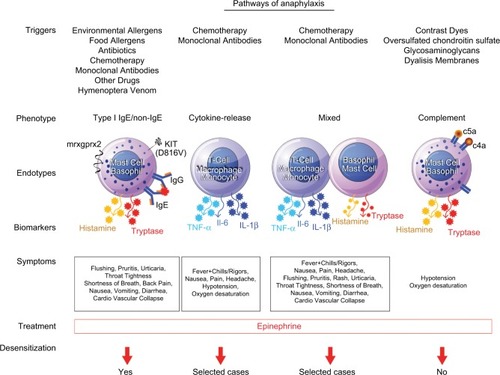
Type I
IgE-mediated anaphylaxis is the major mechanism underlying allergic anaphylaxis.Citation1 After exposure to the allergen, a series of signals trigger the production of allergen-specific IgE by B cells (sensitization phenomenon). In subsequent exposures, the antigen–allergen-specific IgE complex binds to the Fc-epsilon-RI receptor on mast cells and/or basophils and, with adequate signaling, activates and degranulates these cells, thereby releasing preformed mediators, enzymes, and cytokines and facilitating the synthesis of de novo inflammatory mediators (eg, tryptase, histamine, leukotrienes, prostaglandins, platelet-activating factor [PAF], cytokines).Citation15,Citation16
The mediators cause allergic symptoms by directly acting on tissues. The reaction is propagated by recruiting and activating additional inflammatory cells – particularly eosinophils, which release more mediators, including lipid-derived mediators such as prostaglandin D2 and cysteinyl leukotrienes.Citation16
In addition to the classical pathway mediated by IgE, other possible pathways have been described in animal models that are difficult to explore in humans.Citation17 One of these alternative pathways is similar to the IgE-mediated pathway, but involves IgG antibodies. IgG-mediated reactions are mediated by IgG complexes that cross-link to the macrophage low-affinity receptor (FcgRIII) thus stimulating PAF (rather than histamine) release.Citation18
PAF causes platelet aggregation and release of the potent vasoconstrictor thromboxane A2 and serotonin; acts directly on vascular endothelial cells to increase vascular permeability; decreases cardiac output, which can produce hypotension and cardiac dysfunctions; and increases smooth muscle contraction in the airways, gut, and uterus, among other effects.Citation19,Citation20
Although IgG-dependent anaphylaxis has not been demonstrated in humans, it has been hypothesized that IgG antibodies can mediate systemic anaphylaxis if there are large numbers of both IgG and antigen present.Citation21 IgG receptors are capable of activating macrophages and neutrophils to secrete PAF and activate mast cells in vitro, which may contribute to human anaphylaxis.Citation20–Citation23 Chimeric IgG monoclonal antibodies (mAbs), such as rituximab, have been shown to induce anaphylaxis even in the absence of IgE, suggesting IgG-dependent anaphylaxis.Citation24,Citation25
Recent reports with regard to the direct activation of mast cells, independent of those mediated by IgE, indicate that the human G-protein–coupled receptor – MRGPRX2 – may be the receptor for many drugs and cationic proteins, such as quinolone antibiotics (eg, ciprofloxacin, levofloxacin), general anesthetics such as atracuronium and rocuronium, icatibant, and other drugs with tetrahydroisoquinoline (THIQ) motifs.Citation26–Citation29
The endotype for IgE-mediated reactions is mast cell and basophil mediator release that causes flushing, pruritus, hives, angioedema, shortness of breath, wheezing, nausea, vomiting, diarrhea, hypotension, oxygen desaturation, and cardiovascular collapse along with other symptoms.Citation16,Citation30 The common triggers for these reactions include foods, drugs, latex, Hymenoptera venoms, and environmental allergens.Citation3,Citation8–Citation10,Citation31,Citation32 There are important geographic and age-related variations between countries; however, the most common food allergens are peanut, milk, eggs, nuts, shellfish, fruits and vegetables;Citation33 antibiotics such as β-lactams, nonsteroidal anti-inflammatory drugs (NSAIDs), chemotherapeutic agents such as platins and taxanes, chimeric humanized human mAbs, general anesthetics, and immunotherapy allergens are other common allergens both in children and adults.Citation34,Citation35
CRRs
The CRR phenotype is caused by the release of proinflammatory mediators such as tumor necrosis factor alpha (TNF-α), IL-1B, and IL-6, and their target cells (endotype) include monocytes, macrophages, mast cells, and other immune cells with the Fc gamma receptor (FcgR) – an essential participant in many immune system effector functions, including the release of inflammatory mediators and antibody-dependent cellular cytotoxicity.
Triggers for these reactions include chimeric, humanized, and human mAbs and chemotherapeutic agents, including oxaliplatin. These drugs have not only been used to treat neoplastic, autoimmune, and inflammatory diseases but also to treat allergic disorders including allergic asthma, eosinophilic asthma, and chronic urticaria.Citation36,Citation37 HSRs to biologic agents are less common than standard infusion reactions, and they vary based on the biological agents involved.Citation25 Phenotypic symptoms include chills, fever, and pain; these respond to ibuprofen and fluids and have been clinically correlated with IL-6.Citation38 CRRs are typically not as severe as cytokine storm reactions.
Cytokine storm reactions are acute, severe, and potentially lethal systemic complications due to the production of large quantities of cytokines and chemokines, which play a pathological role in the development of systemic symptoms.Citation39,Citation40 IL-6 and other inflammatory cytokines such as IL-8, TNF-α, interferon gamma (IFN-γ), and IL-1β induce the inactivation of cadherin, which mediate cell adhesion, leading to vascular leakage by increased capillary permeability; moreover, this induces the formation of tissue factor (thromboplastin) on the cell surface of monocytes, with subsequent activation of the extrinsic coagulation pathway.Citation41,Citation42 The effects of inflammatory cytokines play a pathological role in the development of pain, tissue hypoxia, hypotension, myocardial dysfunction, and, eventually, disseminated intravascular coagulation (DIC) and multiorgan dysfunction. IL-6 is an excellent biomarker of cytokine storm reactions because of its correlation with the severity of the reaction and its longevity in blood serum.Citation40
This phenotype is characterized by chills, fever, and generalized malaise followed by hypotension, desaturation, and cardiovascular collapse.Citation14,Citation25,Citation37 Premedication with anti-inflammatory COX-1 inhibitors and corticosteroids can decrease the intensity of these symptoms but does not protect from severe reactions.Citation14
Mixed reactions (Type I/CRRs)
Mixed reactions occur as a mixture of Type I and CRR phenotypes and, typically, are observed during chemotherapy and/or mAbs HSR, wherein symptoms of IgE-mediated reactions such as redness, pruritus, urticaria, angioedema, difficulty breathing, wheezing, nausea, vomiting, diarrhea, hypotension, desaturation, cardiovascular collapse, and life-threatening anaphylaxis – occurring secondary to the release of mast cell/basophil mediators (tryptase, histamine, leukotrienes, and prostaglandins) – overlap with symptoms secondary to the release of proinflammatory cytokines and chemokines (IL-1β, IL-6, and TNF-α) such as chills, fever, malaise, hypotension, desaturation, and cardiovascular collapse, thereby making it impossible to differentiate between mechanisms.
Complement/bradykinin-like reactions
Complement reactions involve direct activation of mast cells and other immune cells through complement activation as well as direct and indirect activation of the intrinsic coagulation pathway.Citation16,Citation22,Citation43 Immune complexes can activate the complement system, generating anaphylatoxins such as C3a and C5a, which can bind to complement receptors resulting in release of histamine, leukotrienes, and prostaglandins that can induce flushing, hives, hypoxia, vasodilation, and hypotension.Citation43,Citation44 This mechanism has been described with drugs such as vancomycin,Citation45 contrast media,Citation46 dialysis membranes, and infusions of drugs that are suspended in certain lipid vehicles such as Cremophor EL, polysorbate 80, and polyethylene glycol.Citation17,Citation47 Notably, reports have also suggested that complement can have an important role in vespid-induced anaphylaxis, exacerbating the reaction due to the activation of complement by proteases present in the venom and adding to the IgE-mediated reaction.Citation44,Citation48
The molecular pathway of bradykinin reactions has been elucidated in animal models and involves an increase in heparin and Factor XII-driven contact system that results in the production of bradykinin and ultimately accounts for the increased vascular permeability (clinically, hypotension and desaturation).Citation22 These symptoms have been associated with contamination of heparin with oversulfated chondroitin sulfate.Citation49
Biomarkers for diagnosis of anaphylaxis
Skin tests (STs)
A careful history is important for the identification of culprit allergens. The ST is a highly specific test for Type I reactions, which is used to identify sensitization for numerous allergens including airborne, food-related, drugs (including platins), β-lactams, general anesthetics, and Hymenoptera venom triggers.Citation31,Citation50
Skin testing is considered safe for patients with a history of anaphylaxis and mastocytosis, provided comorbidities such as asthma are controlled. Medications such as β-blockers and angiotensin-converting enzyme (ACE) inhibitors may introduce greater risk for skin testing in case of a serious reaction; therefore, they should be discontinued before testing.Citation51 Patients with CRRs and complement-activation reactions are likely to have negative ST results due to non-IgE mechanisms; however, the use of ST is still useful to identity patients who may convert from CRR to Type I reactions or those with mixed reactions.Citation38,Citation52
There should be a 4- to 6-week gap between anaphylactic reaction and ST due to the temporary loss of cutaneous activity following anaphylaxis; full restoration may take up to 6 weeks. However, due to treatment schedules, exceptions are made to expedite skin testing to 2 weeks post reaction, which should be sufficient to restore cutaneous reactivity; however, negative results may be inconclusive and demand further evaluations.Citation53 For drug allergy, skin testing should be undertaken after resolution of clinical symptoms and clearance of culprit drug and rescue medication from the circulation.Citation54 In case of Hymenoptera hypersensitivity, test reactivity may be falsely negative for approximately 6 weeks following such a reaction; therefore, STs should be performed later.Citation55
Both ST and antigen challenge tests are reliable in vivo tests. However, not all allergenic compounds are present in the current skin testing extracts; therefore, the gold standard to clear an allergy after negative skin testing is a challenge. Patients with a convincing history of anaphylaxis and evidence of sensitization to a specific food should not undergo oral food challenges due to the high risk of anaphylaxis. For those with an equivocal history, low or moderate evidence of sensitization, or both, might benefit from a physician-monitored incremental oral food challenge. A positive (failed) challenge provides a sound basis for continued avoidance of the food. A negative (passed) challenge allows introduction or reintroduction of the specific food into the patient’s diet.Citation56
For evaluation of penicillin allergy, skin testing of both major and minor determinants is required because patients are likely sensitized to minor determinants that are not currently commercially available in the US.Citation57,Citation58 For patients with a distant history of penicillin allergy and symptoms inconsistent with Type I reactions, a direct oral challenge with penicillin may be indicated.Citation59–Citation63 Reactions to beta-lactams can be by reactions to the beta-lactam ring or by reactions to its side-chain, so that there may be cross-reactivity dependent on each of these structures. It has been described patients capable of tolerating penicillin, who present anaphylactic reactions to other β-lactams that share the same side-chain, such as some aminopenicillins and cephalosporins.Citation64,Citation65 Efforts are currently underway to generate suitable ST reagents and establish the predictive value of cephalosporin STs.Citation66
The specificity and sensitivity of mAb skin testing have not been defined because the allergenic components/epitopes are not yet defined and non–IgE-mediated mechanisms are believed to play a role. For chimeric mAbs, such as rituximab and infliximab, mouse epitopes are thought to be involved in the allergic response, and ST results are positive in 60–70% of patients with Type I and mixed reactions for rituximab; however, only 50% of patients with infliximab-induced Type I reactions have positive ST results.Citation67 The negative predictive value for most mAbs is not known. Of 23 patients desensitized to trastuzumab, infliximab, or rituximab, 13 had positive ST results, although all had symptoms compatible with Type I or mixed reactions.Citation67 Another study showed that 58/106 patients treated with mAbs had STs, and 41% of those were positive. Per phenotype, ST was positive in 44% of Type I reactions, 11% of CRRs, and 54% of the mixed reactions. A positive ST was associated with greater severity of initial reaction. These results indicate the value of ST for mAbs as well as the need for continued research.Citation38
Serology and specific IgE
Many patients with allergic disorders (eg, atopic dermatitis, atopic asthma, allergic rhinitis) have elevated levels of total IgE.Citation68 This elevation is not specific and low levels cannot be used to exclude the presence of atopic disease, because patients can still have local production of allergen-specific IgE in the tissues, and total IgE by itself is rarely adequate to diagnose allergic disease.Citation69–Citation71
Measurement of allergen-specific IgE is guided by the results of skin and/or serum testing.Citation69 Specific IgE levels can be measured for food, environmental allergens, Hymenoptera venom, and drugs, including antibiotics and chemotherapy.Citation72,Citation73 They are not especially useful to identify and monitor food allergies because of their crude nature, lack of potency assessment, and variability among manufacturers and lots.Citation74 Furthermore, skin testing may not be indicated due to diffuse skin diseases, significant dermatographism, inability to wean off medications interfering with the testing, or use of an extract believed to have a high probability of inducing a systemic reaction in the subject to be tested.Citation69,Citation75
A poor correlation between the IgE titer with ST result and the risk of anaphylaxis has been shown in previous studies; therefore, there is no evidence that either diagnostic test could accurately predict a severe allergic reaction in children or adults.Citation76
Hymenoptera venom–specific IgE has high sensitivity and specificity, and components of the venoms are under recent evaluation, with a few components commercially available for clinical use. It has become apparent that patients treated with venom extracts may have severe anaphylaxis when re-stung in the field, which may be attributed to sensitization to minor determinants, such as Api m 10, which is absent in most current vaccines.Citation77,Citation78 Patients with specific IgE to Hymenoptera venoms who have been sensitized to major and minor determinants might not be protected after vaccination, and increasing the dose of venom has been recommended.Citation79 The most effective test to determine the effectiveness of immunotherapy is the sting challenge test with live Hymenoptera; however, its safety is controversial.Citation80–Citation82
Penicillin-specific IgE has low sensitivity and is currently reserved for patients with near-fatal anaphylaxis, in whom skin testing is deemed unsafe.Citation83 Measurement of specific IgE to other β-lactams, such as cephalosporins, has been used to assess cross-reactivity, but its clinical value has not been defined.Citation84 Specific platin IgE has been shown to have lower sensitivity than skin testing, but has a higher specificity.Citation85–Citation87 A major advantage of specific IgE for platins is the ability to detect IgE antibodies shortly after the reactions and without the need to wait several weeks to determine ST reactivity, which can delay treatment to chemotherapy.Citation88 Another advantage of specific IgE is that it can detect cross-reactivity with other similar drugs, for example, patients sensitized to oxaliplatin had specific IgE for other platins (carboplatin and cisplatin) without previous exposure.Citation89
Component-resolved diagnostics
Advances in precision medicine have made it possible to identify and characterize some molecules present in the allergenic extracts, and this has resulted in component-resolved diagnosis which has changed our understanding of sensitization profiles and cross-reactivityCitation90 as well as improved our ability to identify specific clinical phenotypes. In addition, it has allowed us to determine the relative risk of the severity of reactions in specific cases, such as soy, peanut, and hazelnutCitation91 allergies, as well as predict the severity of allergic reactionCitation92 – as in the case of Ara h 1, 2, and 3 associated with peanut-induced anaphylaxis, in contrast to patients with specific IgE to Ara h 8, 9, and 10 who might have oral allergy syndrome caused by cross-reactivity with tree pollen allergens and might not be at risk for anaphylaxis.Citation93,Citation94 Geographic variations have been seen – for instance, in the Mediterranean area, systemic reactions to hazelnut are generally mediated by Cor a 8, a lipid transfer protein (nsLTP),Citation95–Citation97 whereas reports from the US and Northern Europe have associated sensitization to Cor a 9 (an 11S globulin) and Cor a 14 (a 2S albumin) with severe hazelnut allergy in children.Citation98,Citation99 However, more studies are needed to validate the predictive values across different races, sex, and ethnic backgrounds.
Studies show that component-resolved diagnostics (CRD) offered greater specificity, but decreased sensitivity, in foods; therefore, its use is complementary and should not be used alone to rule out food allergy.Citation100–Citation102
In relation to pollen allergies, the utility of CRD focuses on the correct identification of species-specific markers (Bet v 1 can identify individuals allergic to the Betulaceae family; Ole e 1, those allergic to the Oleaceae family; Cup a 1, those allergic to the Cupressaceae family; and Pla a 1, those allergic to the Platanaceae family), especially in patients with multiple sensitization patterns secondary to cross-reactivity due to the presence of panallergens (polcalcin, profiling, or nsLTP).Citation90 In cases of multiple pollen sensitization, responses are better to allergen-specific immunotherapy when it is prescribed on the basis of species-specific molecular markers with clinical relevance for each patient.Citation103
In case of Hymenoptera allergy, CRD has become a very useful tool to distinguish between genuine sensitization and cross reactivity, because allergen sources contain potentially cross-reactive allergens between honey bee and wasp, such as Api m 2, Ves v 2 (hyaluronidases), Api m 5, Ves v 3 (dipeti-dylpeptidases), Api m 12, Ves v 6 (vitellogenins), and marker allergens that are specific for honey bee venom (Api m 1, Api m 3, Api m 4, and Api m 10) or yellow jacket venom (Ves v 1 and Ves v 5); these markers allow discrimination in clinical situations in which patients have a multiple sensitization profile.Citation90
Basophil activation test
Basophils and mast cells originate from a common progenitor cell in bone marrow and share similar phenotypic/biochemical characteristics: they express high-affinity IgE receptors and contain special cytoplasmic granules. Basophils can be easily assessed because they are circulating granulocytes that have responded to allergic stimuli by migrating and accumulating at sites of allergic inflammation. Mast cells, unlike basophils, leave the bone marrow immature and complete their maturation in peripheral tissues, where they ultimately reside. Therefore, evaluation of human mast cells is limited to biopsy specimens from specific tissues.Citation104,Citation105
The basophil activation test (BAT) is a blood test used to diagnose IgE-mediated reactions to allergens such as food, environmental allergens, Hymenoptera venom, or drugs. BAT is a flow-cytometry–based functional assay that detects upregulation of the basophil activation surface markers CD63 and CD203c following exposure to an antigenic stimulus. Because both mast cells and basophils are sensitized with the same repertoire of IgE, BAT is considered a true reflection of sensitized mast cells.Citation14
Currently, BAT is commercially available but has not been standardized or approved by the US Food and Drug Administration.
BAT has had success in evaluating immediate reactions to β-lactams, muscle relaxants, other drugs, as well as the diagnosis and monitoring of Hymenoptera venom allergies.Citation13,Citation106–Citation108 BAT is not recommended for patients with a history of multiple reactions, NSAID hypersensitivity, or those with food allergies because of their increased histamine release at baseline.Citation105
In a study with 15 patients allergic to platinum-containing compounds who were undergoing drug desensitization, BAT provided reliable information with regard to sensitization and upregulation of CD203c expression. Furthermore, reaction severity has been correlated with a higher expression of CD63, demonstrating that this technique can be used as a biomarker for desensitization.Citation109
Tryptase
Tryptase is a serine protease and the most abundant secretory mediator formed and stored in human mast cells and basophils. The in vivo biologic activities of mature tryptase remain uncertain, but in vitro activities include generation of complement anaphylatoxins, inactivation of fibrinogen, and stimulation of a variety of different cell types. In addition to its release, it amplifies the allergic response.
The rise of tryptase levels can be detected in serum 30 minutes after the initial symptoms of anaphylaxis and peaks 1–2 hours post initiation of the reaction. Tryptase elevation is transient and typically resolves within 24–48 hours. Commercial immunoassays allow for detection of total (baseline release, reflecting mast cell and basophil burden) and mature tryptase (released only at the time of activation); however, they do not discriminate between the two. Increases above the normal range of 11.4 ng/mL are indicative of either acute mast cell/basophil activation or increase in total mast cell number;Citation110 therefore, a baseline tryptase level is required 2 weeks post anaphylaxis. Tryptase levels ≥2 ng/mL + 1.2× baseline are considered significantly increased for patients with a low baseline tryptase.Citation111,Citation112
Total tryptase levels can be elevated (>20 ng/mL) in most patients with systemic mastocytosis (SM) – a clonal disorder associated with mutation of the membrane tyrosine kinase KIT; however, it is seen in other conditions such as hematologic disorders (acute myelocytic leukemia, myelodysplastic syndromes), immunologic disorders (hypereosinophilic syndrome), severe renal failure, or inherited variations such as familial tryptasemia – a recently characterized disease which is associated with multisystem complaints including cutaneous flushing and pruritus, dysautonomia, functional gastrointestinal symptoms, chronic pain, and connective tissue abnormalities, including joint hypermobility, due to the expression of more than two α-tryptase genes.Citation113,Citation114
Tryptase specificity is high whereas its sensitivity is low due to its release from different mast cell and basophil subsets, depending on the trigger. Tryptase levels are lower in mucosal mast cells than in cutaneous and perivascular mast cells, and anaphylactic reactions to intravenous drugs can elicit greater and more persistent increases than oral triggers, such as foods.
If anaphylaxis is suspected, blood sampling for tryptase measurement should be done between the first 30 minutes and 3 hours of reaction initiation to capture the maximum tryptase value.Citation115 Elevations correlate with hypotension and support the diagnosis of anaphylaxis, although normal levels do not exclude the diagnosis.Citation116,Citation117
Other inflammatory mediators
Other mast cell/basophil mediators can be released during anaphylaxis including histamine, which is the only preformed granule mediator of these cells with vasoactive activity and direct spasmogenic action on the smooth muscle.
Elevation in plasma/urine histamine is consistent with anaphylaxis, although it is not exclusive, because other conditions can also have elevated levels without mast cell/basophil activation.Citation115 Both histamine and its metabolite methylhistamine can be measured in 24-hour urine collection; however, the sensitivity is low because of the difficulties in timing 24-hour urine collections to symptom onset.
The contribution of PAF to anaphylaxis, which has been studied in mice, suggests a synergistic effect between histamine and PAF.Citation16 A recent human study indicated an inverse correlation between serum PAF and acetylhydrolase levels (the enzyme that metabolizes and inactivates PAF) with the severity of anaphylaxis: low enzymatic levels were associated with high PAF levels, severe hypotension, and fatal and near-fatal anaphylaxis.Citation118
Levels of other serum inflammatory mediators such as TNF-α, IL-6, and IL-1b can be elevated in patients with CRRs and anaphylaxis, but their sensitivity or specificity have not yet been validated.Citation38 These inflammatory mediators provide insight into the phenoendotype of reactions and can help guide recommendations for desensitization protocols and premedications.Citation40
Prostaglandin D2 and leukotrienes E4 and C4 can be measured in 24-hour urine collection. Despite high specificity, the sensitivity might be low due to the difficulties in timing 24-hour urine collections to symptom onset. However, prostaglandin D2 levels cannot be useful in patients receiving cyclooxygenase inhibitors.
Other mast cell proteases, such as chymase and carboxypeptidase, have been detected during anaphylaxis, although no commercial assays are available to date.
Patients at risk
Food-induced anaphylaxis and food-triggered exercise-induced anaphylaxis
Predictors for anaphylaxis risk have not been elucidated; one of the most important known risk factors associated with fatal anaphylaxis is asthma.Citation119–Citation121 Food-induced anaphylaxis affects both sexes equally, and fatal food-induced anaphylaxis occurs most often in adolescents and young adults who are unaware of their allergy, with the largest culprit being tree nuts.Citation122
Anaphylaxis can be induced by many foods, the most common including cow’s milk, hen’s eggs, peanuts, tree nuts, fish, shellfish, soy and wheat, with variations depending on geography and local dietary patterns. Food-induced anaphylaxis is most common in children, but can occur in people of any age.Citation123
In a study of 1,094 patients with peanut and tree nut allergy conducted in the UK, severe atopic disease was associated with the most severe reactions. Severe pharyngeal edema was associated with severe rhinitis; life-threatening bronchospasm was associated with severe asthma; and altered mental status was associated with severe eczema. These associations were independent of sex and age, although adults were 9 times more likely to have severe reactions than children and, of 122 patients in whom ACE and aminopeptidase levels were measured, patients with low levels had a greater number of severe reactions, highlighting the role of bradykinins in food-induced anaphylaxis as well as the need to discontinue ACE inhibitors in patients with food allergies.Citation124
In a systematic review and meta-analysis that involved data from North America, Europe, and Australia, the estimated incidence of fatal food anaphylaxis in a food-allergic individual was 1.81 (95% CI 0.94–3.45) per million person-years, confirming that the incidence of fatal food-related anaphylaxis had little impact on the overall mortality risk in this group.Citation125
In an Australian study, 4,453 infants with eczema were evaluated to characterize their risk of challenge-proven food allergy. They found that eczema was a strong risk factor for IgE-mediated food allergy, and the severity of eczema was associated with greater risk of food-induced allergies. They hypothesized that increased cutaneous exposure to food allergens in infants with weakened skin-barrier function may increase the risk of food sensitization.Citation126
Recently, a multicenter study in the US evaluated allergic reactions in oral challenges for individuals with food allergies. Of the 6,377 oral provocations undertaken, the rate of allergic reactions was 14% (95% CI 13–16%) and the pooled estimate of anaphylaxis was 2% (95% CI 1–3%). Peanut was the most frequently involved allergenic food in the Northeast, Midwest, and West, whereas egg was the most frequent in the South. This showed that the likelihood of anaphylactic reaction in allergic individuals is low and geographical variations between trigger foods are present.Citation127
Food-dependent exercise-induced anaphylaxis (FDEIA) is a disorder in which symptoms develop only if exertion takes place within a few hours of eating certain food(s) to which the patient is sensitized.Citation128,Citation129 This has been reported in patients of all ages, although most cases are described in adolescents and young adults, with wheat and omega-5 gliadin identified as the dominant triggers. The mechanisms are not fully understood; however, elimination of wheat before exercise reduces reactions.Citation130–Citation132
More recently, food ingestion associated with other cofactors, such as alcohol or the menstrual period, was found to induce anaphylaxis, expanding the understanding of FDEIA as a syndrome in which exercise might not be necessary.Citation133
Mast cell activation disorders
Mast cell disorders are conditions in which mast cells are either increased in number (clonal mast cell disorders), hyperreactive (non-clonal mast cell disorders), or both ().Citation134
Figure 2 Mast cell activation syndrome.
Abbreviations: AHN, associated hematologic neoplasm; BM, bone marrow; EDS, Ehlers-Danlos Syndrome; GI, gastro-intestinal; IgE, immunoglobulin E; IgG, immunoglobulin G; IL, interleukin; MC, mast cell; MCAS, mast cell activation syndrome; MCL, mast cell leukemia; MMAS, monoclonal mast cell activation syndrome; NSAIDs, nonsteroidal anti-inflammatory drugs; PAF, platelet-activating factor; POTS, Postural Orthostatic Tachycardia Syndrome; RCM, radio contrast medium; SM, systematic mastocytosis; TMPE, telangiectasia macularis eruptiva perstans; TNF-α, tumor necrosis factor alpha; WHO, World Health Organization.
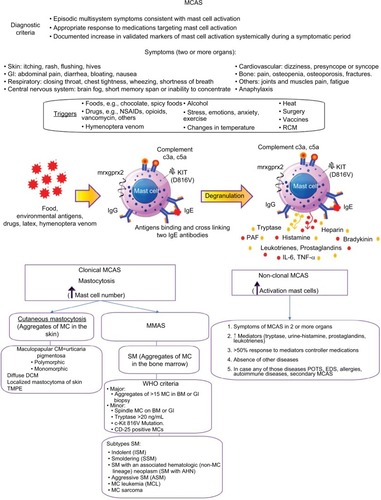
Mast cells activate and accumulate in tissues due to a gain-of-function mutation, commonly in the KIT receptor gene that codes a tyrosine kinase transmembrane receptor involved in the regulation of crucial mast cell functions such as differentiation, activation, and survival. Upon receiving a trigger, they release mediators inappropriately, interfering with normal function, causing a variety of localized and systemic symptoms.Citation134,Citation135
Mast cell activation syndrome (MCAS) applies to all disorders (including anaphylaxis) that appear as a consequence of the release of vasoactive mediators by activated mast cells. Based on previous consensus, MCAS can be classified into three categories:Citation112,Citation136
Primary MCAS, including SM and monoclonal MCAS (MMAS).
Secondary MCAS.
Idiopathic MCAS.
All of the criteria should be present to make a diagnosis of MCAS ().
Table 1 Proposed criteria for mast cell activation syndrome (all 3 must be present)
In primary MCAS (increased mast cells number), the presence of mast cell clones in the bone marrow of the affected individual must be demonstrated. In most patients (>95%) with SM and MMAS, mast cell clonality can be established by detecting KIT receptor-activating mutations. On the other hand, mast cells in secondary and idiopathic MCAS (increased activation) are normal, and symptoms of mast cell activation are related to other, secondary underlying conditions that activate these cells (allergies, neoplasms, inflammatory or autoimmune diseases, or unknown factors).Citation137
Overall, 30% of patients with mastocytosis can present with anaphylaxis with known (including hymenoptera venom–induced anaphylaxis) or unknown triggers.Citation138–Citation140 Due to the lack of cutaneous manifestations (flushing, maculopapular rash in the form of urticaria pigmentosa, etc.), the diagnosis is often missed or delayed.Citation141 Patients with syncope, hypotension, or cardiovascular collapse with severe anaphylaxis after Hymenoptera sting even with normal baseline tryptase levels should be evaluated for the KIT D816V mutation, which is present in >95% of patients with SM. In patients with positive KIT D816V mutation, a bone marrow biopsy is recommended.Citation142–Citation145 A tryptase level >20 ng/mL at baseline 4–6 weeks after an anaphylactic event is a minor criterion of SM and an indication for bone marrow biopsy.Citation143 Other criteria for the diagnosis of SM are shown in . Systemic symptoms of mast cell mediator release such as osteoporosis, bone fractures, and gastrointestinal symptoms, including bloating and diarrhea, can be present for many years and are typically unrecognized.Citation14
Table 2 WHO criteria for SM
Mastocytosis and MCAS can be identified as a cause of idiopathic anaphylaxis due to the fact that they can be controlled with tyrosine-kinase inhibitors.Citation26
Treatment with venom immunotherapy (VIT) – if an IgE mechanism can be demonstrated, with or without omalizumab – provides a dramatic increase in patient safety when re-stung.Citation143,Citation146,Citation147 Although there is no increase in the prevalence of drug allergy in patients with clonal mast cell disorders,Citation148 reactions to NSAIDs are greater in these patients and can present as anaphylaxis.Citation149
Drug allergy
The increase in anaphylaxis to drugs in the last 20 years is thought to be due to the increase in the variety and use of drugs. Some of these drugs have relatively high rates of HSRs including antibiotics, chemotherapeutic agents, mAbs, replacement factors, and biological agents. The pattern of presentation for HSRs continues to evolve, and new classifications have been created to encompass them.Citation14,Citation36,Citation38
Medications are typically cited as among the most common triggers of fatal HSRs without age restriction and have been documented frequently in both pediatric and elderly populations.Citation150 According to the European Anaphylaxis Registry, drugs were the third most frequent elicitor of anaphylaxis after food and insect venoms in the pediatric age group.Citation151 Recently, a study from Turkey reported that 84 (15%) of 561 children were diagnosed with drug allergy; the most common drugs included β-lactam antibiotics (33%; penicillin, amoxicillin–clavulanic acid, ceftriaxone, cefixime, cefaclor, and piperacillin–tazobactam), NSAIDs (25%), chemotherapeutics (19%; asparaginase, oxaliplatin, etoposide, and docetaxel), biologic agents and enzymes (12%; rituximab, eculizumab, and enzymes like galsulfase and idursulfase), anesthetic and neuromuscular blocking agents (5%; propofol, midazolam, and rocuronium), and others (6%; ranitidine, triptorelin, cyclopentolate, iopromide, and Factor 9).Citation34
On the other hand, it has been reported that older patients have both – higher rates of drug-induced anaphylaxis and an increased risk of severe reactions.Citation152–Citation154
Antibiotics are still the most common drug to cause anaphylaxis. IgE-mediated mechanisms are most commonly implicated in β-lactam allergy,Citation63 and certain populations, such as patients with cystic fibrosis and patients treated with multiple courses of antibiotics, are at high risk.Citation155
Patients presenting with anaphylaxis during anesthesia or after quinolone use for whom an IgE mechanism cannot be demonstrated by skin testing may have either increased protein levels or functional MRGPRX2 receptors that can bind and activate mast cells without IgE mediation; however, the participation of MRGPRX2 receptors has not been confirmed in human subjects.Citation27–Citation29
Due to the increased use of chemotherapies and targeted therapies, including mAbs, drug HSRs and anaphylaxis have increased dramatically worldwide, preventing the use of first-line therapies and affecting patient survival and quality of life.Citation156 Some of the most immunogenic chemotherapies are carboplatin, cisplatin, oxaliplatin, and taxanes for ovarian, lung, breast, colon, and prostate cancers.Citation157
Platins can elicit Type I reactions and typically require repeated exposures.Citation85,Citation88,Citation158 Oxaliplatin is an exception to this, and first lifetime exposure reactions have been documented as well as CRRs and mixed reactions.Citation159 Taxanes can induce similar reactions with atypical symptoms, such as back pain, through IgE- and non–IgE-mediated mechanisms. Cremophor and Polysorbate 80, used as diluents, are thought to be implicated in non–IgE-mediated reactions, which can occur at first or second lifetime exposures.Citation156,Citation158,Citation160 In a recent study, STs conducted on 145/164 patients treated with taxane hypersensitivity were used to establish HSR risk and treatment protocol.Citation161
Furthermore, mAbs are used in the treatment of neoplastic, autoimmune, and inflammatory diseases, and their clinical applications are becoming broader. Targets for these drugs include CD20, HER-2, epidermal growth factor receptor, IL-6 receptor, TNF-a, CD30, vascular endothelial growth factor A, programmed cell death protein 1/programmed death ligand L, IL-4, IL-5, IL-13, and IgE, among others.Citation162 Although chimeric mAbs, such as rituximab and infliximab, present with the highest incidence of reaction, humanized and human mAbs – although less immunogenic – are engineered with mouse glycosylation patterns, which can result in allergenic determinants. Even with fully human mAbs, such as adalimumab and ofatumumab, anaphylaxis has been reported.Citation18,Citation163 Clinical presentation included a spectrum of atypical symptoms such as CRRs, infusion-related reactions, and mixed reactions that present with chills, fever, and generalized malaise followed by hypotension, desaturation, and cardiovascular collapse.Citation106,Citation164 These reactions can occur at first exposure but have also been seen after several exposures.Citation67,Citation163–Citation165 Mixed reactions occur when these symptoms are associated with Type I symptoms and mast cells/basophils involvement or positive ST results, demonstrating an IgE mechanism in addition to the release of IL-6.Citation89
Anaphylaxis at first exposure has been observed with cetuximab in patients with preformed carbohydrate galactose-a-1,3-galactose IgE antibodies due to tick exposure (Amblyomma americanum).Citation166 Galactose-a-1,3-galactose is expressed on non-primate mammalian proteins and is present on the cetuximab heavy chain.Citation167
Patients under treatment with β-blockers and ACE inhibitors
Evidence for β-blockers and ACE inhibitor treatment with anaphylaxis is sporadic and limited to retrospective data. There are no prospective trials to explain the relationship between these drugs and anaphylaxis.Citation168 Theoretically, both β-blockers and ACE inhibitors may affect the patient’s likelihood of developing anaphylaxis and their ability to respond to treatment.Citation169–Citation171
The relationship between antihypertensive medication and the severity of anaphylaxis was associated with the conclusion that β-blocker, ACE-inhibitor, diuretic, and antihypertensive medication used in combination was significantly associated with grade 3 anaphylaxis (three or more organ/system involvement; OR, 2.8; 95% CI, 1.5–5.2; P = 0.0008), showing that the use of these drugs increased the severity of anaphylaxis.Citation172
A telephonic survey conducted on German patients with a history of severe anaphylaxis had identified monotherapy with β-blockers or ACE inhibitors as a risk factor for severe anaphylaxis, which was more pronounced with combination therapy. In mouse models, the role of potential cofactors on anaphylactic responses confirmed that β-blocker and ACE inhibitors have modest anaphylaxis-promoting activities as single substances, but clearly display potentiating effects when administered in combination.Citation173
The current recommendation is to avoid β-blockers and ACE inhibitors in patients at risk for anaphylaxis with no cardiovascular disease. Patients with cardiovascular disease should continue with β-blockers and ACE inhibitors, due to the proven decrease in mortality and increased life expectancy of this therapy.Citation168
Latex allergy
Latex in gloves, condoms, and surgical materials has been shown to induce anaphylaxis,Citation174 and cross-reactivity has been demonstrated with fruit allergens.Citation175 In a recent publication, which looked at patients undergoing surgical procedures, latex was reported as the second most common cause of anaphylaxis (20%) after neuromuscular blocking agents (47%),Citation176 and the risk increases with exposure.Citation177
Although STs for latex have higher specificity (96–100%) and sensitivity (95–99%) than in vitro tests, they have been associated with anaphylactic events and are, therefore, discontinued in the US;Citation178 diagnosis relies on serum IgE.
With the advent of latex-free facilities and the use of non-latex gloves, the incidence of latex-induced anaphylaxis has decreased.Citation179
Hymenoptera venom
Epidemiological studies have estimated the prevalence of systemic reaction to Hymenoptera stings from 0.3 to 8.9%, and insect stings as 1.5–34.1% of all-cause anaphylaxis. Cases of fatal insect-sting anaphylaxis account for approximately 20% of fatal anaphylaxis from any cause.Citation82
Risk factors for severe HSRs were determined from a European study of 962 patients with systemic reactions to Hymenoptera sting: elevated baseline tryptase levels (suggestive of MCAS), ACE inhibitor therapy, male, vespid sting, history of milder reactions, and systemic reactions to honey bee stings.Citation180 Other factors determining the severity of reaction to Hymenoptera sting include advanced age as well as preexisting cardiovascular and respiratory disease.Citation142 Mastocytosis may provoke fatal anaphylaxis despite VIT; however, so far, this has been observed only after discontinuation of treatment.Citation82
Hymenoptera anaphylaxis may be the presenting symptom of mastocytosis in an otherwise healthy individual.Citation142 The mechanism of increased susceptibility to Hymenoptera venom anaphylaxis in mastocytosis has not been elucidated, but explanations include the following: 1) increased number of mast cells amplifying the severity of the reaction resulting from high mast cell mediator release; 2) perivascular location of the mast cells, providing direct access to the intravascular compartment; 3) D816V-mutant KIT amplifying the IgE-mediated reaction; and 4) additive direct (non–IgE-mediated) mast cell-activating properties of the Hymenoptera venom, including phospholipase A2 ().Citation142
Figure 3 Mast cell defects may collectively lead to increased risk for anaphylaxis in response to hymenoptera venom in patients with clonal mast cell disease.
Abbreviations: IgE, immunoglobulin E; LTC4, leukotriene C4; PGD2, prostaglandin D2.
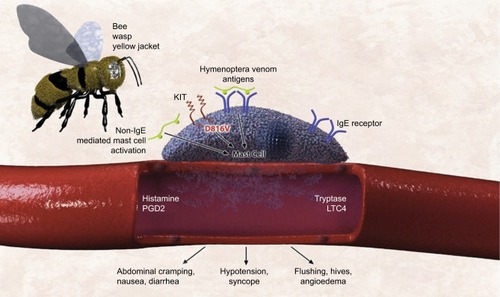
Furthermore, VIT with premedication has been suggested to increase VIT safety. Similarly, omalizumab has been shown to decrease the severity of allergic anaphylaxis following VIT administration in patients who had not previously tolerated immunotherapy,Citation181 including patients with mastocytosis.Citation146,Citation147,Citation182
Progestogen hypersensitivity
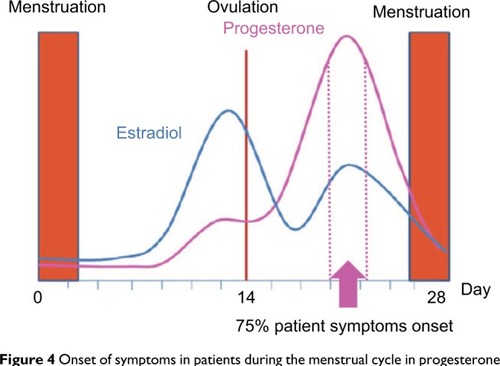
Progestogen hypersensitivity is a new terminology proposed for the already known autoimmune progesterone dermatitis – a rare and under-recognized disease that affects women of childbearing age.Citation183 Symptoms can appear at any age, but most frequently appear in the third decade of life. A broader presentation of cyclical symptoms, including asthma and anaphylaxis, has been described during the progesterone surge.Citation184,Citation185 According to the cases previously reported, the onset of symptoms in 75% of patients occurs during the progesterone peak of the menstrual cycleCitation183,Citation186 (). The lack of specific IgE against progesterone does not necessarily rule out the diagnosis.Citation187 Endogenous and exogenous sources of progesterone can be allergenic triggers, and desensitization to progesterone has reversed infertility.Citation183,Citation186
Figure 4 Onset of symptoms in patients during the menstrual cycle in progesterone hypersensitivity.
One publication reports that four of six women with dermatitis or anaphylaxis related to the luteal phase of the menstrual cycle (some of them related to in vitro fertilization) and positive STs to progesterone were successfully desensitized to this hormone; this is the first evidence of hormonal desensitization that has culminated in successful pregnancies or good tolerance to oral contraceptives.Citation186
More recently, a report based on 24 cases provided an algorithm for the classification, study, and management of these patients.Citation183
Cardiovascular diseases
Kounis syndrome is diagnosed when an acute coronary syndrome co-incidentally occurred with a HSR following an allergenic event. It is thought to be caused by inflammatory mediators released by mast cells.Citation188 The syndrome depends on the subtype of the coronary artery disease present, and symptoms reverse without sequelae within a few hours of the initial anaphylactic symptoms. Coronary damage can occur in severe episodes, but is rare. Use of intravenous epinephrine can have a detrimental role in patients with allergic angina, because of worsening myocardial ischemia, prolongation of the QTc interval, induction of coronary vasospasm, and arrhythmias; however, if necessary, intramuscular doses of epinephrine can be given.Citation189
Takotsubo is defined as stress cardiomyopathy during anaphylaxis secondary to a rapid elevation of circulating catecholamines released in middle-aged women and is characterized by transient regional systolic and diastolic dysfunction of the left ventricle leading to a variety of wall motion abnormalities. This can lead to fatal cardiac arrhythmias with heart failure.Citation190 Treatment is focused on stabilizing the acute onset and correct management of complications.Citation191
Treatment and prevention
Epinephrine is the cornerstone in the acute treatment of ana-phylaxis (), and delayed administration is associated with increased mortality.Citation3,Citation7,Citation14,Citation32,Citation35,Citation192–Citation198 The recommended dose of epinephrine is 0.01 mg/kg of a 1 mg/mL (1:1,000) dilution, to a maximum dose of 0.5 mg in an adult or 0.3 mg in a child, and can be repeated three times every 5–15 minutes. Patients should lie down with their legs elevated to avoid the empty inferior vena cava/empty ventricular syndrome and sudden death.Citation4,Citation32
Figure 5 Acute treatment of anaphylaxis.
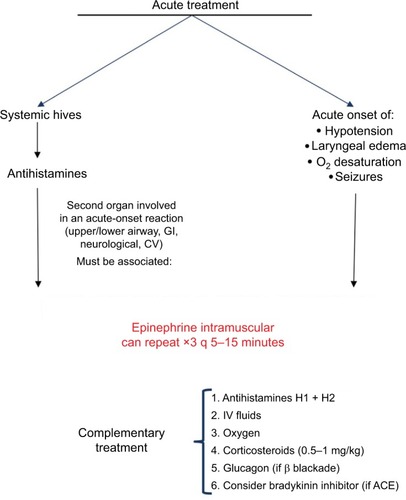
Epinephrine leads to vasoconstriction, increased peripheral vascular resistance, and decreased mucosal edema through the α1-adrenergic receptor, which is present in many tissues (including mast cells). It is the only drug with these effects among the medications used for the treatment of anaphylaxis, and clinically, it manifests as relief of airflow obstruction in the larynx as well as the lower airways, prevention of hypotension, and shock.Citation199
In 2014, a review analyzed how the inadequate use of auto-injectable epinephrine could affect the management of anaphylaxis. Several critical points established by the authors included: problems in properly recognizing and diagnosing the disease; when it was recognized, it was treated with epinephrine, but even in these cases, the use of epinephrine was suboptimal; the patients did not carry auto-injectable epinephrine, or they were not trained for its use and there was no access to this drug in public spaces such as airports, schools, sport stadiums, and restaurants. Based on these findings, suggestions for approval included training health professionals and promoting the availability of epinephrine in public places to prevent cases of fatal anaphylaxis.Citation196
If a patient presents with acute urticaria, antihistamines are recommended without the use of epinephrine unless another organ system is involved. In the case of urticaria with involvement of another organ system, the diagnosis of anaphylaxis should prevail, and epinephrine is the drug of choice.
Long-term treatment after an acute episode of anaphylaxis includes educating and training the patient and their family about the disease and the use of auto-injectable epinephrineCitation32 (). Critically ill patients who have experienced an episode of anaphylaxis should be evaluated by an allergist, and triggers should be identified using allergen STs and/or measurement of allergen-specific IgE levels in serum.Citation200 It is important to determine tryptase, both at baseline and during the reaction, to aid in a differential diagnosis.
Table 3 Preventive treatment of anaphylaxis
Avoidance education based on the known trigger of anaphylaxis should be part of patient education. Anaphylaxis to stinging insects can be prevented by immunotherapy with the relevant insect venom(s), and anaphylaxis to certain medications can be overcome by desensitization.
For food allergy, the first step is to avoid the trigger and treat symptoms in case they appear. Oral immunotherapy for food-induced anaphylaxis is under investigation. In the US, results of a peanut protein oral immunotherapy (OIT) Phase III clinical trial in children aged 9–36 months demonstrated a good safety profile, with a decrease in specific IgE and increased tolerance, while achieving safe dietary reintroduction.Citation201 A similar study reported sustained unresponsiveness following egg OIT, increasing the likelihood of tolerated unbaked eggs in diet,Citation202 and a report from Europe using sublingual immunotherapy (SLIT) with Pru p3 (the primary sensitizer in fruits and responsible for severe HSRs in the Mediterranean area) also demonstrated good tolerance after treatment not only to peach, but also showed immunological changes in allergic patients to Ara h9 – an LTP from peanut.Citation203
Immunotherapy should be considered in all patients who have had a history of anaphylaxis after an insect sting, and who have a positive ST or a positive result to an in vitro test for venom-specific IgE antibodies.Citation204 Immunotherapy in patients with mastocytosis has been shown to significantly reduce the risk of anaphylaxis after a re-sting.Citation80,Citation205 VIT is recommended indefinitely in patients with mastocytosis and Hymenoptera allergy because mastocytosis is a chronic disorder with no curative options and systemic reactions and sting-related fatalities have been reported in patients who discontinued immunotherapy.Citation142
Omalizumab – an anti-IgE mAb – has been shown to be a successful treatment for idiopathic anaphylaxis with IgE-mediated disease, effectively reducing the number of episodes and improving quality of life.Citation206
Several cases have demonstrated the benefits of omalizumab as a pretreatment in patients who have not previously tolerated immunotherapy – both in healthy individuals and MCAS patients.Citation146,Citation181,Citation207
Drug desensitization is a groundbreaking procedure for the management of immediate drug HSRs. It protects patients against anaphylaxis – maintaining patients on first-line therapy and thus representing an important advance in the treatment and prognosis of their medical condition.Citation14
Desensitization to drugs has been used, for the last 15 years, in thousands of cases, with safety and great efficacy in patients with anaphylaxis to chemotherapeutic agents, mAbs, and antibiotics without any deaths. Powerful inhibitory mechanisms are initiated at low antigen doses, which can dominate the activator pathways and prevent anaphylaxis.Citation118,Citation208–Citation210 Drug desensitization should be considered the standard of care when allergic patients require first-line therapy.
In a 2018 study, 526 desensitizations were carried out with 16 different mAbs in 104 patients, showing that reactions during the procedure are rare (23%) and the majority are mild (Grade I), with a decrease in HSR severity. STs may be useful as a predictor of severity when results are positive. The study demonstrated that desensitization provides a safe and effective re-treatment option to continue first-line therapy.Citation38
The largest desensitization study worldwide has reported that 370 highly allergic patients received 2,177 successful desensitizations to 15 drugs. Most importantly, carboplatin-desensitized patients had a non-statistically significant lifespan advantage over non-allergic control subjects, indicating that the efficacy of carboplatin was not reduced in allergic patients and that rapid drug-desensitization protocols are as effective as standard infusions.Citation211 Based on the results of the 2,177 desensitizations, 93% had no or mild reactions, whereas 7% had moderate-to-severe reactions that did not preclude the completion of the treatment; there were no deaths.Citation211 Desensitization to mAbs and antibiotics has been successfully undertaken in targeted populations, such as patients with cystic fibrosis, with similar protocols. On the other hand, desensitization to aspirin for aspirin-exacerbated respiratory disease has induced an increased sense of smell, prevented the regrowth of polyps, and helped stabilize asthma symptoms.Citation212–Citation214
Current issues with available treatment modalities
Glucocorticoids and antihistamines should be used as complementary treatment in anaphylaxis. They are not life-saving (like epinephrine) and do not prevent or relieve upper airway obstruction, hypotension, or shock.Citation32 Glucocorticoid and antihistamine administration should never delay the administration of epinephrine. The use of glucocorticoids and antihistamines alone as prophylactic agents cannot be recommended, as they do not prevent life-threatening reactions and their use has only been studied with radiocontrast HSRs.Citation215
As mentioned previously, omalizumab has been demonstrated to be effective in patients who have episodes of food-induced, VIT-induced, and/or idiopathic anaphylaxis. In a selected group of patients, pretreatment with omalizumab is cost effective and allows the patient to be treated with fewer reactions.
Social aspects of treatment of anaphylaxis and quality of life
Quality of life of patients experiencing one or more episodes of food, medication, Hymenoptera venom, or exercise-induced anaphylaxis is a critical aspect of the patient’s treatment and management. It affects not only the patient but also their family, school, and workplace, as well as their traveling and other social interactions.Citation216 Up to 12% of people who experience an anaphylactic shock can develop post-traumatic stress disorder, as well as long-term effects such as fear and anxiety that can dominate and restrict social, family, and professional interactions in adults and children.Citation217–Citation219 In particular, adolescents can present with high-risk behavior.Citation220 Education of patients, health-care providers, and ED personnel can have a positive effect in all of these areas.Citation221
Conclusion
Anaphylaxis should be considered when symptoms in two or more organs and/or sudden changes in vital signs occur in patients with or without evident exposure to allergens such as food, drugs including antibiotics, chemotherapy and mAbs, Hymenoptera stings, or environmental factors (ie, latex, animal dander, and pollen). Clinical manifestations do not always include skin and mucosal changes, and hypotension and shock may not be present. Non-typical symptoms such as pain, chills, rigors, and fever have been recognized as part of a new phenotypic expression of anaphylaxis during reaction to drugs such as chemotherapy and mAbs.
A new classification system based on precision medicine using phenotypes, endotypes, and biomarkers has expanded the Gell and Coombs classification to better identify and treat anaphylaxis.
Skin testing is a safe tool to identify sensitization to numerous allergens, even in high-risk patients, such as those with cancer, cystic fibrosis, and mastocytosis. Other biomarkers such as specific IgE, component-resolved diagnostics, and BAT are evolving as useful tools for the future.
The tryptase level – which can be detected from 30 minutes to 3 hours after the onset of a reaction – is the best biomarker at the present time which can also identify patients with clonal mast cell disorders.
Epinephrine is the cornerstone in the acute treatment of anaphylaxis, and delayed administration is associated with increased mortality.
Patients with drug-induced anaphylaxis should be considered candidates for desensitization if in need of first-line therapies. Desensitization can increase life expectancy and quality of life of patients with drug-induced anaphylaxis.
Allergists should educate patients and provide them with action plans and tools, including auto-injectable epinephrine. Education of all specialists and health-care providers in the symptoms, presentation, and acute management of anaphylaxis is key to increasing awareness of anaphylaxis.
Precision medicine requires further research on new biomarkers and exploration of new treatment modalities such as anti-IgE.
Acknowledgments
The authors did not receive compensation nor was the content of the article influenced in any way. Adamis Pharmaceuticals paid publication fees for the articles in this special issue on anaphylaxis.
Disclosure
Dr Teodorikez Wilfox Jimenez-Rodriguez has received grants from the Spanish Society of Allergy and Clinical Immunology, the Valencian Association of Allergy and Clinical Immunology, and the Medical Association of Alicante, Spain.
Marlene Garcia-Neuer reports no conflicts of interest in this work.
Leila A Alenazy has been sponsored by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University.
Dr Mariana Castells has received consultancy fees from Sanofi, Genentech, Lytix Biopharma, Bentham Science, and UpToDate. The authors report no other conflicts of interest in this work.
References
- RingJBehrendtHde WeckAHistory and classification of anaphylaxisChem Immunol Allergy20109511120519878
- RojidoGMCien años de anafilaxiaAlergol Inmunol Clin200116364368
- SimonsFEAnaphylaxisJ Allergy Clin Immunol20101252 Suppl 2S161S18120176258
- SimonsFEArdussoLRBilòMBInternational consensus on (ICON) anaphylaxisWorld Allergy Organ J201471924920969
- SimonsFEArdussoLRBilòMBWorld Allergy Organization2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxisCurr Opin Allergy Clin Immunol201212438939922744267
- SampsonHAMuñoz-FurlongACampbellRLSecond symposium on the definition and management of anaphylaxis: summary report – second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposiumAnn Emerg Med200647437338016546624
- MuraroARobertsGWormMEAACI Food Allergy and Anaphylaxis Guidelines GroupAnaphylaxis: guidelines from the European Academy of Allergy and Clinical ImmunologyAllergy20146981026104524909803
- SimonsFEAnaphylaxis: recent advances in assessment and treatmentJ Allergy Clin Immunol20091244625636 quiz 637–63819815109
- SimonsFEAnaphylaxisJ Allergy Clin Immunol20081212 SupplS402S407 quiz S42018241691
- ClarkSCamargoCAJrEpidemiology of anaphylaxisImmunol Allergy Clin North Am2007272145163 v17493495
- JohanssonSGBieberTDahlRRevised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003J Allergy Clin Immunol2004113583283615131563
- SampsonHAMuñoz-FurlongABockSASymposium on the definition and management of anaphylaxis: summary reportJ Allergy Clin Immunol2005115358459115753908
- MuraroALemanskeRFJrCastellsMPrecision medicine in allergic disease-food allergy, drug allergy, and anaphylaxis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and ImmunologyAllergy20177271006102128122115
- CastellsMDiagnosis and management of anaphylaxis in precision medicineJ Allergy Clin Immunol2017140232133328780940
- DombrowiczDBriniATFlamandVAnaphylaxis mediated through a humanized high affinity IgE receptorJ Immunol19961574164516518759751
- ReberLLHernandezJDGalliSJThe pathophysiology of anaphylaxisJ Allergy Clin Immunol2017140233534828780941
- FinkelmanFDKhodounMVStraitRHuman IgE-independent systemic anaphylaxisJ Allergy Clin Immunol201613761674168027130857
- GillisCMJönssonFMancardiDAMechanisms of anaphylaxis in human low-affinity IgG receptor locus knock-in miceJ Allergy Clin Immunol2017139412531265.e1427568081
- MontrucchioGAlloattiGCamussiGRole of platelet-activating factor in cardiovascular pathophysiologyPhysiol Rev20008041669169911015622
- GillPJindalNLJagdisAVadasPPlatelets in the immune response: revisiting platelet-activating factor in anaphylaxisJ Allergy Clin Immunol201513561424143226051949
- FinkelmanFDAnaphylaxis: lessons from mouse modelsJ Allergy Clin Immunol20071203506515 quiz 516–51717765751
- Sala-CunillABjörkqvistJSenterRPlasma contact system activation drives anaphylaxis in severe mast cell-mediated allergic reactionsJ Allergy Clin Immunol2015135410311043.e625240785
- KajiwaraNSasakiTBraddingPActivation of human mast cells through the platelet-activating factor receptorJ Allergy Clin Immunol2010125511371145.e620392487
- VultaggioAMatucciANenciniFAnti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactionsAllergy201065565766119951375
- CastellsMCAnaphylaxis to chemotherapy and monoclonal antibodiesImmunol Allergy Clin North Am201535233534825841555
- LiebermanPGarveyLHMast cells and anaphylaxisCurr Allergy Asthma Rep20161632026857018
- SubramanianHGuptaKAliHRoles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseasesJ Allergy Clin Immunol2016138370071027448446
- SpoerlDNigolianHCzarnetzkiCHarrTReclassifying anaphylaxis to neuromuscular blocking agents based on the presumed patho-mechanism: IgE-mediated, pharmacological adverse reaction or “innate hypersensitivity”?Int J Mol Sci2017186 piiE122328590439
- ZhangTCheDLiuRTypical antimicrobials induce mast cell degranulation and anaphylactoid reactions via MRGPRX2 and its murine homologue MRGPRB2Eur J Immunol201747111949195828688196
- GalliSJTsaiMIgE and mast cells in allergic diseaseNat Med201218569370422561833
- AltmanAMCamargoCAJrSimonsFEAnaphylaxis in America: a national physician surveyJ Allergy Clin Immunol2015135383083325577592
- SimonsFEArdussoLRDimovVWorld Allergy OrganizationWorld Allergy Organization anaphylaxis guidelines: 2013 update of the evidence baseInt Arch Allergy Immunol2013162319320424008815
- MostmansYBlykersMMolsPGutermuthJGrosberMNaeijeNAnaphylaxis in an urban Belgian emergency department: epidemiology and aetiologyActa Clin Belg20167129910626243353
- CavkaytarOKaraatmacaBCetinkayaPGCharacteristics of drug-induced anaphylaxis in children and adolescentsAllergy Asthma Proc2017385566328814352
- TurnerPJJerschowEUmasuntharTLinRCampbellDEBoyleRJFatal anaphylaxis: mortality rate and risk factorsJ Allergy Clin Immunol Pract2017551169117828888247
- GalvãoVRCastellsMCHypersensitivity to biological agents-updated diagnosis, management, and treatmentJ Allergy Clin Immunol Pract201532175185 quiz 18625754718
- KhanDAHypersensitivity and immunologic reactions to biologics: opportunities for the allergistAnn Allergy Asthma Immunol2016117211512027499538
- IsabweGGarcia NeuerMde las Vecillas SánchezLLynchDMMarquisKCastellsMNovel evidence-based phenotypes and endotypes of hypersensitivity reactions to 16 monoclonal antibodies: management with 526 desensitizationsJ Allergy Clin Immunol2017
- SchulertGSGromAAMacrophage activation syndrome and cytokine-directed therapiesBest Pract Res Clin Rheumatol201428227729224974063
- TanakaTNarazakiMKishimotoTImmunotherapeutic implications of IL-6 blockade for cytokine stormImmunotherapy20168895997027381687
- Gomez-SalineroJMRafiiSPlasmin regulation of acute cytokine stormBlood201713015628684447
- NeumannFJOttIMarxNEffect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activityArterioscler Thromb Vasc Biol19971712339934059437185
- Muñoz-CanoRMPicadoCValeroABartraJMechanisms of anaphylaxis beyond IgEJ Investig Allergol Clin Immunol20162627382 quiz 2p following 83
- FregoneseLSwanFJvan SchadewijkAExpression of the anaphylatoxin receptors C3aR and C5aR is increased in fatal asthmaJ Allergy Clin Immunol200511561148115415940127
- ChopraNOppenheimerJDerimanovGSFinePLVancomycin anaphylaxis and successful desensitization in a patient with end stage renal disease on hemodialysis by maintaining steady antibiotic levelsAnn Allergy Asthma Immunol200084663363510875494
- SimonRASchatzMStevensonDDRadiographic contrast media infusions. Measurement of histamine, complement, and fibrin split products and correlation with clinical parametersJ Allergy Clin Immunol197963428128885650
- WeiszhárZCzúczJRévészCRosivallLSzebeniJRozsnyayZComplement activation by polyethoxylated pharmaceutical surfactants: Cremophor-EL, Tween-80 and Tween-20Eur J Pharm Sci201245449249821963457
- van der LindenPWHackCEKerckhaertJAStruyvenbergAvan der ZwanJCPreliminary report: complement activation in wasp-sting anaphylaxisLancet199033687209049061976931
- KishimotoTKViswanathanKGangulyTContaminated heparin associated with adverse clinical events and activation of the contact systemN Engl J Med2008358232457246718434646
- HeinzerlingLMariABergmannKCThe skin prick test – European standardsClin Transl Allergy201331323369181
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and ImmunologyThe diagnosis and management of anaphylaxis: an updated practice parameterJ Allergy Clin Immunol20051153 Suppl 2S483S52315753926
- SloaneDGovindarajuluUHarrow-MortellitiJSafety, costs, and efficacy of rapid drug desensitizations to chemotherapy and monoclonal antibodiesJ Allergy Clin Immunol Pract20164349750426895621
- PascalDJean BousquetPRomanoAAntibiotic-induced anaphylaxisCastellsMCAnaphylaxis and Hypersensitivity ReactionsBoston, MAHumana Press2011171182
- BrockowKRomanoABlancaMRingJPichlerWDemolyPGeneral considerations for skin test procedures in the diagnosis of drug hypersensitivityAllergy20025714551
- GoldbergAConfino-CohenRTiming of venom skin tests and IgE determinations after insect sting anaphylaxisJ Allergy Clin Immunol199710021821849275138
- Nowak-WegrzynAAssa’adAHBahnaSLBockSASichererSHTeuberSSAdverse Reactions to Food Committee of American Academy of Allergy, Asthma & ImmunologyWork Group report: oral food challenge testingJ Allergy Clin Immunol20091236 SupplS365S38319500710
- LinESaxonARiedlMPenicillin allergy: value of including amoxicillin as a determinant in penicillin skin testingInt Arch Allergy Immunol2010152431331820185923
- FernándezJTorresMJCamposJArribas-PovesFBlancaMDAP-Diater GroupProspective, multicenter clinical trial to validate new products for skin tests in the diagnosis of allergy to penicillinJ Investig Allergol Clin Immunol2013236398408
- BlumenthalKGShenoyESVarugheseCAHurwitzSHooperDCBanerjiAImpact of a clinical guideline for prescribing antibiotics to inpatients reporting penicillin or cephalosporin allergyAnn Allergy Asthma Immunol20151154294300.e226070805
- DemolyPAdkinsonNFBrockowKInternational Consensus on drug allergyAllergy Eur J Allergy Clin Immunol2014694420437
- FoxSParkMAPenicillin skin testing in the evaluation and management of penicillin allergyAnn Allergy Asthma Immunol201110611721195938
- MacyESchatzMLinCPoonKYThe falling rate of positive penicillin skin tests from 1995 to 2007Perm J2009132121821373225
- MacyERomanoAKhanDPractical management of antibiotic hypersensitivity in 2017J Allergy Clin Immunol Pract20175357758628365277
- AntúnezCMartínECornejo-GarcíaJAImmediate hypersensitivity reactions to penicillins and other betalactamsCurr Pharm Des200612263327333317017927
- AntunezCBlanca-LopezNTorresMJImmediate allergic reactions to cephalosporins: evaluation of cross-reactivity with a panel of penicillins and cephalosporinsJ Allergy Clin Immunol2006117240441016461141
- WheatleyLMPlautMSchwaningerJMReport from the National Institute of Allergy and Infectious Diseases workshop on drug allergyJ Allergy Clin Immunol20151362262271.e226254053
- BrennanPJRodriguez BouzaTHsuFISloaneDECastellsMCHypersensitivity reactions to mAbs: 105 desensitizations in 23 patients, from evaluation to treatmentJ Allergy Clin Immunol200912461259126619910036
- GouldHJSuttonBJBeavilAJThe biology of IGE and the basis of allergic diseaseAnnu Rev Immunol200321157962812500981
- StoneKDPrussinCMetcalfeDDIgE, mast cells, basophils, and eosinophilsJ Allergy Clin Immunol20101252 Suppl 2S73S8020176269
- SmithJKKrishnaswamyGHDykesRReynoldsSBerkSLClinical manifestations of IgE hypogammaglobulinemiaAnn Allergy Asthma Immunol19977833133189087159
- WittigHJBelloitJDe FillippiIRoyalGAge-related serum immunoglobulin E levels in healthy subjects and in patients with allergic diseaseJ Allergy Clin Immunol19806643053137419833
- PaganelliRAnsoteguiIJSastreJSpecific IgE antibodies in the diagnosis of atopic disease. Clinical evaluation of a new in vitro test system, UniCAP (TM), in six European allergy clinicsAllergy19985387637689722225
- SampsonHAUtility of food-specific IgE concentrations in predicting symptomatic food allergyJ Allergy Clin Immunol2001107589189611344358
- JohnsonTLMcCleskeyPERathkopfMMeffertJJHaganLLPathergy response to skin prick testingJ Allergy Clin Immunol200711951270127217353037
- KrzysztofKLawrenceDOverview of in vitro allergy testsUpToDate Online2016 Available form: https://www-uptodate-com.sangva.a17.csinet.es/contents/overview-of-in-vitro-allergy-tests?search=Overview%20of%20in%20vitro%20allergy%20tests.&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1Accessed March 26, 2018
- HarlemanLSieAHistory, blood tests or skin prick testing? Is it possible to predict the severity of allergic reactions in children with IgE-mediated food allergy?Arch Dis Child20151006594598
- KöhlerJBlankSMüllerSComponent resolution reveals additional major allergens in patients with honeybee venom allergyJ Allergy Clin Immunol2014133513831389 1389.e1–e624440283
- CifuentesLVosselerSBlankSIdentification of hymenoptera venom-allergic patients with negative specific IgE to venom extract by using recombinant allergensJ Allergy Clin Immunol2014133390991024290287
- NurmatovUDhamiSArasiSAllergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysisAllergy20177281133114728058751
- RuëffFWenderothAPrzybillaBPatients still reacting to a sting challenge while receiving conventional hymenoptera venom immunotherapy are protected by increased venom dosesJ Allergy Clin Immunol200110861027103211742283
- Alfaya AriasTSoriano GómisVSoto MeraTHymenoptera Allergy Committee of the SEAICKey issues in hymenoptera venom allergy: an updateJ Investig Allergol Clin Immunol20172711931
- BilòMBAnaphylaxis caused by Hymenoptera stings: from epidemiology to treatmentAllergy201166Suppl 95353721668850
- ZhaoYQiaoHDetection of specific IgE antibodies to major and minor antigenic determinants in sera of penicillin allergic patientsChin Med J (Engl)2003116121904191014687482
- RomanoAGaetaFValluzziRLZaffiroACarusoCQuaratinoDNatural evolution of skin-test sensitivity in patients with IgE-mediated hypersensitivity to cephalosporinsAllergy201469680680924673580
- CaiadoJVenemalmLPereira-SantosMCCostaLBarbosaMPCastellsMCarboplatin-, oxaliplatin-, and cisplatin–specific IgE: cross-reactivity and value in the diagnosis of carboplatin and oxaliplatin allergyJ Allergy Clin Immunol Pract20131549450024565621
- CaiadoJPicardMDiagnostic tools for hypersensitivity to platinum drugs and taxanes: skin testing, specific IgE, and mast cell/basophil mediatorsCurr Allergy Asthma Rep201414845124951237
- ThamEHChengYKTayMHAlcasabasAPShekLPEvaluation and management of hypersensitivity reactions to chemotherapy agentsPostgrad Med J201591107314515025659930
- HesterbergPEBanerjiAOrenERisk stratification for desensitization of patients with carboplatin hypersensitivity: clinical presentation and managementJ Allergy Clin Immunol2009123612621267.e119501233
- CaiadoJCastellsMPresentation and diagnosis of hypersensitivity to platinum drugsCurr Allergy Asthma Rep20151541526130472
- MatricardiPMKleine-TebbeJHoffmannHJEAACI molecular allergology user’s guidePediatr Allergy Immunol201627Suppl 23125027288833
- SichererSHDhillonGLaugheryKAHamiltonRGWoodRACaution: the Phadia hazelnut ImmunoCAP (f17) has been supplemented with recombinant Cor a 1 and now detects Bet v 1-specific IgE, which leads to elevated values for persons with birch pollen allergyJ Allergy Clin Immunol20081222413414 414.e218586316
- TuanoKSDavisCMUtility of component-resolved diagnostics in food allergyCurr Allergy Asthma Rep20151563226141579
- NicolaouNPoorafsharMMurrayCAllergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnosticsJ Allergy Clin Immunol20101251191197e1e1320109746
- EigenmannPALackGMazonAManaging nut allergy: a remaining clinical challengeJ Allergy Clin Immunol Pract20175229630027793601
- OrtolaniCBallmer-WeberBKHansenKSHazelnut allergy: a double-blind, placebo-controlled food challenge multicenter studyJ Allergy Clin Immunol2000105357758110719310
- FlintermanAEAkkerdaasJHden Hartog JagerCFLipid transfer protein-linked hazelnut allergy in children from a non-Mediterranean birch-endemic areaJ Allergy Clin Immunol20081212423428.e218036652
- KattanJDSichererSHOptimizing the diagnosis of food allergyImmunol Allergy Clin North Am2015351617625459577
- De KnopKJVerweijMMGrimmelikhuijsenMAge-related sensitization profiles for hazelnut (Corylus avellana) in a birch-endemic regionPediatr Allergy Immunol2011221 Pt 2e139e14921342279
- BeyerKGrishinaGBardinaLGrishinASampsonHAIdentification of an 11S globulin as a major hazelnut food allergen in hazelnut-induced systemic reactionsJ Allergy Clin Immunol2002110351752312209105
- D’UrbanoLEPellegrinoKArtesaniMCPerformance of a component-based allergen-microarray in the diagnosis of cow’s milk and hen’s egg allergyClin Exp Allergy201040101561157020633029
- GámezCSánchez-GarcíaSIbáñezMDTropomyosin IgE-positive results are a good predictor of shrimp allergyAllergy201166101375138321651567
- AlessandriCZennaroDScalaEOvomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen’s egg and an increased risk to progress to multiple environmental allergen sensitisationClin Exp Allergy201242344145022168465
- DouladirisNSavvatianosSRoumpedakiISkevakiCMitsiasDPapadopoulosNGA molecular diagnostic algorithm to guide pollen immunotherapy in southern Europe: towards component-resolved management of allergic diseasesInt Arch Allergy Immunol2013162216317223921568
- OcmantAMulierSHanssensLBasophil activation tests for the diagnosis of food allergy in childrenClin Exp Allergy20093981234124519549026
- McGowanECSainiSUpdate on the performance and application of basophil activation testsCurr Allergy Asthma Rep201313110110923188565
- SanzMLGamboaPMMayorgaCBasophil activation tests in the evaluation of immediate drug hypersensitivityCurr Opin Allergy Clin Immunol20099429830419483617
- HausmannOVGentinettaTBridtsCHEboDGThe basophil activation test in immediate-type drug allergyImmunol Allergy Clin North Am200929355556619563997
- ArmstrongNWolffRvan MastrigtGA systematic review and cost-effectiveness analysis of specialist services and adrenaline auto-injectors in anaphylaxisHeal Technol Assess201317171117vvi
- Giavina-BianchiPGalvãoVRPicardMCaiadoJCastellsMCBasophil activation test is a relevant biomarker of the outcome of rapid desensitization in platinum compounds-allergyJ Allergy Clin Immunol Pract20175372873628034549
- SchwartzLBMetcalfeDDMillerJSEarlHSullivanTTryptase levels as an indicator of mast-cell activation in systemic anaphylaxis and mastocytosisN Engl J Med198731626162216263295549
- AkinCSotoDBrittainETryptase haplotype in mastocytosis: relationship to disease variant and diagnostic utility of total tryptase levelsClin Immunol2007123326827117449330
- ValentPAkinCArockMDefinitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposalInt Arch Allergy Immunol2012157321522522041891
- McNeilHPAdachiRStevensRLMast cell-restricted tryptases: structure and function in inflammation and pathogen defenseJ Biol Chem200728229207852078917504754
- LyonsJJYuXHughesJDElevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy numberNat Genet201648121564156927749843
- SchwartzLB[webpage on the Internet] Laboratory tests to support the clinical diagnosis of anaphylaxisUpToDate Online2016 Available from: https://www.uptodate.com/contents/laboratory-tests-to-support-the-clinical-diagnosis-of-anaphylaxis?source=search_result&search=Laboratory tests to support the clinical diagnosis of anaphylaxis&selectedTitle=1~150Accessed November 27, 2017
- SampsonHAMendelsonLRosenJPFatal and near-fatal anaphylactic reactions to food in children and adolescentsN Engl J Med199232763803841294076
- van der LindenPWStruyvenbergAKraaijenhagenRJHackCEvan der ZwanJKAnaphylactic shock after insect-sting challenge in 138 persons with a previous insect-sting reactionAnn Intern Med199311831611688417633
- VadasPGoldMPerelmanBPlatelet-activating factor, PAF acetylhydrolase, and severe anaphylaxisN Engl J Med20083581283518172172
- LouroERomano-LieberNSRibeiroEAdverse events to antibiotics in inpatients of a university hospitalRev Saude Publica200741610421048 Portuguese [with English abstract]17992357
- BirdJABurksAWFood allergy and asthmaPrim Care Respir J200918425826519588055
- FoongRXdu ToitGFoxATAsthma, food allergy, and how they relate to each otherFront Pediatr201758928536690
- BockSAMuoz-FurlongASampsonHAFatalities due to anaphylactic reactions to foodsJ Allergy Clin Immunol2001107119119311150011
- DhamiSSheikhAAnaphylaxis: epidemiology, aetiology and relevance for the clinicExpert Rev Clin Immunol201713988989528562113
- SummersCWPumphreyRSWoodsCNMcDowellGPembertonPWArkwrightPDFactors predicting anaphylaxis to peanuts and tree nuts in patients referred to a specialist centerJ Allergy Clin Immunol20081213632638.e218207562
- UmasuntharTLeonardi-BeeJTurnerPJIncidence of food anaphylaxis in people with food allergy: a systematic review and meta-analysisClin Exp Allergy201545111621163625495886
- MartinPEEckertJKKoplinJJHealthNuts Study InvestigatorsWhich infants with eczema are at risk of food allergy? Results from a population-based cohortClin Exp Allergy201545125526425210971
- AkueteKGuffeyDIsraelsenRBMulticenter prevalence of anaphylaxis in clinic-based oral food challengesAnn Allergy Asthma Immunol20171194339348.e128890356
- FeldwegAMExercise-induced anaphylaxisImmunol Allergy Clin North Am201535226127525841550
- ShefferALAustenKFExercise-induced anaphylaxisJ Allergy Clin Immunol19806621061117400473
- ShadickNALiangMHPartridgeAJThe natural history of exercise-induced anaphylaxis: survey results from a 10-year follow-up studyJ Allergy Clin Immunol1999104112312710400849
- CastellsMCHoranRFShefferALExercise-induced anaphylaxisCurr Allergy Asthma Rep200331152112542988
- MatsuoHKohnoKMoritaEMolecular cloning, recombinant expression and IgE-binding epitope of omega-5 gliadin, a major allergen in wheat-dependent exercise-induced anaphylaxisFEBS J2005272174431443816128812
- FeldwegAMFood-dependent, exercise-induced anaphylaxis: diagnosis and management in the outpatient settingJ Allergy Clin Immunol Pract20175228328828283153
- AkinCMast cell activation syndromesJ Allergy Clin Immunol2017140234935528780942
- ValentPAkinCMetcalfeDDMastocytosis: 2016 updated WHO classification and novel emerging treatment conceptsBlood2017129111420142828031180
- AkinCValentPMetcalfeDDMast cell activation syndrome: proposed diagnostic criteriaJ Allergy Clin Immunol2010126610991104.e421035176
- González-de-OlanoDÁlvarez-TwoseIInsights in anaphylaxis and clonal mast cell disordersFront Immunol2017879228740494
- González De OlanoDDe La Hoz CaballerBNúñez LópezRPrevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: a study of the Spanish network on mastocytosis (REMA)Clin Exp Allergy200737101547155517883734
- ZanottiRLombardoCPassalacquaGClonal mast cell disorders in patients with severe Hymenoptera venom allergy and normal serum tryptase levelsJ Allergy Clin Immunol2015136113513925605272
- BrockowKJoferCBehrendtHRingJAnaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patientsAllergy200863222623218186813
- HartmannKEscribanoLGrattanCCutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; The American Academy of Allergy, Asthma & Immunology; And the European Academy of Allergology and Clinical ImmunologyJ Allergy Clin Immunol20161371354526476479
- CastellsMCHornickJLAkinCAnaphylaxis after hymenoptera sting: is it venom allergy, a clonal disorder, or both?J Allergy Clin Immunol Pract20153335035525858055
- ValentPEscribanoLBroesby-OlsenSEuropean Competence Network on MastocytosisProposed diagnostic algorithm for patients with suspected mastocytosis: a proposal of the European Competence Network on MastocytosisAllergy201469101267127424836395
- EscribanoLÁlvarez-TwoseISánchez-MuñozLPrognosis in adult indolent systemic mastocytosis: a long-term study of the Spanish Network on Mastocytosis in a series of 145 patientsJ Allergy Clin Immunol2009124351452119541349
- Álvarez-TwoseIGonzález de OlanoDSánchez-MuñozLClinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptomsJ Allergy Clin Immunol2010125612691278.e220434205
- CarterMRobynJABresslerPBWalkerJCShapiroGGMetcalfeDDOmalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosisJ Allergy Clin Immunol200711961550155117481708
- González de OlanoDÁlvarez-TwoseIEsteban-LópezMISafety and effectiveness of immunotherapy in patients with indolent systemic mastocytosis presenting with Hymenoptera venom anaphylaxisJ Allergy Clin Immunol2008121251952618177694
- BonadonnaPPaganiMAbererWDrug hypersensitivity in clonal mast cell disorders: ENDA/EAACI position paperAllergy201570775576325824492
- MuraliMRCastellsMCSongJYDudzinskiDMHasserjianRPCase records of the Massachusetts General Hospital. Case 9-2011. A 37-year-old man with flushing and hypotensionN Engl J Med2011364121155116521428772
- KuruvillaMKhanDAAnaphylaxis to drugsImmunol Allergy Clin North Am201535230331925841553
- GrabenhenrichLBDolleSMoneret-VautrinAAnaphylaxis in children and adolescents: The European Anaphylaxis RegistryJ Allergy Clin Immunol2016137411281137.e126806049
- ClarkSWeiWRuddersSACamargoCAJrRisk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitalsJ Allergy Clin Immunol201413451125113024985399
- JerschowELinRYScaperottiMMMcGinnAPFatal anaphylaxis in the United States, 1999–2010: temporal patterns and demographic associationsJ Allergy Clin Immunol2014134613181328.e725280385
- BrownSGStoneSFFatovichDMAnaphylaxis: clinical patterns, mediator release, and severityJ Allergy Clin Immunol2013132511411149.e523915715
- ParmarJSNasserSAntibiotic allergy in cystic fibrosisThorax200560651752015923254
- Bonamichi-SantosRCastellsMDiagnoses and management of drug hypersensitivity and anaphylaxis in cancer and chronic inflammatory diseases: reactions to taxanes and monoclonal antibodiesClin Rev Allergy Immunol Epub2016 68
- BanerjiALaxTGuyerAHurwitzSCamargoCAJrLongAAManagement of hypersensitivity reactions to carboplatin and paclitaxel in an outpatient oncology infusion center: a 5-year reviewJ Allergy Clin Immunol Pract20142442843325017531
- CastellsMCHypersensitivity to antineoplastic agentsCurr Pharm Des200814272892290118991707
- WongJTLingMPatilSBanerjiALongAOxaliplatin hypersensitivity: evaluation, implications of skin testing, and desensitizationJ Allergy Clin Immunol Pract201421404524565767
- BoulangerJBoursiquotJNCournoyerGComité de l’évolution des pratiques en oncologieManagement of hypersensitivity to platinum- and taxane-based chemotherapy: CEPO review and clinical recommendationsCurr Oncol2014214e630e64125089112
- PicardMCastellsMCRe-visiting hypersensitivity reactions to taxanes: a comprehensive reviewClin Rev Allergy Immunol201549217719124740483
- FanGWangZHaoMLiJBispecific antibodies and their applicationsJ Hematol Oncol2015813026692321
- PuxedduIGioriLRocchiVHypersensitivity reactions during treatment with infliximab, etanercept, and adalimumabAnn Allergy Asthma Immunol2012108212312422289732
- VultaggioACastellsMCHypersensitivity reactions to biologic agentsImmunol Allergy Clin North Am201434361563225017680
- WarringtonRJLeeKRMcPhillipsSThe value of skin testing for penicillin allergy in an inpatient population: analysis of the subsequent patient managementAllergy Asthma Proc200021529729911061039
- ChungCHMirakhurBChanECetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactoseN Engl J Med2008358111109111718337601
- ComminsSPJamesHRKellyLAThe relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-α-1,3-galactoseJ Allergy Clin Immunol2011127512861293.e621453959
- CoopCASchapiraRSFreemanTMAre ACE inhibitors and beta-blockers dangerous in patients at risk for anaphylaxis?J Allergy Clin Immunol Pract2017551207121128552379
- TenBrookJAJrWolfMPHoffmanSNShould beta-blockers be given to patients with heart disease and peanut-induced anaphylaxis? A decision analysisJ Allergy Clin Immunol2004113597798215131583
- CavigliaAGPassalacquaGSennaGRisk of severe anaphylaxis for patients with Hymenoptera venom allergy: are angiotensin-receptor blockers comparable to angiotensin-converting enzyme inhibitors?J Allergy Clin Immunol201012551171 author reply 1171–117220347124
- Moneret-VautrinDALatarcheCDrugs as risk factors of food anaphylaxis in adults: a case-control studyBull Acad Natl Med20091932351362 discussion 362–363; French [with English abstract]19718892
- LeeSHessEPNestlerDMAntihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxisJ Allergy Clin Immunol201313141103110823453138
- NassiriMBabinaMDölleSEdenharterGRuëffFWormMRamipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell primingJ Allergy Clin Immunol2015135249149925441633
- BousquetJFlahaultAVandenplasONatural rubber latex allergy among health care workers: a systematic review of the evidenceJ Allergy Clin Immunol2006118244745416890771
- CrespoJFRodríguezJJamesJMDarocaPReañoMVivesRReactivity to potential cross-reactive foods in fruit-allergic patients: implications for prescribing food avoidanceAllergy2002571094694912269944
- YunkerNSWagnerBJA pharmacologic review of anaphylaxisPlast Surg Nurs201636417317927922561
- KahnSLPodjasekJODimitropoulosVABrownCWJrNatural rubber latex allergyDis Mon201662151726743990
- KellyKJSussmanGLatex allergy: where are we now and how did we get there?J Allergy Clin Immunol Pract2017551212121628888250
- BrownRHTaenkhumKBuckleyTJHamiltonRGDifferent latex aeroallergen size distributions between powdered surgical and examination gloves: Significance for environmental avoidanceJ Allergy Clin Immunol2004114235836315316516
- RuëffFPrzybillaBBilóMBPredictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase-a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom HypersensitivityJ Allergy Clin Immunol200912451047105419895993
- RicciardiLOmalizumab: a useful tool for inducing tolerance to bee venom immunotherapyInt J Immunopathol Pharmacol201629472672827679679
- SokolKCGhaziAKellyBCGrantJAOmalizumab as a desensitizing agent and treatment in mastocytosis: a review of the literature and case reportJ Allergy Clin Immunol Pract20142326627024811015
- FoerDBuchheitKMGargiuloARLynchDMCastellsMWicknerPGProgestogen hypersensitivity in 24 cases: diagnosis, management, and proposed renaming and classificationJ Allergy Clin Immunol Pract201544723729
- BernsteinILBernsteinDILummusZLBernsteinJAA case of progesterone-induced anaphylaxis, cyclic urticaria/angioedema, and autoimmune dermatitisJ Womens Health (Larchmt)201120464364821417747
- BarrRGWentowskiCCGrodsteinFProspective study of postmenopausal hormone use and newly diagnosed asthma and chronic obstructive pulmonary diseaseArch Intern Med2004164437938614980988
- Prieto-GarciaASloaneDEGargiuloARFeldwegAMCastellsMAutoimmune progesterone dermatitis: clinical presentation and management with progesterone desensitization for successful in vitro fertilizationFertil Steril20119531121e9e13
- LiebermanPCatamenial anaphylaxisJ Allergy Clin Immunol Pract20142335835924811034
- FassioFLosappioLAntolin-AmerigoDKounis syndrome: A concise review with focus on managementEur J Intern Med20163071026795552
- LiebermanPSimonsFEAnaphylaxis and cardiovascular disease: therapeutic dilemmasClin Exp Allergy20154581288129525711241
- KotsiouOSXirogiannisKIGourgoulianisKIKounis syndrome: is it really a takotsubo-like syndrome?J Investig Allergol Clin Immunol2017273198200
- Y-HassanSTornvallPEpidemiology, pathogenesis, and management of takotsubo syndromeClin Auton Res2018281536528917022
- SimonsFEArdussoLRBilòMBWorld Allergy Organization2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxisCurr Opin Allergy Clin Immunol201212438939922744267
- SheikhASimonsFEBarbourVWorthAAdrenaline auto-injectors for the treatment of anaphylaxis with and without cardiovascular collapse in the communityCochrane Database Syst Rev20128CD00893522895980
- MostmansYGrosberMBlykersMMolsPNaeijeNGutermuthJAdrenaline in anaphylaxis treatment and self-administration: experience from an inner city emergency departmentAllergy201772349249727709624
- SimonsFEAnaphylaxis in infants: can recognition and management be improved?J Allergy Clin Immunol2007120353754017765754
- SongTTWormMLiebermanPAnaphylaxis treatment: current barriers to adrenaline auto-injector useAllergy201469898399124835773
- CamargoCSimmonsFwebpage on the Internet] Anaphylaxis: rapid recognition and treatmentUpToDate Online2013141 Available from: http://www.uptodate.com/contents/anaphylaxis-rapid-recognition-and-treatmentAccessed November 27, 2017
- GreenbergerPAFatal and near-fatal anaphylaxis: factors that can worsen or contribute to fatal outcomesImmunol Allergy Clin North Am201535237538625841558
- SimonsFEFirst-aid treatment of anaphylaxis to food: focus on epinephrineJ Allergy Clin Immunol2004113583784415131564
- LiebermanPNicklasRAOppenheimerJThe diagnosis and management of anaphylaxis practice parameter: 2010 updateJ Allergy Clin Immunol20101263477480e1e4220692689
- VickeryBPBerglundJPBurkCMEarly oral immunotherapy in peanut-allergic preschool children is safe and highly effectiveJ Allergy Clin Immunol20171391173181.e827522159
- JonesSMBurksAWKeetCLong-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapyJ Allergy Clin Immunol2017137411171127
- GomezFBogasGGonzalezMThe clinical and immunological effects of Pru p 3 sublingual immunotherapy on peach and peanut allergy in patients with systemic reactionsClin Exp Allergy201747333935028160513
- CasaleTBBurksAWHymenoptera-sting hypersensitivityN Engl J Med2014370151432143924716682
- BonadonnaPGonzalez-de-OlanoDZanottiRVenom immunotherapy in patients with clonal mast cell disorders: efficacy, safety, and practical considerationsJ Allergy Clin Immunol Pract20131547447824565619
- WarrierPCasaleTBOmalizumab in Idiopathic AnaphylaxisAnn Allergy Asthma Immunol2009102325725819354075
- JagdisAVadasPOmalizumab for a case of monoclonal mast cell activation syndrome with recurrent anaphylaxisJ Allergy Clin Immunol20141132AB27
- Prieto-GarciaAZhengDAdachiRMast cell restricted mouse and human tryptase-heparin complexes hinder thrombin-induced coagulation of plasma and the generation of fibrin by proteolytically destroying fibrinogenJ Biol Chem2012287117834784422235124
- CastellsMMast cell mediators in allergic inflammation and mastocytosisImmunol Allergy Clin North Am200626346548516931289
- de LasVecillasSánchezLAlenazyLAGarcia-NeuerMCastellsMCDrug hypersensitivity and desensitizations: mechanisms and new approachesInt J Mol Sci2017186 piiE131628632196
- RomanoATorresMJCastellsMSanzMLBlancaMDiagnosis and management of drug hypersensitivity reactionsJ Allergy Clin Immunol20111273 SupplS67S7321354502
- LaidlawTMBoyceJAPathogenesis of Aspirin-Exacerbated Respiratory Disease and ReactionsImmunol Allergy Clin North Am201333219521023639708
- CahillKNLaidlawTMPathogenesis of aspirin-induced reactions in aspirin-exacerbated respiratory diseaseImmunol Allergy Clin North Am201636468169127712763
- MacyEBernsteinJACastellsMCAspirin Desensitization Joint Task ForceAspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paperAnn Allergy Asthma Immunol200798217217417304886
- TramerMRvon ElmELoubeyrePHauserCPharmacological prevention of serious anaphylactic reactions due to iodinated contrast media: systematic reviewBMJ2006333757067516880193
- WarrenCMOttoAKWalknerMMGuptaRSQuality of life among food allergic patients and their caregiversCurr Allergy Asthma Rep20161653827048239
- ChungMCWalshADennisITrauma exposure characteristics, past traumatic life events, coping strategies, posttraumatic stress disorder, and psychiatric comorbidity among people with anaphylactic shock experienceCompr Psychiatry201152439440421081226
- ChenHHuangNLiWJClinical and laboratory features, and quality of life assessment in wheat dependent exercise-induced anaphylaxis patients from central ChinaJ Huazhong Univ Sci Technolog Med Sci201636341041527376813
- Oude ElberinkJNDuboisAEQuality of life in insect venom allergic patientsCurr Opin Allergy Clin Immunol20033428729312865773
- WarrenCMDyerAAOttoAKFood allergy-related risk-taking and management behaviors among adolescents and young adultsJ Allergy Clin Immunol Pract201652381390.e13
- MuraroARobertsGClarkAEAACI Task Force on Anaphylaxis in ChildrenThe management of anaphylaxis in childhood: position paper of the European Academy of Allergology and Clinical ImmunologyAllergy200762885787117590200
