Abstract
Background:
Histamine is an important mediator in the development of allergic reactions. The biological effects of histamine are mediated through four histaminergic receptors. In recent years, an important role has been assigned to the proinflammatory functions of histamine regarding the H4 receptor. Previously, we have demonstrated that injection of immature dendritic cells treated with histamine into allergic mice promotes an increase in CD8+ Tc2 lymphocytes, which are involved in the worsening of allergy symptoms during the chronic phase of the disease. The aim of this study was to evaluate the role of the H3/H4 receptor antagonist, thioperamide, in allergy.
Methods:
Ovalbumin-allergized mice and nonallergized mice were injected with phosphate-buffered saline, dendritic cells, or thioperamide-treated dendritic cells. After treatment, the lungs of the mice were obtained and analyzed for changes in the populations of dendritic cells and T lymphocytes, as well as the expression of H and H4 receptors in mononuclear lung cells.
Results:
We found an increase in regulatory T cells in the lungs of allergic mice intratracheally injected with dendritic cells which had their H3/H4 receptors blocked with thioperamide. We also found an increase in the production of interleukin-10 by dendritic cells of the lung. Finally, we observed a decrease in serum levels of specific anti-IgE and a reduction of eosinophils in bronchoalveolar lavage from allergic mice.
Conclusion:
Thioperamide induces a significant improvement in symptoms of allergic reaction perhaps via induction of regulatory T lymphocytes. These findings may become relevant in the understanding of type 1 hypersensivity reactions.
Introduction
Dendritic cells are antigen-presenting cells with a unique ability to initiate the adaptive response by activating naïve T lymphocytes. However, these cells are not only able to prime immune responses, but also induce self-tolerance.Citation1,Citation2 The recognition of inflammatory stimuli or pathogens in the periphery imposes several changes in dendritic cells, leading to their maturation. These changes enable dendritic cells to activate T lymphocytes and to determine their profile of polarization.Citation3,Citation4
Histamine plays a central role in the regulation of both inflammatory and immune responses. It is also able to modulate the function of dendritic cells. Histamine stimulates the chemotaxis of immature dendritic cells and promotes differentiation of T CD4+ lymphocytes into a Th2 profile.Citation5 In addition, in immature dendritic cells from mice, histamine induces the cross-presentation of soluble antigens, such as ovalbumin, acting through H3/H4 receptors.Citation6 Recently, we found a higher recruitment of CD11c+/CD8+ dendritic cells and Tc2 type T CD8+ cells in the lungs of allergic mice receiving dendritic cells treated with histamine and pulsed with ovalbumin.Citation7 In addition, these mice showed increased levels of serum-specific IgE antibodies and a more persistent presence of eosinophils in bronchoalveolar lavage compared with mice treated with ovalbumin-pulsed control dendritic cells. This suggests that histamine may influence the severity of allergies by acting on dendritic cells.
Allergic diseases, including conjunctivitis, allergic rhinitis, asthma, and atopic dermatitis, are inflammatory disorders in which susceptible individuals respond by producing allergen-specific Th2 lymphocytes.Citation8,Citation9 Histamine is one of the most important mediators of allergy and inflammation. Although its effects are mediated via four receptors,Citation10 signaling through H1R seems to play a central role in the induction of allergy, but the use of antagonists of this receptor, like ketotifen,Citation11 did not prove to be effective in most of these pathologies because the symptoms did not disappear completely.Citation12
It was shown that the H4 receptor plays an essential role in inflammatory pathologies. H4R was involved in the amplification of allergic symptoms as well as the maintenance of chronic inflammation.Citation13 In this study, we evaluated the effect of injection of dendritic cells pretreated with thioperamide and pulsed with ovalbumin in allergic mice in vitro. Interestingly, we found a significant increase in CD4+ CD25+ FOXP3+ lymphocytes, as well as increased levels of interleukin-10 in lung dendritic cells of allergic mice. In addition, the levels of specific anti-IgE antibodies as the percentages of eosinophils are decreased. Therefore, H3/H4 receptors would be central to the induction of chronic inflammatory responses. Future studies may determine whether the use of antagonists of these receptors could offer an alternative in the treatment of inflammatory disorders through the induction of a regulatory profile.
Methods
Mice
All experiments were carried out using two-month-old virgin female BALB/c mice raised at the National Academy of Medicine, Buenos Aires, Argentina. They were housed as 4–6 animals per cage and kept at 20°C ± 2°C on an automatic 12-hour light-dark schedule. Animal care was in accordance with institutional guidelines.
Sensitization and challenge with ovalbumin
Mice (4–6 per experiment) were sensitized using a standard protocol, as described previously.Citation14 Briefly, mice were injected intraperitoneally with 20 μg of ovalbumin (Grade V, Sigma-Aldrich, St Louis, MO) in 2 mg of aluminum hydroxide at days 0 and 7. Control mice received a saline injection instead of ovalbumin/aluminum hydroxide solution (Sigma, St Louis, MO). On day 14, sensitized mice were challenged intranasally with 50 μL of phosphate-buffered saline containing 3% ovalbumin for five days. Control mice were instilled with phosphate-buffered saline.
Dendritic cell generation from bone marrow cultures
The procedure used to obtain dendritic cells was performed as described by Inaba et al,Citation15 with minor modifications.Citation16 Briefly, bone marrow was flushed from the long bones of the limbs using 2 mL of RPMI 1640 medium (Invitrogen, Carlsbad, CA) with a syringe and 25-gauge needle. Red cells were lysed with ammonium chloride. After washing, the cells were suspended at a concentration of 1.5 × 106 cells/mL in 70% RPMI 1640 medium supplemented with 10% fetal calf serum, and 5.5 × 10−5 mercaptoethanol (complete medium [Gibco, Buenos Aires, Argentina]) from Sigma and 30% J588-GM cell line supernatant. The cultures were fed every two days by gently swirling the plates, aspirating 50% of the medium, and adding back fresh medium with J588-GM cell line supernatant. At day 9 of the culture, more than 80% of the harvested cells expressed major histocompatibility class (MHC) II, CD40 and CD11c, but not GR-1 (not shown).
Intratracheal injection of dendritic cells
Dendritic cells obtained from bone marrow precursors were incubated in the absence or presence of thioperamide 100 μM (dendritic cells and thioperamide-pretreated dendritic cells, respectively) for 30 minutes at 37ºC. The cells were then incubated for three hours at 37ºC in the presence or absence of ovalbumin 100 μg/mL. Finally, the dendritic cells were washed and injected intratracheally into BALB/c mice after intranasal challenge of sensitized mice with ovalbumin. For this purpose, the mice were anesthetized with pentobarbital sodium (Nembutal, [Abbott Laboratories, Buenos Aires, Argentina]) (2% v/v in phosphate-buffered saline), and 100 μL of phosphate-buffered saline, dendritic cells, or thioperamide-pretreated dendritic cells (5 × 105 cells) were injected.
Treatment of lung tissues to obtain a cellular suspension
The lungs were cut into small pieces and treated with Type I collagenase 250 U/mL (Roche, Buenos Aires, Argentina) for 30 minutes at 37°C. At the end of the incubation time, the reaction was stopped by addition of phosphate-buffered saline supplemented with 5% fetal calf serum. Subsequently, the fragments were incubated with DNase I 50 U/mL (Invitrogen, Buenos Aires, Argentina) for 40 minutes at 37°C. Finally, the cell suspensions were collected through a gauze mesh and washed with cold phosphate-buffered saline. For the analysis of H3 and H4 receptors, lung suspensions were seeded in a Ficoll-Hypaque (Amersham Biosciences, Buenos Aires, Argentina) gradient and centrifuged for 40 minutes at 1600 rpm to obtain the mononuclear cell monolayer.
Bronchoalveolar lavage
At 15 or 30 days, after treatment of the allergic mice with phosphate-buffered saline, dendritic cells, or thioperamide-pretreated dendritic cells, the lungs were washed via a tracheal tube with phosphate-buffered saline. Cells were washed and leukocyte counts were determined by optical microscopy. Cytospin slides were stained with toluidine to determine the percentages of eosinophils in bronchoalveolar lavage.
Flow cytometry
Cells were stained for cell surface markers with the following monoclonal antibodies: anti-CD11c, anti-CD40, anti-CD86, anti-MHC class II, anti-CD4+, anti-CD25+, and anti-GR1 (FITC, PE or PERCP, Pharmingen, San Diego, CA). The staining was analyzed by flow cytometry on FACScan using Cellquest software (BD Biosciences, Buenos Aires, Argentina).
Analysis of serum levels of IgE antibodies directed to ovalbumin
Serum samples were obtained from the mice at the end of the experiments by cardiac puncture. Ovalbumin-specific IgE antibodies were determined using plates coated overnight with 1 μg/mL ovalbumin in sodium carbonate buffer (pH 9.5, Sigma-Aldrich, St Louis, MO). Plates were pretreated with Tween 0.5% in phosphate-buffered saline supplemented with 1% bovine serum albumin for two hours at room temperature. Serial dilutions of sera were added and, after two hours, the plates were washed three times with Tween 0.5% in phosphate-buffered saline, and an appropriate dilution of biotinylated detection antibody (rat anti-mouse IgE, BD Pharmingen, Buenos Aires, Argentina) was added for one hour. After washing the plates, the enzyme avidine-peroxidase (eBiosciences) was added for 20 minutes. 3, 3′, 5, 5′-tetramethylbenzidine was used as a substrate. Absorbance was measured at 450 nm.
Cell purification
T cells and dendritic cells were purified from lung cell suspensions using an autoMACS separator in accordance with the manufacturer’s protocol (Miltenyi Biotec, Hanover, Germany). Dendritic cells and T cells were purified by positive selection using magnetic beads coupled to anti-CD11c+ and anti-CD3, respectively.
Intracellular FOXP3 staining
Purified T cells were fixed in 4% paraformaldehyde and permeabilized with saponin (0.1% in phosphate-buffered saline). The cells were then incubated with FITC-conjugated anti-FOXP3 in phosphate-buffered saline 0.5% bovine serum albumin or a similarly labeled isotype-matched control antibody for 30 minutes. Antibodies against FOXP3 was purchased from BD Pharmingen. Cells were subsequently washed with phosphate-buffered saline and stained for surface cell markers with the monoclonal antibodies anti-CD4+ and anti-CD25+. The stained cells were washed with saponin buffer twice and resuspended in isoflow. The staining was analyzed by flow cytometry on FACscan using Cellquest software.
Interleukin-10 production
Dendritic cells purified from the lungs were stimulated for 18 hours with ovalbumin 10 ng/mL. Interleukin-10 production was then analyzed by enzyme-linked immunosorbent assay (eBiosciense, San Diego, CA) in the supernatants of CD11c+ cells (1.5 × 105 cells/200 μL cultured for 18 hours).
Confocal microscopy
Mononuclear cells from lung tissue of BALB/c mice were used for receptor expression analysis, and were fixed with a mixture of 2% paraformaldehyde (Merck, Germany) and 0.125% glutaraldehyde (Electron Microscopy Science, Hartfield, PA) in 0.2 M phosphate buffer at pH 7.4 for two hours at room temperature. Fixed cells were processed for ultrathin cryosectioning, immunogold-labeled and contrasted as described previously.Citation17 Cells (5 × 104) were incubated for 30 minutes at room temperature. Then cells were fixed in 4% paraformaldehyde for 10 minutes at 4°C, after which the cells were treated with 0.1 M of glycine to blocked aldehyde groups. Cells were washed with cold medium and fixed as above. At the optimal antibody concentrations, no background labeling of the nucleus and mitochondria was detected (data not shown). Sections were observed and photographed under a Philips CM120 electron microscope (FEI Company, Eindoven, The Netherlands). Images were acquired using the ITEM program. The quantification of H3 and H4 receptor expression was performed using the Image J program.
T lymphocyte proliferation assay
For the autologous Ag-specific proliferation assay, 2.5 × 105 T lymphocytes from the lungs of BALB/c mice previously allergized were cocultured with 5 × 104 purified dendritic cells from BALB/c mice that were treated in vitro with ovalbumin. At 72 hours, cells were pulsed with [3H] thymidine (1 μCi/well, DuPont-New England Biolabs, Boston, MA). Then cells were harvested using a semiautomatic harvester (Skatron Instruments), and the amount of [3H]thymidine incorporation was determined in a Wallac 1414 Winspectral beta scintillation counter (PerkinElmer). Unspecific T cell proliferation was measured by stimulation of 2.5 × 105 splenocytes with 1 μg/mL lipopolysaccharide (Sigma-Aldrich) for 96 hours.
Statistical analysis
Statistically significant differences between means were assessed by the Student’s t-test. P < 0.05 was taken as indicating statistical significance.
Results
Previously we have demonstrated that histamine increases the cross-presentation of extracellular antigens by dendritic cells acting through H3R/H4R.Citation18 Moreover, we found in a mouse model of allergic airway inflammation that injection of dendritic cells treated with histamine in vitro increases recruitment of Tc2 CD8+ T cells in the lungs of allergic mice, suggesting an effect on the chronicity of the process.Citation7 In light of these findings, we decided to investigate the role of H4/H3 receptors in the development of the allergic process. Airway inflammation was induced in BALB/c mice by intraperitoneal injection of ovalbumin in aluminum hydroxide followed by challenge with aerosolized ovalbumin, as described in the Methods section. Dendritic cells differentiated from bone marrow precursors were incubated for 30 minutes at 37°C with thioperamide (100 μM), an antagonist of H3R/H4R, or phosphate-buffered saline. Either dendritic cells or thioperamide-pretreated dendritic cells were then pulsed with ovalbumin 10 μg/mL for three hours at 37ºC and, after washing, the cells (5 × 105) were intratracheally injected into mice after five days of challenge with aerosolized ovalbumin. Control mice were inoculated intratracheally with phosphate-buffered saline instead of dendritic cells. shows the phenotype of the injected dendritic cells. Incubation with ovalbumin did not change the maturity status of the dendritic cells and, as shown, expression of CD40, CD86, and MHC class II was not affected.
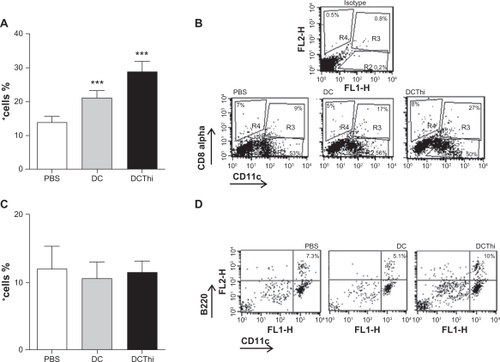
Figure 1 Thioperamide, a H3R/H4R antagonist acting on dendritic cells, increases the recruitment of Treg lymphocytes in lungs of allergic mice. BALB/c mice were allergized as described in the Methods section. Dendritic cells obtained from bone marrow precursors were pretreated or not pretreated with thioperamide 100 μM and pulsed with ovalbumin, and were then injected intratracheally (5 × 105 cells/mouse). Control mice were injected with phosphate-buffered saline. (A) Representative histograms of the phenotype of dendritic cells injected into allergic mice. After 14 days, we obtained a population of lung lymphocyte cells by treatment of cell suspensions with a PanT antibody (anti-CD3) and later purification through an affinity column. The different T cells were analyzed by flow cytometry. (B) Bar graph representing the percentage mean of positive cells ± standard error of the mean [n = 4] from three independent experiments. *P ≤ 0.05, ***P ≤ 0.001. Phosphate-buffered saline versus dendritic cells versus thioperamide-treated dendritic cells. (C) Representative dot plot.
![Figure 1 Thioperamide, a H3R/H4R antagonist acting on dendritic cells, increases the recruitment of Treg lymphocytes in lungs of allergic mice. BALB/c mice were allergized as described in the Methods section. Dendritic cells obtained from bone marrow precursors were pretreated or not pretreated with thioperamide 100 μM and pulsed with ovalbumin, and were then injected intratracheally (5 × 105 cells/mouse). Control mice were injected with phosphate-buffered saline. (A) Representative histograms of the phenotype of dendritic cells injected into allergic mice. After 14 days, we obtained a population of lung lymphocyte cells by treatment of cell suspensions with a PanT antibody (anti-CD3) and later purification through an affinity column. The different T cells were analyzed by flow cytometry. (B) Bar graph representing the percentage mean of positive cells ± standard error of the mean [n = 4] from three independent experiments. *P ≤ 0.05, ***P ≤ 0.001. Phosphate-buffered saline versus dendritic cells versus thioperamide-treated dendritic cells. (C) Representative dot plot.](/cms/asset/32cee91f-16e5-4fe6-bcff-3c3604888408/djaa_a_23507_f0001_b.jpg)
Two weeks later, we obtained the population of T lymphocytes by treatment of lung cells suspensions with a PanT antibody (anti-CD3) and subsequent purification through an affinity column. Finally, we analyzed regulatory T lymphocytes by cytometry. In , we found as already describedCitation19 that injection of immature dendritic cells induced an increase in regulatory T cells (CD4+ CD25+ FOXP3+) in the lungs of allergic mice. Interestingly, in the allergic mice that received dendritic cells with receptors H3 and H4 blocked by thioperamide, we found high recruitment of regulatory T cells in the lungs, which was significantly greater than that observed for untreated dendritic cells (40%). We noted that while the percentage of CD4+ T lymphocytes decreased with both treatments, the population of regulatory T cells was even greater, perhaps as a result of inhibition of the effector cell population.
It is known that plasmacytoid and lymphoid dendritic cells are able to induce T regulatory cells in the lung,Citation20,Citation21 so we decided to evaluate whether use of thioperamide affects a particular subset of dendritic cells in the lungs of allergic mice. Dendritic cells were purified from lung suspensions from allergic mice that had been inoculated with ovalbumin-pulsed dendritic cells or thioperamide-pretreated dendritic cells, by positive selection, using magnetic beads attached to anti-CD11c. As shown in , lymphoid dendritic cells (CD11c+ CD8α+) were increased both by dendritic cells and thioperamide-pretreated dendritic cells, but no significant differences between dendritic cells and thioperamide-pretreated dendritic cells were observed, so we can assume that the increase was at the expense of dendritic cells alone. Moreover, we observed no difference between treatments for the plasma-cytoid dendritic cell subset ().
Figure 2 Thioperamide-pretreated dendritic cells stimulate recruitment of CD11c+ CD8α+ dendritic cells in the lung tissue of allergic mice. Dendritic cells pretreated or not pretreated with thioperamide 100 μM and pulsed with ovalbumin were intratracheally inoculated (5 × 105 cells/allergized mouse). After 15 days, the mice were sacrificed and the lungs were processed. Lung cell suspensions were enriched using antibodies directed to CD11c coupled with magnetic beads. Isolated cells were analyzed for the expression of CD11c, B220, GR1, and CD8α using specific antibodies labeled with PE, PerCP, and FITC. (A) Lymphoid dendritic cells (CD11c+ CD8α+) and (C) plasmacytoid dendritic cells (CD11c+B220+). Results are expressed as the mean ± standard error of the mean of six experiments. (B) and (D) show representative experiments in myeloid and plasmacytoid dendritic cells, respectively.
Considering that induction of tolerance by dendritic cells is associated mainly with release of interleukin-10,Citation22 we also investigated this issue. As shown in , we found that CD11c (+) dendritic cells isolated from the lung tissue of mice treated with thioperamide-pretreated dendritic cells released higher levels of interleukin-10 compared with CD11c (+) cells isolated from the lung tissue of mice that received untreated dendritic cells. This result may reflect that the blockade of H3/H4 receptors, inhibits the cellular response through the induction of regulatory T cells in the lungs of allergic mice, could be a mechanism mediated in part, by the interleukin-10 secreted from thioperamide-pretreated dendritic cells. We investigated this possibility two weeks after injection of allergic mice with phosphate-buffered saline, dendritic cells, or thioperamide-pretreated dendritic cells, by purifying CD11c+ cells from lungs and coculturing them with autologous T lymphocytes from allergized mice. As shown in , thioperamide-stimulated dendritic cells did not induce proliferation of T cells, and a ratio of 5:1 (effector:target) strongly inhibited this action.
Figure 3 Thioperamide induces interleukin-10 production in dendritic cells and inhibition of T lymphocyte proliferation. We obtained a population of lung dendritic cells by treatment of cell suspensions with anti-CD11c and later purification through an affinity column. Production of interleukin-10 was analyzed in the supernatants of CD11c+ cells (1.5 × 105 cells/200 μL cultured for 18 hours) by enzyme-linked immunosorbent assay. (A) Bar graph representing the arithmetic mean of interleukin-10 concentration (pg/mL) ± standard error of the mean, n = 5. *P ≤ 0.05 for phosphate-buffered saline versus dendritic cells versus thioperamide-pretreated dendritic cells. To investigate for differences in T lymphocyte stimulatory ability of lung dendritic cells, we purified lung dendritic cells from treated allergic mice after 12 days. Cells were washed and cocultured with autologous lymphocytes from allergic mice (5 × 104 dendritic cells/well versus 2.5 × 105 splenocytes/well). Dendritic cells stimulated for 48 hours with lipopolysaccharides were included as a positive control. (B) T cell proliferation was measured 72 hours after starting cocultures by [3H]thymidine incorporation. Data are expressed as the mean [3H]thymidine incorporation and are representative of five independent experiments. *P < 0.05. (C) Forty-eight hours after starting coculture, the supernatants were collected and analyzed for interleukin-10 production by quantitative enzyme-linked immunosorbent assay.
![Figure 3 Thioperamide induces interleukin-10 production in dendritic cells and inhibition of T lymphocyte proliferation. We obtained a population of lung dendritic cells by treatment of cell suspensions with anti-CD11c and later purification through an affinity column. Production of interleukin-10 was analyzed in the supernatants of CD11c+ cells (1.5 × 105 cells/200 μL cultured for 18 hours) by enzyme-linked immunosorbent assay. (A) Bar graph representing the arithmetic mean of interleukin-10 concentration (pg/mL) ± standard error of the mean, n = 5. *P ≤ 0.05 for phosphate-buffered saline versus dendritic cells versus thioperamide-pretreated dendritic cells. To investigate for differences in T lymphocyte stimulatory ability of lung dendritic cells, we purified lung dendritic cells from treated allergic mice after 12 days. Cells were washed and cocultured with autologous lymphocytes from allergic mice (5 × 104 dendritic cells/well versus 2.5 × 105 splenocytes/well). Dendritic cells stimulated for 48 hours with lipopolysaccharides were included as a positive control. (B) T cell proliferation was measured 72 hours after starting cocultures by [3H]thymidine incorporation. Data are expressed as the mean [3H]thymidine incorporation and are representative of five independent experiments. *P < 0.05. (C) Forty-eight hours after starting coculture, the supernatants were collected and analyzed for interleukin-10 production by quantitative enzyme-linked immunosorbent assay.](/cms/asset/2a5f2dbb-e392-40be-95ea-a1a97ab29e87/djaa_a_23507_f0003_b.jpg)
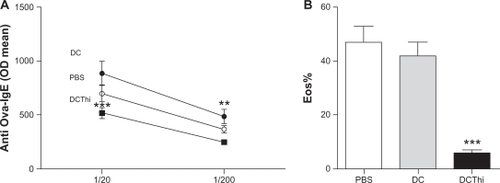
In order to determine whether H3/H4 receptor blockade leads to an improvement in allergy symptoms, we then measured serum levels of ovalbumin-IgE antibodies and the presence of eosinophils in bronchoalveolar lavage from allergic mice 15 days after intratracheal injection. As shown in , both of these parameters were significantly decreased in allergic mice inoculated with ovalbumin-pulsed thioperamide-pretreated dendritic cells compared with the allergic controls.
Figure 4 Injection of thioperamide-pretreated dendritic cells into allergic mice decreases serum levels of specific IgE antibodies directed to ovalbumin and infiltration of the lungs by eosinophils. Dendritic cells pretreated or not pretreated with histamine and pulsed with ovalbumin were intratracheally inoculated (5 × 105 cells/allergized mouse). (A) After 15 days, serum samples were obtained and levels of serum IgE antibodies directed to ovalbumin were determined by enzyme-linked immunosorbent assay using serum dilutions of 1/20 and 1/200. Data are expressed as the arithmetic mean of the optical density ± standard error of the mean (n = 6). After 15 and 30 days, amounts of eosinophils in bronchoalveolar lavage were determined (B). Data are expressed as the percentage of eosinophils in bronchoalveolar lavage (arithmetic mean ± standard error of the mean, n = 6).
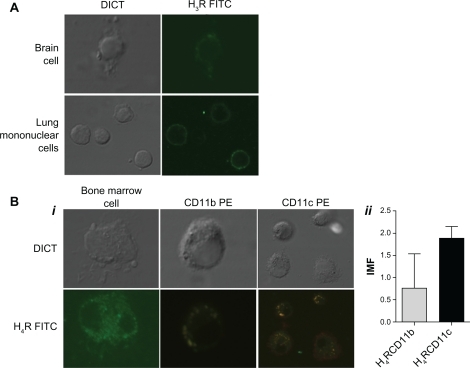
In light of these results, we were interested in investigating whether these receptors, which are known to be implicated in the allergic process, are represented in the lung tissue of allergic mice. Therefore, we analyzed the expression of H3R and H4R in mononuclear lung cells of allergic mice by confocal microscopy (see Methods section) using positive control brain tissue (for H3R) and bone marrow precursors (for H4R). We used specific antibodies against these receptors and membrane markers associated with dendritic cells and macrophages, such as CD11c and CD11b.Citation23 As shown in i–ii, we found that while expression of H4R was associated with cells that express CD11c+ and CD11b+, H3R showed only weak expression in the fraction corresponding to mononuclear lung cells not associated with expression of CD11c and CD11b markers (ii).
Figure 5 H4R is expressed in mice lung CD11b and CD11c cells. H3R (A) and H4R (Bi) expression was evaluated in mice lung mononuclear cells and in positive controls (mice brain and bone marrow) by confocal microscopy. Specific antibodies were used against H3R and H4R (conjugated with fluorescein isothiocyanate, green) and CD11b and CD11c (conjugated with PE, red). The colocalization of the histamine rceptor and CD11c or CD11b on the cellular membrane is visualized in yellow. (Bii) shows the average of expression intensity for both receptors (n = 4).
Discussion
It has been demonstrated in a mouse model of food allergy that subcutaneous administration of synthetic peptides comprising immunodominant T cell determinants of ovalbumin to sensitized BALB/c mice decreases allergy symptoms.Citation24 This therapy reduced the hypersensitivity reaction, ie, serum histamine and ovalbumin-specific IgE levels. Interestingly, an increase in mRNA expression of both regulatory molecules, TGF-β and FOXP3, was detected in intestinal tissues, contributing to the suppressive effect to the allergy. In our research, we showed in a widely accepted mouse model of acute airway inflammation in response to ovalbumin, that intratracheal injection of dendritic cells with H3R/H4R blocked by thioperamide increased the proportions of CD4+ CD25+ FOXP3+ cells in the lungs of allergic mice, demonstrating a role of endogenous histamine in the modulation of regulatory T cells. It should be noted that dendritic cells constitutively produce histamine,Citation6 although the presence of inflammatory stimuli strongly increases the secretory capacity of dendritic cells,Citation25 suggesting that histamine could play an autocrine role during genesis of the inflammatory response.
Functionally, this model is divided in two phases, ie, sensitization and provocation.Citation26,Citation27 In the sensitization phase, the experimental allergen, ie, ovalbumin, is taken up by dendritic cells that induce a polarized Th2 response and IgE production. Re-exposure to ovalbumin is referred to as provocation that induces the acute allergic reaction. It is now widely accepted that recruitment of CD8+ Tc2 cells is crucial in maintaining airway hyperresponsiveness and eosinophilia in the later phase of the asthma process.Citation28,Citation29 Massive amounts of histamine are released in allergic reactions like asthma, rhinitis, and conjunctivitis.Citation30 In this sense, the published research supports the essential role of this biogenic amine in the inflammatory reaction, acting through four histamine receptors, referred to as H1R, H2R, H3R, and H4R.Citation31,Citation32
We have previously demonstrated in vitro that histamine increases the cross-presentation of soluble ovalbumin by immature murine dendritic cells.Citation12 In a recent paper, we also showed that histamine acting on dendritic cells can favor the recruitment of TCD8+ cells in lung tissue of allergic mice, indicating a likely role of this amine in the chronic phase.Citation9 This was demonstrated by the fact that mice with histamine-treated dendritic cells showed increased levels of serum-specific IgE antibodies directed to ovalbumin, and a more persistent presence of eosinophils in bronchioalveolar lavage fluid compared with mice treated with ovalbumin-pulsed control dendritic cells. An interesting finding was that blocking of H3R/H4R on dendritic cells reduced the numbers of eosinophils and specific IgE antibodies in allergic mice, as reported by Dunford et al using an H4R-selective antagonist, JNJ 7777120 or H4R-deficient mice.Citation25 However, the point of balance between dendritic cells and T cells at which symptom improvement occurs is not clearly defined. When we analyzed regulatory T cells in the lungs of allergic mice, we found a greater increase in CD4+/FOXP3+ cells, indicating that H4R blockade on dendritic cells with thioperamide caused an improvement in asthmatic symptoms by induction of T regulatory cells which suppress the effector response via production of interleukin-10. It has been shown that activation of regulatory T cells in allergy not only leads to suppression of lymphocyte proliferation, but also downregulates production of specific IgE antibodies and reduces inflammation by inhibiting the activity of inflammatory cells, including eosinophils, basophils, and mast cells. In addition, the amounts required for this function are low, considering that, once specifically activated, their suppressor function operates on all effector lymphocytes independently of their specificity.Citation33,Citation34
Yang et al showed that increased mRNA expression of transforming growth factor beta and FOXP3 in intestinal tissues is consistent with low serum histamine levels.Citation24 In line with this, our results reinforce the idea that endogenous histamine acting on dendritic cells negatively regulates induction of Treg cells in the lung tissue of allergic mice, being dependent on H4R for this action. Morgan et al studied a mouse model of airway hypersensitivity and demonstrated that intratracheal administration of 4MeHA, an H4R-selective ligand, is associated with a reduction in asthma-like symptoms attributed to accumulation of FOXP3+ T cells in the lung.Citation35 These authors assumed an anti-inflammatory function of H4R. However, before discovery of H4R, 4MeHA was used as a specific ligand for H2R, so it is possible that the anti-inflammatory effects observed were due to activation of H2R rather than H4R.Citation36,Citation37 In rhinitis or conjunctivitis, the H1R antagonist is effective in controlling clinical symptoms, but is not relevant in asthma. This may be explained by the fact that asthma has a multifactorial pathogenesis. In this regard, development of a Th2 response is essential for sensitization and the early inflammatory response, but the chronicity of the process depends on recruitment of CD8+ T cells.Citation29 Our results and those of other research groups reinforce the idea that the action of histamine on dendritic cells has a proinflammatory role. In this way, H4R blockade or deficiency has been effective in reducing asthma symptoms in several studies, perhaps through an increase in regulatory T cells. Forward et alCitation38 investigated the consequences of mast cell and regulatory CD4+ CD25+ T cell interaction, and concluded that histamine signaling via H1 receptors leads to reduction in the suppressor function of these regulatory T cells, and this function is restored when histamine levels decrease. Thus, local histamine produced by mast cells and dendritic cells could modulate the development of inflammatory reactions at different levels, ie, by acting via H1R on innate cells present in tissues and by maintenance of the inflammatory response via H4R on dendritic cells.
An interesting observation was that expression of the H4 receptor is restricted to CD11c+ lung cells. Recently, Lundberg et alCitation39 showed that blockade of H4R in monocyte-derived dendritic cells from allergic donors inhibited allergen-specific T cell responses. Given that alveolar macrophages and dendritic cells are the most abundant cell populations expressing CD11c+ in the lung mucosa,Citation40 and considering that dendritic cells are potential targets in allergic therapy because they define polarization of T lymphocytes under the influence of microenvironment, it is possible that use of H4R antagonists may enable more effective treatment of allergic diseases.
Conclusion
We conclude that treatment of dendritic cells with thioperamide, an H3/H4 receptor antagonist, increases CD4+ CD25+ FOXP3+ regulatory T cells in the lungs of allergic mice, suggesting that endogenous histamine exerts autocrine control of dendritic cells during the inflammatory response. Considering that mucosal dendritic cells in the lung express the H4 receptor, we believe that its blockade may favor the induction of a beneficial anti-inflammatory response to counteract allergic effects.
Acknowledgements
This work was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas and the Agencia Nacional de Promoción Científica y Tecnológica, Argentina. We thank Beatriz Loria and Edith Mabel Horvat for their technical assistance with this research.
Disclosure
The authors report no conflicts of interest in this work.
References
- JoffreONolteMASpörriRReis e SousaCInflammatory signals in dendritic cell activation and the induction of adaptive immunityImmunol Rev2009227123424719120488
- CarboneFRBelzGTHeathWRTransfer of antigen between migrating and lymph node-resident DCs in peripheral T-cell tolerance and immunityTrends Immunol2004251265565815530835
- Reis e SousaCToll-like receptors and dendritic cells: for whom the bug tollsSemin Immunol2004161273414751761
- SabattéJMagginiJNahmodKInterplay of pathogens, cytokines and other stress signals in the regulation of dendritic cell functionCytokine Growth Factor Rev2007181–251717321783
- GutzmerRDiestelCMommertSHistamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cellsJ Immunol200517495224523215843518
- BäumerWWendorffSGutzmerRHistamine H4 receptors modulate dendritic cell migration through skin immunomodulatory role of histamineAllergy200863101387139418782117
- AmaralMMAlvarezCLangellottiCGeffnerJVermeulenMHistamine-treated dendritic cells improve recruitment of type 2 CD8 T cells in the lungs of allergic miceImmunology2010130458959620406304
- KayABOverview of allergy and allergic diseases: with a view to the futureBr Med Bull200056484386411359624
- HawrylowiczCRyannaKAsthma and allergy: the early beginningsNat Med201016327427520208515
- AkdisCASimonsFEHistamine receptors are hot in imunopharmacologyEur J Pharmacol20065331–3697616448645
- PhanHMoellerMLNahataMCTreatment of allergic rhinitis in infants and children: efficacy and safety of second-generation antihistamines and the leukotriene receptor antagonist montelukastDrugs200969182541257619943707
- AkdisCAAllergy and hypersensitivity: mechanisms of allergic diseaseCurr Opin Immunol200618671872617029937
- JablonowskiJACarruthersNIThurmondRLThe histamine H4 receptor and potential therapeutic uses for H4 ligandsMini Rev Med Chem200449993100015544559
- LloydCMGonzaloJACoyleAJGutierrez-RamosJCMouse models of allergic airway diseaseAdv Immunol20017726327311293118
- InabaKInabaMRomaniNGeneration of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factorJ Exp Med19921766169316971460426
- VermeulenMGiordanoMTrevaniASAcidosis improves uptake of antigens and MHC I-restricted presentation by dendritic cellsJ Immunol200417253196319914978127
- JancicCSavinaAWasmeierCRab27a regulates phagosomal pH and NADPH oxidase recruitment to dendritic cell phagosomesNat Cell Biol20079436737817351642
- AmaralMMDavioCCeballosAHistamine improves antigen uptake and cross-presentation by dendritic cellsJ Immunol200717963425343317785776
- HuangHDawickiWZhangXTownJGordonJRTolerogenic dendritic cells induce CD4+ CD25+ Foxp3+ regulatory T cell differentiation from CD4+CD25−/loFoxp3 effector T cellsJ Immunol201018595003501020870943
- MattaBMCastellanetaAThomsonAWTolerogenic plasmacytoid DCEur J Immunol201040102667267620821731
- LangloisRALeggeKLRespiratory dendritic cells: mediators of tolerance and immunityImmunol Res2007391–312814517917061
- WikstromMEStumblesPAMouse respiratory tract dendritic cell subsets and the immunological fate of inhaled antigensImmunol Cell Biol200785318218817262055
- GeurtsvanKesselCHLambrechtBNDivision of labor between dendritic cell subsets of the lungMucosal Immunol20081644245019079211
- YangMYangCMineYMultiple T cell epitope peptides suppress allergic responses in an egg allergy mouse model by the elicitation of forkhead box transcription factor 3 and transforming growth factor-beta-associated mechanismsClin Exp Allergy201040466867820082619
- DunfordPJO’DonnellNRileyJPWilliamsKNKarlssonLThurmondRLThe histamine H4 receptor mediates allergic airway inflammation by regulating the activation of CD4+ T cellsJ Immunol2006176117062707016709868
- ShinYSTakedaKGelfandEWUnderstanding asthma using animal modelsAllergy Asthma Immunol Res200911101820224665
- TakedaKGelfandEWMouse models of allergic diseasesCurr Opin Immunol200921666066519828303
- GelfandEWDakhamaACD8+ T lymphocytes and leukotriene B4: novel interactions in the persistence and progression of asthmaJ Allergy Clin Immunol2006117357758216522456
- MiyaharaNTakedaKKodamaTContribution of antigen-primed CD8+ T cells to the development of airway hyperresponsiveness and inflammation is associated with IL-2J Immunol200417242549245814764728
- YehYCXieLLangdonJMThe effects of overexpression of histamine releasing factor (HRF) in a transgenic mouse modelPLoS One201056e1107720552026
- CowdenJMRileyJPMaJYThurmondRLDunfordPJHistamine H4 receptor antagonism diminishes existing airway inflammation and dysfunction via modulation of Th2 cytokinesRespir Res2010118620573261
- NeumannDBeermannSSeifertRDoes the histamine H4 receptor have a pro- or anti-inflammatory role in murine bronchial asthma?Pharmacology201085421722320215812
- BragaMQuecchiaCCavallucciET regulatory cells in allergyInt J Immunopathol Pharmacol2011241 Suppl55S64S21329567
- OzdemirCAkdisMAkdisCAT regulatory cells and their counterparts: masters of immune regulationClin Exp Allergy200939562663919422105
- MorganRKMcAllisterBCrossLHistamine 4 receptor activation induces recruitment of FoxP3+ T cells and inhibits allergic asthma in a murine modelJ Immunol2007178128081808917548646
- DurantGJGanellinCRParsonsMEChemical differentiation of histamine H1- and H2-receptor agonistsJ Med Chem1975189905909240025
- MitsuhashiMMitsuhashiTPayanDMultiple signaling pathways of histamine H2 receptors. Identification of an H2 receptor dependent Ca2+ mobilization pathway in human HL-60 promyelocytic leukemia cellsJ Biol Chem19892643118356183622553705
- ForwardNAFurlongSJYangYLinTJHoskinDWMast cells down-regulate CD4+CD25+ T regulatory cell suppressor function via histamine H1 receptor interactionJ Immunol200918353014302219667094
- LundbergKBroosSGreiffLBorrebaeckCALindstedtMHistamine H(4) receptor antagonism inhibits allergen-specific T-cell responses mediated by human dendritic cellsEur J Pharmacol20106511–319720421093429
- LambrechtBNHammadHBiology of lung dendritic cells at the origin of asthmaImmunity200931341242419766084