Abstract
Introduction
Respiratory infections have significant effects on childhood asthma. Viral respiratory infections, such as rhinovirus and respiratory syncytial virus are likely to be important in the development and exacerbation of asthma. In this study, we investigated the nasopharyngeal colonization in children with asthma to determine the prevalence of pathogens and their contribution to respiratory symptoms and airway resistance during winter.
Methods
From December 2016 to March 2017, 50 nasopharyngeal specimens were collected from 18 patients (age, 5.0±1.1 years) with asthma and 9 specimens from 9 control children (age, 4.9±1.0 years). Samples were tested for 19 viruses and 7 bacteria, using multiplex real-time PCR. Respiratory disease markers included the Global Asthma Network Questionnaire, the Common-Cold Questionnaire, the Global Initiative for Asthma assessment of asthma control, and the airway resistance at 5 Hz by forced-oscillation technique.
Results
The most commonly isolated organisms in both groups (patients and controls) were Streptococcus pneumoniae, Haemophilus influenzae, and rhinovirus. Most patients had multiple isolates (median, 3.5; range, 1–5), which changed during the study period. Types of isolates were 4 bacteria (S. pneumoniae, H. influenzae, Bordetella pertussis, and Bordetella parapertussis) and 6 viruses (rhinovirus, enterovirus, metapneumovirus, adenovirus, coronaviruses, and parainfluenza viruses). Similar isolates, including influenza A-H3 virus and bocavirus, were detected in the controls. Of the 9 patients with “wheezing disturbing sleep ≥1 per week”, 6 had rhinovirus, 2 coronaviruses, and 1 no detectable viruses. Patients with mild common cold symptoms had significantly higher airway resistance at 5 Hz z-score (P=0.025).
Conclusion
Multiple respiratory pathogens were isolated from many patients with asthma, which appeared to contribute to disease symptoms and airway resistance. Minimizing children’s exposure to respiratory pathogens might be beneficial, especially during winter.
Introduction
Viruses and bacteria contribute to the pathogenesis and natural history of childhood asthma.Citation1 Respiratory syncytial virus (RSV) predominates in infants and toddlers, while human rhinovirus (hRV), influenza (Flu) viruses, parainfluenza virus (PIV), adenovirus (AdV), coronavirus (CoV), and human enterovirus (hEV) are more prevalent in older children.Citation2
It is common for children with asthma to develop respiratory symptoms, especially during winter. Many of their clinical findings, however, result from respiratory infections that require supportive care and minimizing exposure to respiratory pathogens.Citation3,Citation4 The prevailing practice, nevertheless, is escalating the use of short-acting β-agonists (SABAs), long-acting β-agonists (LABAs), inhaled corticosteroids (ICSs), and leukotriene receptor antagonist. These medications are expensive and may impose serious adverse events, especially in young children.Citation5 Therefore, identifying and controlling triggers of asthma deserve further studies.
There are no studies from the United Arab Emirates (UAE) or surrounding countries that have evaluated respiratory infections or nasopharyngeal isolates in patients with asthma. However, few studies have addressed respiratory viral infections at community level. For example, in Saudi Arabia, most cases of RSV occur from November through March and some cases have been reported at other months of the year.Citation6 In Kuwait, a study that has investigated the causative agents in >1,000 patients with lower respiratory tract infections using PCR revealed that RSV and hRV are the major isolates among hospitalized children from October to March. PIV-2 and human CoV were not detected in any of the patients’ samples.Citation7
Regular surveillance, especially during the winter is necessary if pathogens are to be identified with a view to possible prevention. This prospective, case–control pilot study aimed to estimate the prevalence of nasopharyngeal isolates in children with asthma during winter season and identify their clinical impact on respiratory symptoms and function. Its main objective was to address the importance of controlling respiratory pathogens in the treatment of childhood asthma.
Materials and methods
This study was conducted between December 2016 and March 2017 in the Pediatric Pulmonary Clinic at Tawam Hospital (Al Ain, UAE). It was approved by the Research Ethics Committee of the UAE University – College of Medicine and Health Sciences (ERH 2015 3235 15 111). Written informed consent was obtained from the parents of all participants.
Children 3–6 years of age, with a diagnosis of asthma, were eligible to participate in the study. The clinical diagnosis of asthma was made by the pediatric pulmonologist, based on the Global Initiative for Asthma (GINA) criteria.Citation8 Asthma staging ranged from mild to moderate, according to severity level and if the children required either ICS alone or in association with either LABA or montelukast. The study was explained to the parents of all eligible patients, and, if they agreed to the procedures, their children were enrolled in the study. The control group consisted of age-matched children without asthma, mainly relatives of our co-workers.
Immunization was up-to-date in all the studied participants. Children were excluded from the study if they had significant illnesses, such as chronic lung disease of prematurity, cystic fibrosis, congenital heart disease, immune deficiency, or upper airway anomaly. Demographic, medical, and vaccination data were reviewed. History of asthma symptoms was obtained using a modified global asthma network questionnaire.Citation9 For the assessment of symptoms of viral infection, we used a validated Common Cold Questionnaire (CCQ).Citation10 This questionnaire inquired about the presence of fever, chills, muscle pain, watery eyes, runny nose, sneezing, sore throat, cough, and chest pain during 2 days prior to specimen collection; each complaint was scored as none (0), mild (1), moderate (2), or severe (3). The GINA assessment of asthma control was used to assess the level of asthma control over the past 4 weeks.Citation8 This instrument scored the presence (1) or absence (0) of each of the 4 clinical variables, day symptoms, night symptoms, reliever use, and exercise limitation. A score of zero indicated well-controlled asthma, 1–2 partly-controlled asthma, and 3–4 uncontrolled asthma.
Forced oscillation technique (FOT) was used to assess airway resistance using a commercial device (tremoFlo™ C-100, tremoFlo software, version 1.0.34 build 32; Thorasys Medical Systems, Montreal, QC, Canada), as previously described.Citation11 Briefly, measurements were performed with the child sitting upright with the head in a neutral position, the cheeks supported, and the nose clipped. The child was instructed to breathe tidally through a mouthpiece. Data were collected over several seconds and the average of 3 acceptable measurements (coefficient of variation ≤15%) was taken. Airway resistance at 5 Hz (R5, in cmH2O.s.L−1) was expressed as R5 z-score.Citation11
Sample collection and processing
Multiple (1–3 and 4–6 weeks apart) nasopharyngeal specimens were collected from each patient between December 2016 and March 2017 (, , S1, and S2). For controls, each participant had only 1 sample collected between January and March 2017 (). The specimens were stored at −70°C until analysis, which was performed using the Allplex™ Respiratory Full Panel Assay (Seegene Biotechnology Inc., Seoul, Korea) as instructed by the manufacturer.Citation12 The assay was composed of four panels and utilized a multiplex one-step real-time PCR to identify 16 viruses (with three influenza A subtypes) and seven bacteria. The viruses were influenza (Flu A, B, A-H1, A-H1pdm09, and A-H3), PIV (1–4), RSV (A and B), AdV, hEV, human metapneumovirus (MPV), human bocavirus (hBoV), hRV, and CoV NL63, CoV 229E, and CoV OC43. The bacteria were Streptococcus pneumoniae (SP), Haemophilus influenzae (HI), Mycoplasma pneumoniae, Chlamydophila pneumoniae, Legionella pneumophila, Bordetella pertussis (BP), and Bordetella parapertussis (BPP).
Table 1 Nasopharyngeal isolates, airway resistance (R5), “common cold” symptoms (CCQ), and GINA assessment of asthma control in the 18 patients with asthma (33 pathogens of 10 types); December 2016–January 2017.
Table 2 CCQ score, GINA assessment of asthma control score, R5 z-score, nasopharyngeal isolates, and escalation of asthma prophylaxis
Table 3 Nasopharyngeal isolates, airway resistance, and “common cold” symptoms in the 9 control children (23 isolates of 8 different types); December 2016–March 2017.
Statistical analysis
The data were analyzed with the SPSS statistical package (version 20). Descriptive statistics included the number and percentage, or the measured value of the observed variables, as appropriate. ANOVA was used to compare the R5 z-score on one hand, with the number of isolated pathogens, the GINA asthma control score, and the CCQ score on the other hand. The unpaired t-test was used to compare the R5 z-score between the children with asthma and the controls. For all tests, statistical significance was defined by a 2-sided P-value <0.05.
Results
Fifty nasopharyngeal specimens were collected between December 2016 and March 2017 from 18 patients with asthma (age, 5.0±1.1 years). As patient 18 was recruited into the study only in February 2017 (), no data are available on him prior to that time, and his later results are included in Table S1. The results of studies performed between December 2016 and January 2017 are shown in , between January 2017 and February 2017 in Tables S1, and in March 2017 in Tables S2. Patients’ responses to the Global Asthma Network Questionnaire are shown in Table S3. Only 6 patients used daily asthma prophylaxis (Table S3). In addition, 9 nasopharyngeal specimens were also collected from 9 control children who had no history of asthma (age, 4.9±1.0 years); their results are shown in .
The prevalence of each isolate in patients and controls are shown in and S1. The most common organisms were SP, HI, and hRV for both groups.
Figure 1 Nasopharyngeal pathogens isolated from the 18 patients with asthma and the 9 healthy children during the studied winter periods.
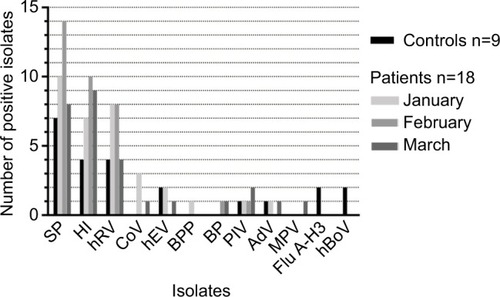
Patients (December 2016–January 2017): Thirty-three pathogens (18 bacteria and 15 viruses) of 10 different types were isolated from 16 (94%) of 17 patients with asthma; Patient 7 had no detectable isolate (). These organisms were SP (10 patients), HI (7 patients), BPP (1 patient), rhinovirus (7 patients), CoV (2 patients), hEV (2 patients), AdV (1 patient), and PIV-3 (1 patient). Four (23%) patients had 4 pathogens, 1 (6%) had 3 pathogens, 3 (18%) had 2 pathogens, 8 (47%) had 1 pathogen, and 1 (6%) had none. Six (75%) of 8 patients with rhinovirus had a treatment modification, such as starting ICS (3 patients), increased ICS dosing (2 patients), or adding montelukast or salmeterol to the ICS (3 patients), .
Nine (52%) patients had mild common cold symptoms (CCQ scores ranged from 1 to 7 of a maximum score of 27), 4 (24%) had moderate symptoms (CCQ scores ranged from 8 to 16), and 4 (24%) had no symptoms (CCQ score =0), . Isolates from patients with CCQ score ≥7 included SP (Patients 4, 9, and 12), HI (Patients 4, 12, and 17), BPP (Patient 4), and hRV (Patients 4, 12, and 13).
Twelve (80%) of 15 patients had GINA assessment of asthma control score of ≥2 (maximum score, 4; ). The R5 z-score (airway resistance) was not significantly different when compared among children with different number of pathogens isolated (P=0.2, ANOVA). Similarly, the R5 z-score was not significantly different among children with different CCQ scores (P=0.1, ANOVA) or GINA control scores (P=0.5, ANOVA).
Patients (February–March 2017): Isolate profiles changed in the majority of patients (Tables S1 and S2). For example, Patient 3 acquired BP and Patient 6 rhinovirus (Table S1). Many of previously detected viruses were not present on the subsequent testing; eg, Patients 6 and 8 lost their colonizations with CoV (Table S1). Different strains of PIV also appeared in March 2017 (PIV1 in Patient 11, and PIV4 in Patient 12; Table S2); while the strain detected in December–January was PIV3 (Patient 14, ).
It is worth noting that BP and BPP were detected during the entire study period. The 2 patients with BP (Patients 2 and 3, Tables S1 and S2) had increased ICS dosing.
shows the pathogens isolated from patients sorted by varying degrees of CCQ and GINA assessment of asthma control scores. The 4 patients with CCQ scores =0 and GINA assessment of asthma control scores ≥2 had increased airway resistance (high mean R5 z-scores), suggesting poor asthma control. However, only 2 of these patients had treatment escalation.
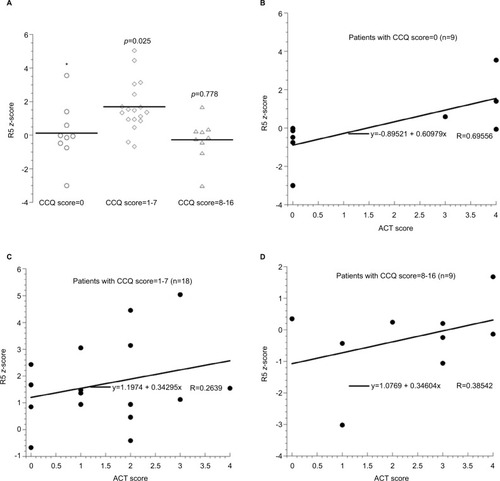
The impact of common cold symptoms on airway resistance is shown in . Patients with a CCQ score of 1–7 had significantly higher R5 z-score (P=0.025).
Figure 2 The horizontal lines are mean values. The P-values are Mann–Whitney (2-tailed) between CCQ score =0 and the other groups. Kruskal–Wallis test that compared 3 CCQ groups revealed an asymptotic significance (2-tailed) of 0.013. Panel (A): R5 z-scores in the patients with asthma as function of CCQ score. Panels (B–D): R5 z-scores as function of Global Initiative of Asthma assessment of asthma control score in the patients with asthma grouped by their CCQ score.
Nine of the 18 patients had “wheezing disturbing sleep ≥1 per week” (Table S3). Six of these patients had rhinovirus, 2 had CoV, and 1 had severe atopy (Patient 18), .
Treatment modifications: The treatment modifications, based on clinical findings, were made at the discretion of their pediatric pulmonologist and are detailed in . Many patients were already on controller medications prior to the study as shown in Table S3.
Controls (December 2016–March 2017): Twenty-three pathogens isolated from the control children: SP (7 children), HI (4 children), rhinovirus (4 children), Flu A-H3 (2 children), enterovirus (2 children), hBoV (2 children), PIV4 (1 child) and AdV (1 child), and . Two (22%) children had 4 pathogens, 2 (22%) had 3 pathogens, 4 (45%) had 2 pathogens, and 1 (11%) had 1 pathogen. The results of their airway resistance and CCQ scores are detailed in . The R5 z-score (airway resistance) was not significantly different between children with asthma and controls (P=0.8, unpaired t-test).
Discussion
The nasopharynx is a reservoir for pathogens associated with respiratory diseases, such as asthma. Airway microbiome studies have shown that bacteria may play a substantial role in the onset, evolution, and severity of asthma.Citation13
This study was conducted to test the hypothesis that nasopharyngeal colonizations with community-acquired pathogens have an adverse impact on the natural history of asthma in young children. The results show that the majority of patients with asthma had several viral pathogens, which contributed to symptoms and airway resistance ( and ). The clinical impact of the respiratory pathogens on asthma control is shown in . Patients 2 and 3 had increased CCQ scores in association with colonizations with HI and BP on the second sampling. The increased airway resistance in Patient 5 on the second sample collection could be due to colonization with HI or adverse events of prior AdV. Similarly, the increased airway resistance in Patient 8 could be due to prior CoV OC43. The high airway resistance in Patient 14 could be due to PIV-3. As the course and severity of asthma are also related to the role of many environmental factors (such as air pollution, humidity, and smoking), medication adherence, and poor inhaler technique, it is not totally unexpected that the role of respiratory pathogens cannot be considered in isolation and cannot be solely responsible for asthma control. With respect to the controls (), Children 1 and 6 both had high airway resistance associated with multiple colonizations. Child 1 was found subsequently to have symptoms of allergic rhinitis and mild night cough.
The 2 patients who were found to have pertussis had increased symptoms, possibly falsely attributed to worsening asthma control, leading to inappropriate escalation of their medications before the infection was diagnosed (Tables S1 and S2). In addition, patients with asthma are at increased risk for pertussis infection. This was highlighted during the 2004 pertussis outbreak in MN, USA where the population attributable risk percentage of asthma for risk of pertussis was calculated to be 17%.Citation14 Consistently, humoral immunoreaction to BP could be suppressed in patients with asthma.Citation15 Therefore, targeting patients with asthma for pertussis surveillance and vaccination as a selective high-risk group might be an appropriate strategy.
Children with asthma may have an increased risk of pneumonia.Citation16 This might be facilitated by their use of ICS, as these medications also inhibit mucosal immune responses, thus encouraging colonization with organisms.Citation5
In children with no common cold symptoms, 33% had asthma prophylaxis therapy escalated in association with an elevated GINA assessment of asthma control score and increased airway resistance (probably justifiably; ). In those with mild common cold symptoms, 20% had asthma prophylaxis therapy increased in association with increased airway resistance, although 50% had low GINA assessment of asthma control score. In those with more severe common cold symptoms, 50% had asthma prophylaxis therapy escalated, regardless of the GINA assessment of asthma control score and without increase in airway resistance (probably intensification triggered by the severity of cold symptoms instead of asthma score or airway resistance; probably not justified here), .
In the northern hemisphere, most asthma-related emergency department visits are higher in September than other months. In late fall, there is often a “second wave” with fluctuations throughout winter probably coinciding with rhinovirus episodes.Citation17,Citation18 Symptomatic rhinovirus infections are found to be an important contributor to asthma exacerbations in children.Citation19 In this study, rhinovirus (hRV) was detected in the majority of patients (). Future studies are needed to address important variables relevant to this organism, such as serotypes (especially HRV-16), upper vs lower respiratory colonization, and host susceptibility (eg, variability in expression of intercellular adhesion molecule-1).Citation2
Airway inflammation has been reported in infections with Flu viruses, which are well known to induce severe exacerbation in adults.Citation4 M. pneumoniae and C. pneumoniae, on the other hand, seem to be involved in asthma persistence.Citation1,Citation4 The atypical bacteria C. pneumoniae and M. pneumoniae were not detected in our studied population. These pathogens may be more important in adults with chronic asthma. Similarly, RSV and MPV were not detected in the patients. This finding could be due to the small sample size of the study or to the regional epidemic pattern of these pathogens. Thus, surveil-lances that cover the entire year are necessary.
In 1 study, children (3 months to 16 years of age) with asthma exacerbation had a high prevalence of respiratory pathogens that included RSV, hRV, M. pneumoniae, and C. pneumoniae. Most hospitalizations were associated with seasonal hRV and RSV.Citation4 MPV and hBoV were previously reported in children with asthma exacerbation.Citation12,Citation13,Citation20,Citation21 In this current study, hBoV was detected in 1 healthy child () and MPV in 1 patient (Table S2).
It is worth noting that R5 z-score in patients with a CCQ score ≥8 was similar to those with CCQ score =0 (). In addition, R5 z-scores correlated best with GINA assessment of asthma control scores in patients with CCQ score =0 (R>0.695) (). These findings reflect a limitation in using FOT in patients with significant upper respiratory symptoms, which have been shown to influence FOT resistance measurements.
The study finding supports the need for strategies that limit children’s exposure to respiratory viruses and bacteria, especially those with poorly controlled disease. Specific measures that have been suggested to minimize recurrent infections in children with asthma include hand hygiene, a healthy balanced diet, active probiotic supplements, and the immunostimulant OM-85.Citation22 Further studies, however, are needed to investigate whether reducing exposure to pathogens would improve asthma control. In addition, the role of other environmental factors (such as air pollution, humidity, and smoking), medication adherence, and poor inhaler technique has to be taken into consideration in future studies.
Conclusion
The majority of patients with asthma had viral and bacterial pathogens contributing to their disease. Regular surveillance could play a role in asthma care, especially in those with poorly controlled disease. Effective strategies to minimize exposure to respiratory pathogens, such as hand hygiene should be incorporated in childhood asthma guidelines. Such approach would emphasize the importance of environmental control measures rather than relying solely on escalating asthma drug therapy with its potential toxicity. Further studies are needed to evaluate whether strategies that minimize children’s exposure to pathogens would improve asthma control.
Author contributions
ARA conceived the study, participated in its design and coordination, and drafted the manuscript. AMA, AA, and GA recruited participants and collected clinical data. JG and SMK coordinated and performed PCR experiments. HN and AKS participated in the data analysis and manuscript preparation. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors are grateful to all participating children and their parents. The contribution of Mrs Sania M Al-Hamad toward data collection is greatly appreciated. The study was funded by a grant from the College of Medicine and Health Sciences, UAE University (31M252). The funding body had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
Supplementary materials
Figure S1 Percent positive nasopharyngeal pathogens isolated from the 18 patients with asthma (A) and the 9 healthy children (B) during the studied winter periods.
Note: “x”, not detected.
Abbreviations: AdV, adenovirus; BP, Bordetella pertussis; BPP, Bordetella parapertussis; CoV coronavirus; Flu A-H3, influenza A virus-H3; hBoV, human bocavirus; hEV, human enterovirus; HI, Haemophilus influenzae; hRV, human rhinovirus; PIV1, parainfluenza virus 1; PIV3, parainfluenza virus 3; SP, Streptococcus pneumoniae.
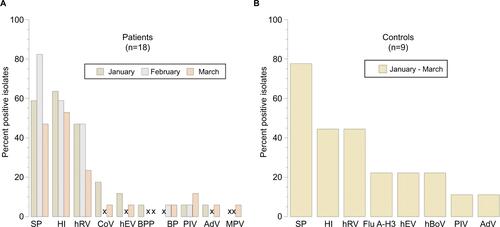
Table S1 Nasopharyngeal isolates, airway resistance (R5), “common cold” symptoms (CCQ), and GINA score in the 18 patients with asthma
Table S2 Nasopharyngeal isolates, airway resistance (R5), “common cold” symptoms (CCQ), and GINA in the 18 patients with asthma
Table S3 Responses to the Global Asthma Network Questionnaire (based on symptoms in the past 12 months) in the 18 patients with asthma
Disclosure
The authors report no conflicts of interest in this work.
References
- PapadopoulosNGChristodoulouIRohdeGViruses and bacteria in acute asthma exacerbations--a GA2 LEN-DARE systematic reviewAllergy201166445846821087215
- ToveyERStelzer-BraidSToelleBGRhinoviruses significantly affect day-to-day respiratory symptoms of children with asthmaJ Allergy Clin Immunol2015135366366925476729
- Halmø HürdumSZhangGKhooSKRecurrent rhinovirus detections in children following a rhinovirus-induced wheezing exacerbation: A retrospective studyInt J Pediatr Child Health201531101828018912
- MaffeyAFBarreroPRVenialgoCViruses and atypical bacteria associated with asthma exacerbations in hospitalized childrenPediatr Pulmonol201045661962520503289
- ZhangLPrietschSOMendesAPInhaled corticosteroids increase the risk of oropharyngeal colonization by Streptococcus pneumoniae in children with asthmaRespirology201318227227723039314
- Al-AlaiyanSPollackPNotarioGFSafety and pharmacokinetics of extended use of palivizumab in Saudi Arabian infants and childrenDrugs Context20154 pii: 212270
- KhadadahMEssaSHigaziZBehbehaniNAl-NakibWRespiratory syncytial virus and human rhinoviruses are the major causes of severe lower respiratory tract infections in KuwaitJ Med Virol20108281462146720572084
- GINAGlobal Initiative for Asthma, Global Strategy for Asthma Management and Prevention2015 Available from: https://www.ginasthma.orgAccessed on October 24, 2016
- Global Asthma Network Available from: http://www.globalasthmanetwork.org/surveillance/manual/study6.phpAccessed October 24, 2016
- PowellHSmartJWoodLGValidity of the common cold questionnaire (CCQ) in asthma exacerbationsPLoS One2008193e1802
- AlblooshiAAlkalbaniANarchiHRespiratory function in healthy Emirati children using forced oscillationsPediatr Pulmonol201853793694129528572
- ParkSOhKCKimKSRole of Atypical Pathogens and the Antibiotic Prescription Pattern in Acute Bronchitis: A Multicenter Study in KoreaJ Korean Med Sci201530101446145226425041
- Pérez-LosadaMAlamriLCrandallKAFreishtatRJNasopharyngeal Microbiome Diversity Changes over Time in Children with AsthmaPLoS One2017121e017054328107528
- CapiliCRHettingerARigelman-HedbergNIncreased risk of pertussis in patients with asthmaJ Allergy Clin Immunol2012129495796322206778
- NakamuraAIwashimaYTakakuwaOSatoSSensitivity to bordetella pertussis in asthmatic patientsEur Respir J201138p2515
- MartinMShawDEffect of inhaled corticosteroids on the microbiology of the respiratory tractRespirology201318220120223347106
- LarsenKZhuJFeldmanLYThe Annual September Peak in Asthma Exacerbation Rates. Still a Reality?Ann Am Thorac Soc201613223123926636481
- CohenHABlauHHoshenMBatatEBalicerRDSeasonality of asthma: a retrospective population studyPediatrics20141334e923e93224616356
- KhetsurianiNKazerouniNNErdmanDDPrevalence of viral respiratory tract infections in children with asthmaJ Allergy Clin Immunol2007119231432117140648
- JarttiTvan den HoogenBGarofaloRPOsterhausADRuuskanenOMetapneumovirus and acute wheezing in childrenLancet200236093431393139412423987
- AllanderTJarttiTGuptaSHuman bocavirus and acute wheezing in childrenClin Infect Dis200744790491017342639
- AhanchianHJonesCMChenY-ShengSlyPDRespiratory viral infections in children with asthma: do they matter and can we prevent them?BMC Pediatr20121214722974166
