Abstract
Background
Patients with severe, uncontrolled asthma experience debilitating symptoms that result in meaningful reductions to health-related quality of life. Benralizumab is an interleukin-5 receptor alpha–directed cytolytic monoclonal antibody that reduces exacerbations and improves asthma symptoms for patients with severe, uncontrolled eosinophilic asthma.
Objective
The objective of this study was to evaluate improvements in daily asthma-related health status outcomes following treatment with benralizumab.
Methods
Pooled results from the SIROCCO (NCT01928771) and CALIMA (NCT01914757) Phase III studies were analyzed. Patients aged 12–75 years with severe, uncontrolled asthma, and blood eosinophil counts (BEC) ≥300 and ≥150 cells/µL were evaluated. Patients received subcutaneous benralizumab 30 mg every 4 weeks (Q4W) or every 8 weeks (Q8W, first three doses Q4W) or placebo and completed a daily diary reporting rescue medication use, night-time awakening requiring rescue medication use, perceived tiredness, and asthma-related activity impairment. Outcome measures were compared across treatment arms from baseline to end of treatment (EOT) using a mixed-effect model for repeated measures analyses.
Results
Patients with BEC ≥300 cells/µL receiving benralizumab Q8W had greater improvements in all patient-reported outcomes at EOT relative to baseline than patients receiving placebo (all nominal P≤0.013). Effects were reported as early as 3 days following the initial dose and sustained throughout treatment for daily and night-time rescue medication use and night-time awakenings requiring rescue medication. For patients with BEC ≥300 and ≥150 cells/ µL, sustained improvements in activity impairment items (all nominal P<0.05) were achieved with benralizumab Q8W at week 2.
Conclusion
Benralizumab produces sustained reductions by as early as 3 days in rescue medication use and activity impairment for patients with severe, uncontrolled eosinophilic asthma.
Introduction
Patients with severe, uncontrolled asthma experience serious health-related quality of life impairments,Citation1–Citation4 reporting difficulties in performing daily activities and engaging in social interactions, as well as increased tiredness.Citation2–Citation5 Patients often make adjustments to their daily activities in response to their asthma symptoms, such as pacing themselves during activities or avoiding activities entirely.Citation2–Citation4 Difficulties with daily activities can be particularly impactful for patients with severe asthma.Citation6 Symptoms such as shortness of breath, coughing, and night-time awakenings can occur on a daily basis.Citation2,Citation5,Citation6 This can result in a decrease in patients’ health-related quality of life that can be challenging for physicians to manage. Treatments to alleviate symptoms, reduce activity impairment, and improve salient aspects of day-to-day life are needed for patients with severe, uncontrolled asthma.
Benralizumab is an interleukin-5 receptor alpha–directed cytolytic monoclonal antibody that has received marketing approval in various countries, including the United States and Europe.Citation7,Citation8 In the United States, benralizumab is indicated for the add-on maintenance treatment of patients with severe asthma, aged ≥12 years with an eosinophilic phenotype.Citation7 Benralizumab targets eosinophilic inflammation by inducing direct, rapid, and nearly complete depletion of eosinophils via enhanced antibody-dependent cell-mediated cytotoxicity, an apoptotic process of eosinophil elimination involving natural killer cells.Citation9,Citation10 Eosinophilic inflammation is present in 50% of patients with asthma and is associated with decreased lung function and increased disease severity, exacerbation frequency, and symptom burden.Citation11–Citation13 In two Phase III trials, SIROCCO (NCT01928771) and CALIMA (NCT01914757), benralizumab significantly reduced asthma exacerbations and improved lung function and disease symptom control for patients with severe, uncontrolled asthma and blood eosinophil counts (BECs) ≥300 cells/µL vs placebo in combination with high-dosage inhaled corticosteroids plus long-acting β2-agonists (ICS/LABA).Citation14,Citation15 Benralizumab also improved asthma control and health-related quality of life for these patients, as measured by the Asthma Control Questionnaire 6 (ACQ-6) and the Asthma Quality of Life Questionnaire (standardized) for 12 years and older (AQLQ[S]+12).Citation14,Citation15
This study evaluated the effect of benralizumab on daily measures of health status, including rescue medication use, asthma-related activity impairment, and patient-perceived tiredness, for patients with severe, uncontrolled asthma with eosinophilic inflammation through a pooled analysis of the SIROCCO and CALIMA studies.
Methods
Study design and participants
SIROCCO and CALIMA were randomized, double-blind, parallel-group, placebo-controlled, global Phase III studies.Citation14,Citation15 The study design comprised an enrollment visit (week –4), 4-week screening/run-in phase, randomization phase (week 0), 48- (SIROCCO) or 56-week (CALIMA) treatment period, and final follow-up visit 8 (SIROCCO) or 4 (CALIMA) weeks following the end-of-treatment (EOT) period. Enrollment criteria for the studies have been described previously.Citation14,Citation15 The studies included male and female patients aged 12–75 years with a weight of ≥40 kg and physician-diagnosed asthma that required treatment with medium- to high-dosage ICS/LABA for ≥12 months before enrollment. Inclusion criteria included ≥2 asthma exacerbations within 12 months before enrollment that required systemic corticosteroid therapy or a temporary increase in the usual maintenance dosage of oral corticosteroid. Patients must also have had documented treatment with high-dosage ICS/LABA (>500 µg/d fluticasone propionate or equivalent daily dosage) with or without oral corticosteroids and additional asthma controllers for ≥3 months before enrollment. Additional inclusion criteria included a pre-bronchodilator FEV1 of <80% predicted (<90% predicted for patients aged 12–17 years) at screening (week –3), a documented post-bronchodilator reversibility of ≥12% and ≥200 mL in FEV1 within 12 months prior to enrollment or demonstrated at screening, and an ACQ-6 score ≥1.5 at enrollment. Studies were conducted in accordance with the Declaration of Helsinki, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use/Good Clinical Practice guidelines, and the ethics committee at each participating site.
In both studies, patients were randomized 1:1:1 to receive either subcutaneous benralizumab 30 mg every 4 weeks (Q4W), benralizumab 30 mg Q4W for the first three doses followed by every 8 weeks (Q8W) for the remainder of the treatment period, or placebo Q4W. Patients with baseline BECs ≥300 and <300 cells/µL were recruited at a ratio of ~2:1, respectively.
Outcomes
Patients completed an Asthma Daily Diary twice daily at home using a handheld device. Daily patient-reported outcome (PRO) assessments included daytime and night-time symptoms reported previously,Citation14,Citation15 as well as daytime and night-time rescue medication use; night-time awakenings requiring rescue medication; activity limitations, activity avoidance, need to self-pace during activities; and perceived tiredness. Content of the Asthma Daily Diary was informed by a previously reported patient interview study that indicated these concepts were salient, distinct, and variable.Citation3 The Asthma Daily Diary was completed in the morning upon awakening and evening before going to bed from the evening of the screening visit to the last morning of the treatment period (SIROCCO, week 48; CALIMA, week 56). Night-time rescue medication use and night-time awakenings requiring rescue medication were recorded in the morning diary, whereas daytime rescue medication use, activity impairments, and perceived tiredness were captured in the evening diary.
Patient responses to the three activity impairment questions and the perceived tiredness item were captured using a 5-point categorical response scale of 0–4, in which 0 indicated no limitation/none of the time and 4 indicated totally limited/all of the time. Rescue medication use was recorded as the total number of puffs used in each recall period (night-time and daytime). Daily rescue medication use was the sum of the two daily measures. Night-time awakening requiring rescue medication use was assessed with a yes (1)/no (0) response. Baseline for daily measures was the 10-day period before randomization at week 0. Daily assessments were summarized as biweekly means if ≥7 of 14 daily assessments were available. On days 3 and 7, 3-day and 7-day averages were calculated if ≥2 and ≥4 daily assessments were available, respectively. Night-time awakening requiring rescue medication use was calculated as the percentage of nights with awakening requiring rescue medication use during the summary time periods (ie, 3 days, 7 days, and 2 weeks). Outcome measures consisted of changes in biweekly mean item scores from baseline to EOT and changes in mean item scores from baseline to days 1, 3, and 7 of the study. Analyses were performed for patient populations with BECs ≥ and < 300 cells/µL and ≥ and < 150 cells/µL. Data are reported and discussed for the indicated benralizumab Q8W dosage; results for the benralizumab Q4W dosage are presented in tables and figures to allow for evaluation of data consistency with previous studies.
Statistical analysis
Analyses were based on the full analysis set according to the intention-to-treat principle for the pooled SIROCCO and CALIMA studies. This set included all randomized patients who received any study treatment and continued participation in the study, regardless of protocol adherence. Analyses were performed with SAS version 9.2 and above (SAS Institute Inc, Cary, NC, USA).
Each PRO measure was compared across treatment arms from baseline to EOT for patients in the pooled data set using a mixed-effect model for repeated measures analysis, with adjustments for treatment, study code, baseline value, region, oral corticosteroids use, visit, and treatment × visit. For days 1, 3, and 7 analyses, comparisons across treatment arms used analysis of covariance, with adjustments for treatment, study code, baseline value, oral corticosteroid use, and geographic region. Least squares (LS) means, treatment differences in LS means, 95% confidence intervals, and P-values were calculated. Because these analyses were not part of the formal testing strategy, all P-values were nominal. To account for the 2:1 stratification for baseline BECs (≥300 and <300 cells/µL) in analyses for all patients, patients with baseline BECs <300 cells/µL were reweighted using the ratio of the number of patients with baseline BECs ≥300 cells/µL to the number of those who had counts <300 cells/µL. Similar reweighting was performed for patients with BECs <150 cells/µL.
Ethics approval
Before any patient was enrolled, an independent ethics committee or institutional review board at each study center approved the clinical study protocol, and the national regulatory authority either approved the clinical study protocol or received a notification according to local regulations. All patients provided written informed consent at enrollment.
Results
A total of 1,537 (515 placebo, 516 benralizumab Q4W, 506 benralizumab Q8W) and 1,941 (648 placebo, 647 benralizumab Q4W, 646 benralizumab Q8W) patients with BECs ≥300 and ≥150 blood cells/µL, respectively, receiving high-dosage ICS/LABA were evaluated from the SIROCCO and CALIMA studies. Demographics and clinical characteristics were similar between the placebo and benralizumab Q8W groups.Citation14,Citation15 Only the indicated Q8W dosage of benralizumab is discussed here, with Q4W results provided in the – and – for completeness, as they were part of the SIROCCO and CALIMA studies.
Figure 1 Reduction in rescue medication use with benralizumab and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts ≥300 cells/µL).
Abbreviations: EOT, end of treatment; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W).
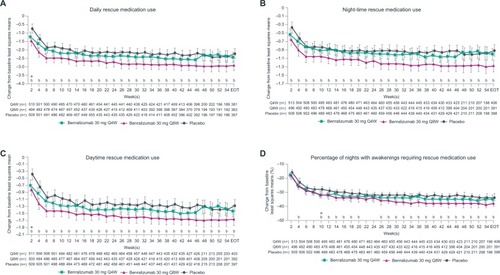
Figure 2 Improvement in patient-reported outcomes with benralizumab and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts ≥300 cells/µL)
Abbreviations: EOT, end of treatment; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W).
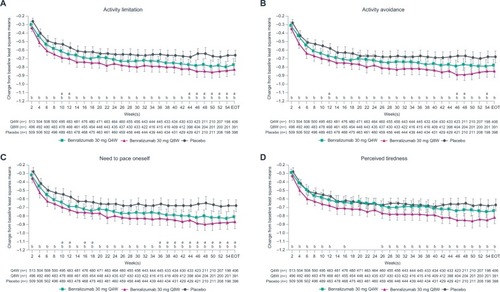
Figure 3 Reduction in rescue medication use with benralizumab and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts ≥150 cells/µL).
Abbreviations: EOT, end of treatment; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W).
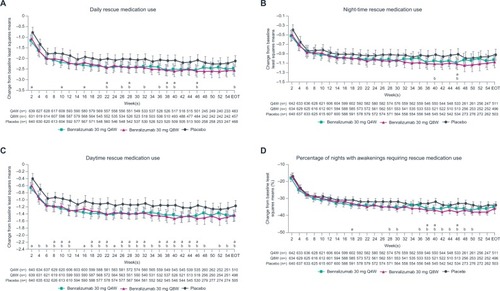
Figure 4 Improvement in patient-reported outcomes with benralizumab and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts ≥150 cells/µL).
Abbreviations: EOT, end of treatment; ICS, inhaled corticosteroids; LABA, long-acting β2-agonists; Q4W, every 4 weeks; Q8W, every 8 weeks (first three doses Q4W).
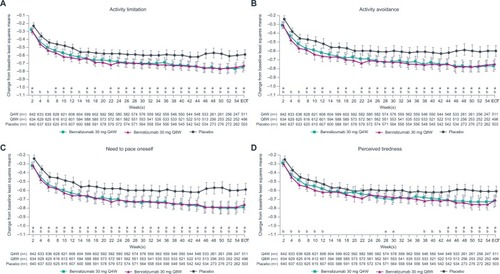
Table 1 Patient-reported outcome assessment changes from baseline to EOT for patients receiving benralizumab vs placebo and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts ≥300 cells/µL)
Table 2 Patient-reported outcome assessments on days 1, 3, and 7 for patients receiving benralizumab Q8W vs placebo and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts ≥300 cells/µL)
Table 3 Patient-reported outcome assessment changes from baseline to EOT for patients receiving benralizumab vs placebo and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts ≥150 cells/μL)
Table 4 Patient-reported outcome assessments on days 1, 3, and 7 for patients receiving benralizumab Q4W vs placebo and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts ≥300 cells/μL)
Table 5 Patient-reported outcome assessment changes from baseline to EOT for patients receiving benralizumab vs placebo and high-dosage ICS/LABA (full analysis set, pooled, blood eosinophil counts <300 or <150 cells/µL)
Improvements from baseline to EOT were observed for all daily PROs for patients receiving benralizumab Q8W and with BECs ≥300 cells/µL when compared with placebo (all nominal P≤0.013) (). Daily rescue medication use was reduced by 0.71 puffs (95% CI: −1.12 to −0.29, nominal P≤0.001) with benralizumab Q8W vs placebo. Overall, daily rescue medication use for patients receiving benralizumab Q8W decreased by a mean of 2.93, from 4.4 puffs at baseline to 1.47 puffs at EOT. Changes in daytime and night-time rescue medication use for benralizumab Q8W vs placebo contributed similarly to overall daily use (). Reductions in daily and night-time rescue medication use began as early as 3 days with benralizumab Q8W, and decreased daytime rescue medication use was observed at 1 week (). These reductions in rescue medication use were sustained until EOT (). Patients in the benralizumab Q8W and placebo groups with BECs ≥300 cells/µL experienced night-time awakenings requiring rescue medication use on 50% of nights during the baseline period, which was reduced to 12% and 16%, respectively, at EOT ().
Patients receiving benralizumab Q8W showed improvement from baseline to EOT in the three activity impairment items and perceived tiredness compared with those receiving placebo (). The reduction in activity impairments in favor of benralizumab Q8W was observed as soon as week 2 (), whereas continued separation in favor of benralizumab Q8W for perceived tiredness was not achieved until week 26 ().
Patients with BECs ≥150 cells/µL showed greater improvement in all daily PROs compared with placebo, except for night-time rescue medication use and night-time awakenings requiring rescue medication use, at EOT with benralizumab Q8W (all nominal P<0.05; ). Consistently greater reductions vs placebo in activity limitation, activity avoidance, and need for self-pacing began after 2 weeks with benralizumab Q8W (all nominal P<0.05; ). Changes in daily PROs from baseline to EOT for patients with BECs <300 or <150 cells/µL were greater in the benralizumab Q8W group compared with placebo (). However, these differences were less than those observed for groups with BECs ≥300 or ≥150 cells/µL.
Discussion
Patients with severe, uncontrolled asthma experience debilitating symptoms that result in meaningful reductions to health-related quality of life. Asthma symptoms compromise a patient’s ability to perform day-to-day activities and sleep without interruption, and this can manifest as increased feelings of tiredness, anxiety, and depression.Citation3 Patients often have to pace themselves during activities or avoid activities as a response to symptoms or as a way to pre-emptively avoid symptom worsening.Citation3 Treatments that provide symptom relief for patients with severe, uncontrolled asthma need to demonstrate benefits for patients’ well-being and ability to perform daily activities. In this study, we demonstrate that benralizumab Q8W in combination with high-dosage ICS/ LABA can reduce rescue medication use by as early as 3 days and decrease activity impairments for patients with severe, uncontrolled asthma and BECs ≥300 cells/µL compared with placebo. Reductions in daily and night-time medication use were observed as early as day 3 following treatment with benralizumab Q8W vs placebo. Patient-reported activity limitations, activity avoidance, and need for self-pacing were consistently decreased after 2 weeks.
Results from this study are consistent with reported efficacy results for patients with severe, uncontrolled asthma with eosinophilic inflammation receiving benralizumab Q8W and high-dosage ICS/LABA.Citation14,Citation15 In both SIROCCO and CALIMA, significant improvements in prebronchodilator FEV1 occurred after 4 weeks of treatment with benralizumab Q8W relative to placebo for patients with BECs ≥300 cells/ µL.Citation14,Citation15 Overall assessments of patient-reported symptoms and/or health-related quality of life using total asthma symptom score, ACQ-6, and AQLQ[S]+12 also indicated benefits with benralizumab Q8W vs placebo at EOT.Citation14,Citation15 Consistent improvements in total asthma symptom score with benralizumab Q8W vs placebo were observed by week 36 (nominal P<0.5) in SIROCCO and CALIMA, while ACQ-6, and AQLQ(S)+12 were not reported.Citation14,Citation15
For patients with BECs ≥150 cells/µL, all daily PROs, except for night-time rescue medication use and awakenings requiring rescue medication use, improved by EOT with benralizumab Q8W. Decreases in activity avoidance and need for self-pacing were reported after 4 weeks. These findings are consistent with previous findings indicating that benralizumab Q8W reduces exacerbations and improves lung function and asthma symptoms for patients with BECs ≥150 cells/µL.Citation16,Citation17 Changes in daily PROs for patients with BECs <150 or <300 cells/µL receiving benralizumab Q8W were directionally consistent with those observed for patients with BECs ≥150 or ≥300 cells/µL. These findings suggest that BECs may not be an absolute predictor for benralizumab responsiveness. Although BECs are a practical and reliable surrogate measure for eosinophilic airway inflammation, the measure may lack specificity at low BECs.Citation18–Citation20 For patients with low BECs, additional baseline factors may be needed to identify patients who may respond to benralizumab.
To the best of our knowledge, this is the first report evaluating the quantitative effect of an asthma biologic on daily PROs for patients with asthma at early treatment time points. The use of daily measures has many advantages, including reduced recall bias and improved precision, thus enabling assessment at earlier time points. The use of daily PROs for evaluating health status is supported by observations that changes in overall health-related quality of life measures, ACQ-6 and AQLQ(S)+12, strongly correlate with changes from baseline to EOT in daily activity impairment and perceived tiredness and moderately correlate with changes in rescue medication use and night-time awakenings.Citation21 Validated daily PRO measurements that reflect early and sustained responses to benralizumab will be important in obtaining a more complete efficacy profile of benralizumab, and potentially other drugs, early in treatment.
Previously, the humanized anti–interleukin-5 monoclonal antibodies mepolizumab and reslizumab were evaluated for improvements in health-related quality of life measures for patients with severe asthma with eosinophilic inflammation.Citation22,Citation23 Improvements were observed as early as 12 and 16 weeks with mepolizumab and reslizumab, respectively.Citation22,Citation23
This study has some limitations. First, this post hoc analysis was not designed to compare daily PRO changes between different BEC groups or the two benralizumab treatment cohorts. Second, improvements for patients in the placebo group may be explained by their participation in a clinical trial. Patients in clinical trials receive more intensive treatment and are closely monitored. Also, being in a clinical trial may, from a psychological aspect, positively influence a patient’s perceived tiredness and activity impairment. Several studies have demonstrated that patients respond positively to a placebo agent or sham procedure in a clinical trial based on perceived benefit.Citation24–Citation26 In a study of bronchial thermoplasty treatment for severe asthma, 64% of patients undergoing a sham procedure reported improvements in health-related quality of life.Citation25 Wechsler et al demonstrated that although significant objective improvements were achieved for patients with asthma receiving albuterol vs placebo, subjective improvements of 45%–50% were reported by patients receiving either albuterol or placebo.Citation26
Conclusion
Benralizumab Q8W reduces rescue medication use by as early as 3 days and decreases activity impairment and perceived tiredness, as reported by patients with severe, uncontrolled asthma with eosinophilic inflammation.
Author contributions
Sean O’Quinn, Xiao Xu, and Ian Hirsch were involved in the conception and design, data acquisition, analysis, and interpretation for the study. All authors participated in the preparation of the manuscript and gave final approval of the version of the manuscript for publication. All authors agreed to be accountable for all aspects of the work.
Acknowledgments
Funding for this study was provided by AstraZeneca. Writing and editing support, including preparation of the draft manuscript under the direction and guidance of the authors, incorporating author feedback, and manuscript submission, was provided by Alan Saltzman, PhD, of JK Associates, Inc. (Conshohocken, PA, USA), and Michael A Nissen, ELS, of AstraZeneca (Gaithersburg, MD, USA). This support was funded by AstraZeneca.
Data availability
Registry URL and database number: SIROCCO trial: NCT01928771 (URL: https://clinicaltrials.gov/ct2/show/NCT01928771)
CALIMA trial: NCT01914757 (URL: https://clinicaltrials.gov/ct2/show/NCT01914757)
Disclosure
Sean O’Quinn, Xiao Xu, and Ian Hirsch are employees of AstraZeneca.
References
- Global Initiative for AsthmaGlobal strategy for asthma management and prevention Available from: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/Accessed April 5, 2018
- SvedsaterHRobertsJPatelCMaceyJHiltonEBradshawLLife impact and treatment preferences of individuals with asthma and chronic obstructive pulmonary disease: results from qualitative interviews and focus groupsAdv Ther20173461466148128536998
- McLaurinKKPokrsywinskiRO’QuinnSXuXHareendranAConceptualization of symptoms and symptom impacts among adults with inadequately controlled moderate-severe asthmaAm J Respir Crit Care Med2012185A1086
- ChippsBEHaselkornTPaknisBMore than a decade follow-up in patients with severe or difficult-to-treat asthma: The epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) IIJ Allergy Clin Immunol201814151590159728797732
- JuniperEFGuyattGHEpsteinRSFerriePJJaeschkeRHillerTKEvaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trialsThorax199247276831549827
- NelsenLMKimelMMurrayLTQualitative evaluation of the St George’s respiratory questionnaire in patients with severe asthmaRespir Med2017126323828427547
- Fasenra™ (benralizumab) prescribing information Available from: https://www.azpicentral.com/fasenra/fasenra_pi.pdf#page=1Accessed January 10, 2018
- Fasenra™ (benralizumab) summary of product characteristics Available from: http://ec.europa.eu/health/documents/community-register/2018/20180108139598/anx_139598_en.pdfAccessed March 13, 2018
- KolbeckRKozhichAKoikeMMEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity functionJ Allergy Clin Immunol201012561344135320513525
- PhamTHDameraGNewboldPRanadeKReductions in eosinophil biomarkers by benralizumab in patients with asthmaRespir Med2016111212926775606
- PavordIDEosinophilic phenotypes of airway diseaseAnn Am Thorac Soc201310SupplS143S14924313765
- PriceDWilsonAMChisholmAPredicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practiceJ Asthma Allergy2016911226793004
- TaliniDNovelliFBacciESputum eosinophilia is a determinant of FEV1 decline in occupational asthma: results of an observational studyBMJ Open201551e005748
- BleeckerERFitzGeraldJMChanezPEfficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trialThe Lancet20163881005621152127
- FitzGeraldJMBleeckerERNairPBenralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trialThe Lancet20163881005621282141
- FitzGeraldJMBleeckerERMenzies-GowAPredictors of enhanced response with benralizumab for patients with severe asthma: pooled analysis of the SIROCCO and CALIMA studiesLancet Respir Med201861516428919200
- GoldmanMHirschIZangrilliJGNewboldPXuXThe association between blood eosinophil count and benralizumab efficacy for patients with severe, uncontrolled asthma: subanalyses of the Phase III SIROCCO and CALIMA studiesCurr Med Res Opin20173391605161328644104
- FowlerSJTavernierGNivenRHigh blood eosinophil counts predict sputum eosinophilia in patients with severe asthmaJ Allergy Clin Immunol2015135382282425445828
- HastieATMooreWCLiHBiomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjectsJ Allergy Clin Immunol20131321728023706399
- WagenerAHde NijsSBLutterRExternal validation of blood eosinophils, FENO and serum periostin as surrogates for sputum eosinophils in asthmaThorax201570211512025422384
- XuXO’QuinnSHirschIGopalanGAsthma symptom improvements with benralizumab are associated with improvements in activity functions and health-related quality of life for patients with severe, uncontrolled asthma: results of pooled phase III benralizumab studiesAm J Respir Crit Care Med2017A4682
- ChuppGLBradfordESAlbersFCEfficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trialLancet Respir Med20175539040028395936
- CastroMZangrilliJWechslerMEReslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trialsLancet Respir Med20153535536625736990
- DutileSKaptchukTJWechslerMEThe placebo effect in asthmaCurr Allergy Asthma Rep201414845624951239
- CastroMRubinASLavioletteMEffectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trialAm J Respir Crit Care Med2010181211612419815809
- WechslerMEKelleyJMBoydIOActive albuterol or placebo, sham acupuncture, or no intervention in asthmaN Engl J Med2011365211912621751905
