Abstract
Introduction
Most patients with asthma, either allergic or non-allergic, usually exhibit some level of concurrent rhinitis. Treatments for rhinitis and asthma can affect both conditions.
Objective
The present study aimed to examine asthma-specific outcomes in patients with chronic rhinitis (CR) and asthma after surgery for nasal obstruction, and to identify the patient group most likely to experience improved asthma control after surgery.
Methods
Asthmatic patients with CR and nasal obstruction were prospectively recruited for evaluations of nasal and asthma-specific outcomes before and after surgery for nasal obstruction.
Results
Twenty-eight participants were enrolled. There was a significant association between the Asthma Control Test (ACT) and the Sino-Nasal Outcome Test-22 scores, both at the preoperative and 3-month postoperative assessments. Patients demonstrating ACT improvement after nasal surgery had worse preoperative ACT scores and predicted forced expiratory volume in 1 s.
Conclusion
Nasal symptom severity was closely associated with the extent of asthma control in asthmatic patients with CR and nasal obstruction. Assessment of CR and nasal obstruction in patients with poorly controlled asthma should be considered an essential approach to improve the response to treatment and patients’ quality of life.
Plain Language Summary
Most patients with asthma, either allergic or non-allergic, usually exhibit some level of concurrent rhinitis. The airway is a continuous structure that extends from the nose to the alveolar units of the lung. The lower airways may be affected by upper airway inflammation through a systemic reaction of inflammatory mediators or aspiration of inflammatory secretions from the nose. Treatments for rhinitis and asthma can affect both conditions. The present study aimed to examine asthma-specific outcomes in patients with chronic rhinitis and asthma after surgery for nasal obstruction, and to identify the patient group most likely to experience improved asthma control after surgery. Nasal symptom severity was closely associated with extent of asthma control in asthmatic patients with chronic rhinitis and nasal obstruction both at the preoperative and 3-month postoperative assessments. Patients demonstrating Asthma Control Test improvement after nasal surgery had worse preoperative Asthma Control Test scores and predicted forced expiratory volume in 1 s. Assessment of chronic rhinitis in patients with poorly-controlled asthma should be considered an essential approach to improve the response to treatment and patients’ quality of life.
Introduction
The relationship between asthma and rhinitis has been widely studied and recognized.Citation1 Most patients with asthma, either allergic or non-allergic, usually exhibit some level of concurrent rhinitis.Citation2 The airway is a continuous structure that extends from the nose to the alveolar units of the lung. The lower airways may be affected by upper airway inflammation through a systemic reaction of inflammatory mediators or aspiration of inflammatory secretions from the nose.Citation3
Type 2 eosinophilic inflammation resulted from an immunoglobulin E (IgE)-mediated hypersensitivity to aeroallergens in the respiratory tract, presenting as rhinitis in the upper airway and asthma in the lower airway.Citation4 Treatments for rhinitis and asthma can affect both conditions.Citation5 For example, omalizumab, an anti-IgE monoclonal antibody, is effective for both severe asthmaCitation6 and uncontrolled allergic rhinitis.Citation7 In both ARCitation8 and allergic asthma,Citation9 immunotherapy improves symptoms and decreases the need for medication. In population-based studies, treatment of rhinitis with intranasal corticosteroids (INS) is associated with a lower frequency of asthma-related hospitalization and emergency department visits.Citation10
Currently, the management of chronic rhinitis (CR) involves the treatment of patients with maximal medical therapy; however, this unfortunately fails to control nasal symptoms in some patients.Citation11 Nasal surgery is then considered, especially in individuals with persistent symptoms of nasal obstruction due to hypertrophied turbinates and/or nasal septal deviation.Citation12 Therefore, a common clinical question that arises is whether surgery for nasal obstruction will also result in improved outcomes for comorbid asthma. Accordingly, the primary aim of the present investigation was to prospectively study asthmatic patients with CR and nasal obstruction to examine asthma-specific outcomes after nasal surgery. Furthermore, our secondary aim was to identify the patient group most likely to experience improvement in asthma control after surgery.
Methods
Patients
Between August 2013 and June 2016, adult asthmatics (>18 years of age) with CR and nasal obstruction, who were being treated at the authors’ otolaryngology and thoracic medicine departments, were prospectively recruited. The recruited patients fulfilled the diagnostic criteria from the Global Initiative for Asthma (GINA) guidelinesCitation13 and had failed 3 months of maximal medical treatment for their nasal symptoms (eg, INS and/or antihistamines). All patients’ asthma had been treated according to the GINA guidelines for ≥6 months. Patients with major medical disorders, such as diabetes, nephrotic diseases, autoimmune disorders, immunodeficiency, malignancy, and other chronic illnesses, were excluded. The Institutional Review Board of Chang Gung Memorial Hospital (Taoyuan City, Taiwan) approved the study protocol (IRB number: 103-7085B) and all patients provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Objective and Subjective Measurements
All patients underwent thorough nasal endoscopy examination and sinus computed tomography (CT) to confirm the sinonasal diagnosis before nasal surgery; patients with chronic rhinosinusitis (CRS) were excluded. Patients underwent pulmonary function testing, which evaluated forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), and the FEV1/FVC ratio preoperatively and 3 months postoperatively. Percentages of the predicted values for these tests were calculated according to age, sex, height, and ethnicity.Citation14 Additional information regarding the patients’ clinical characteristics was collected from their medical records. Atopy was defined by the presence of elevated levels of total and allergen-specific serum IgE using a commercially available assay (ImmunoCAP, Thermo Fisher Scientific).Citation15
Participants also completed the Asthma Control Test (ACT)Citation16 and the Sino-Nasal Outcome Test-22 (SNOT-22) questionnairesCitation17 at the same time for evaluation of asthma control and nasal symptoms both preoperatively and 3 months postoperatively. The SNOT-22 tool is a validated instrument developed to quantify self-reported measures of symptom severity associated with sinonasal conditions.Citation18 The SNOT-22 tool covers various symptoms, physical problems, functional limitations, and emotional consequences of having a sinonasal disorder. Item scores range from 0 to 5, with higher scores indicating more severe symptoms.
The ACT is also a validated, self-administered questionnaire used to assess patients’ perception of asthma control over the previous 4-week period. The ACT consists of five items including the frequency of asthma-related symptoms, the need for rescue medications, and perceived control of asthma. Higher total scores (range, 0–25) indicate better disease control. The total score is used to indicate perfectly controlled asthma (25 points), well-controlled asthma (20–24 points), or poorly controlled asthma (≤19 points).Citation16
Statistical Analyses
Data were reported as mean ± standard error or number, and were analyzed using GraphPad Prism version 5 (GraphPad Prism Software Inc., San Diego, CA, USA). Categorical variables were analyzed using the chi-squared test or Fisher’s exact test, as appropriate. Continuous variables were compared between the two groups using the Mann–Whitney U-test or Wilcoxon signed-rank test. Correlations between two items were evaluated using Spearman correlation coefficient. A p-value of <0.05 was considered to be statistically significant.
Results
Participants’ Clinical Characteristics
summarizes the participants’ clinical characteristics. Twenty-eight asthmatic patients with CR and nasal obstruction were enrolled in this study. All patients underwent surgery including inferior turbinoplasty with or without septoplasty for nasal obstruction. Twenty-four patients completed postoperative evaluation 3 months after nasal surgery, 13 of whom reported improvement in ACT assessment (higher ACT scores postoperatively than those preoperatively). The patient group demonstrating postoperative ACT improvement had fewer atopy cases.
Table 1 Clinical Characteristics of the Study Population
Asthma and Rhinitis Outcomes After Nasal Surgery
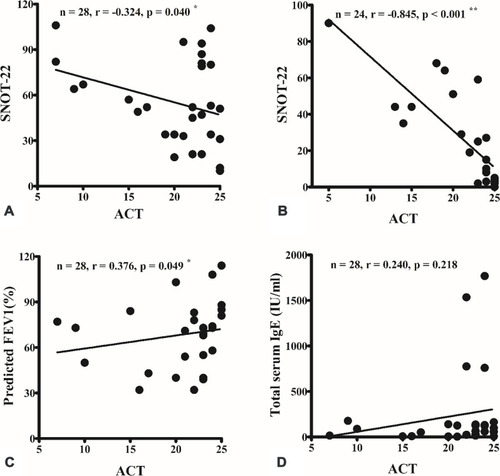
A good correlation between the ACT and SNOT-22 scores was observed at the preoperative () and 3-month postoperative assessments (). The nasal symptoms score according to SNOT-22 had a critical influence on asthma control evaluation using the ACT questionnaire. ACT score was also well correlated with FEV1 predicted (), but not with total serum IgE level ().
Figure 1 A strong correlation was observed between the Asthma Control Test (ACT) and Sino-Nasal Outcome Test-22 (SNOT-22) scores preoperatively (A) and 3 months postoperatively (B). ACT score was also well correlated with the predicted forced expiratory volume in 1 s (FEV1) (C), but not with total serum immunoglobulin (IgE) level (D). *p < 0.05; **p < 0.001 (Spearman correlation).
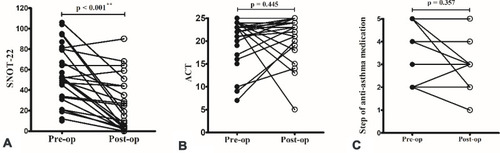
Nasal surgery improved sinonasal symptoms (), but not asthma control (), or the use of anti-asthma medication (). Most patients received the same medication during the study period except for two with step-up therapy (from step 2 to 3) and four with step-down therapy. As a result, the effect of medication change on the improvement of asthma control should be small.
Figure 2 Nasal surgery improved sinonasal symptoms (A), but not asthma control (B) and the use of anti-asthma medication (C). The symbols represented patients with the same step of anti-asthma medication were overlapped. **p < 0.001 (Wilcoxon signed-rank test).
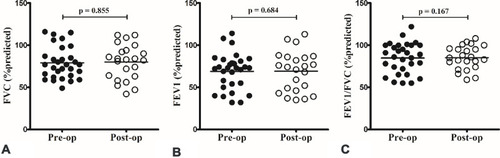
There was no significant difference between the preoperative and postoperative results of pulmonary function tests including predicted FVC (), FEV1 (), and FEV1/FVC ().
Figure 3 There was no significant difference between preoperative (pre-op) and postoperative (post-op) results of pulmonary function testing, including predicted forced vital capacity (FVC) (A), forced expiratory volume in 1 s (FEV1) (B) and FEV1/FVC (C).
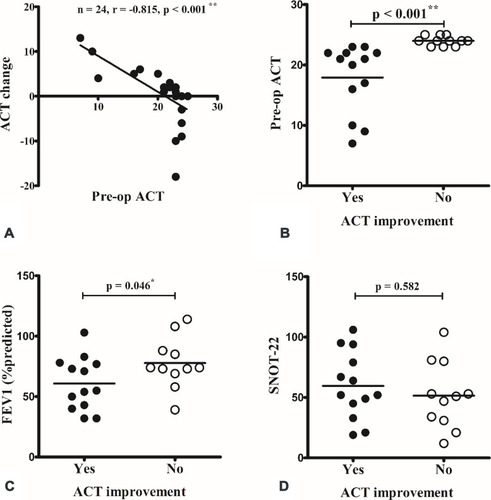
The mean change in ACT score was 0.4 ± 1.3, 4.3 ± 1.0, and −4.2 ± 1.8 in total patients, patients with and without ACT improvement, respectively. The change in ACT score was inversely correlated with preoperative ACT score (). Comparison between patients with and without improvement of ACT score after nasal surgery revealed that patients demonstrating postoperative ACT improvement had a worse preoperative ACT score () and predicted FEV1 (). There was no difference in preoperative SNOT-22 evaluation () and each symptom item score (data not shown).
Figure 4 The change in Asthma Control Test (ACT) scores were inversely correlated with the preoperative (pre-op) ACT score (A). When compared between patients with and without improvement in ACT after surgery, patients with postoperative ACT improvement had a worse pre-op ACT score (B) and predicted forced expiratory volume in 1 s (FEV1) (C). There was no difference in pre-op Sino-Nasal Outcome Test-22 (SNOT-22) score (D). *p < 0.05; **p < 0.001 (Spearman correlation and Mann–Whitney U-test).
In the 13 patients reported improvement in ACT assessment, the mean ACT score was 17.9 ± 1.6 preoperatively and 22.2 ± 0.9 postoperatively (p=0.002). Further analysis with each ACT item revealed “Use of rescue medication”, “Patient rating of control”, and “Shortness of breath” improved significantly after surgery (p=0.001, 0.004, and 0.01, respectively).
Discussion
This study investigated the effect of surgery for nasal obstruction on asthma-specific outcomes. Our study demonstrated a strong correlation between ACT and SNOT-22 scores at the preoperative and 3-month postoperative assessments. Nasal symptoms had a substantial association with asthma control evaluation. Nasal surgery improved sinonasal symptoms, but not asthma control level, the use of anti-asthma medication, or the results of pulmonary function testing. However, in detailed analysis, the change in ACT score was inversely correlated with preoperative ACT score. Patients with postoperative improvement in ACT scores were those with worse preoperative ACT scores. These results suggested a close link between the upper and lower airway inflammation diseases, such as rhinitis and asthma. Furthermore, surgery for nasal obstruction has a positive role in the management of asthma, especially in patients with poorly controlled asthma. Assessment of CR in patients with poorly controlled asthma should be considered an essential approach to improve the response to treatment and patient quality of life.
Based on the united airways concept,Citation1 patients with lower airway inflammation usually exhibit some level of parallel inflammation in the upper airway.Citation19 The upper and lower airway inflammation may be triggered by a systemic response to inflammatory mediators.Citation20 Moreover, changes in the inspired air content (eg, moisture, temperature, and nitric oxide)―caused by nasal obstruction or aspiration of nasal secretions into the lower airways―would all affect the function of the lower airway.Citation21 Therefore, although asthma and sinonasal disorders (eg, CRS and CR) appear to be distinct diseases in different anatomical subsites, we believe that they should be evaluated and treated as common airway diseases. Our previous study and those reported in the literatureCitation22 demonstrated the significant role of sinus surgery for CRS in the management of asthma. In the present study, we further investigated the role of surgery in treating nasal obstruction in patients with asthma and CR.
The current study demonstrated that patients with worse preoperative asthma control level―evaluated according to ACT score―were more prone to experience improved asthma control than those with well-controlled asthma. This result is similar to that of a previous study by Schlosser et al,Citation23 who investigated the effect of sinus surgery for CRS on asthma control and reported that there was more opportunity for additional improvement after nasal surgery. Twenty-two of the total of 28 asthmatic patients in the current study were perfectly or well-controlled asthma (ACT≧20 points). This may be the reason why nasal surgery did not improve asthma control in the total study group but patients with worse preoperative asthma control level were more prone to experience improved asthma control. Additionally, we report that nasal disease is a neglected area responsible for refractoriness in patients with poorly controlled asthma. Although it is difficult because of the concern of increased risk of surgery, future study to recruit more asthmatic patients with poor asthma control would be necessary.
It is known that the allergic inflammation of the airways may trigger by the contact of patients with aeroallergens, air-pollutants, microbes and irritants. Thus, asthma control usually fluctuates. Of 24 patients who completed postoperative evaluation 3 months after nasal surgery, only five patients had miner ACT change, meant ACT score got worse after surgery, and none of them was poorly controlled asthma. This indicated the benefit of nasal surgery in patients with poorly controlled asthma may be greater than the data shown.
When patients with and without improvement in ACT score after nasal surgery were compared, the patient group with postoperative ACT improvement had fewer cases of atopy. Type 2 helper T cell-driven airway inflammation and IgE-mediated immune response have been considered the major drivers of allergic airway diseases including allergic rhinitis and asthma.Citation24 Thus, a corticosteroid-based regimen has been the standard therapy for these allergic airway diseases and has typically yielded acceptable improvement. However, individuals with hypertrophied turbinates, with or without nasal septal deviation, would experience persistent nasal symptoms, especially nasal obstruction and refractoriness to medical treatment. As a consequence, these patients may benefit from nasal surgery the most, both for nasal disease and asthma control.
This study demonstrated no significant difference between the preoperative and short-term postoperative results of pulmonary function tests including predicted FVC, FEV1, and FEV1/FVC (). Though pulmonary function usually fluctuates in asthmatic patients, it is better to evaluate with multiple measurements in a longer period to clarify the influence of nasal surgery on the pulmonary function.
The present study had several limitations that warrant consideration. One weakness of our study was the lack of a control group of asthmatic patients who did not undergo nasal surgery. However, it is difficult to recruit appropriate and matched control patients because of the diverse triggers of asthma and CR exacerbation.Citation25 Furthermore, our sample size was small, which did not allow for subgroup analysis for rhinitis and asthma. Subgroup analysis of different phenotypes of CR and asthma requires larger-scale studies. This study focused on the effect of surgery for nasal obstruction on asthma control. Thus, we enrolled asthmatic patients with CR and persistent nasal symptoms requiring surgical intervention, and evaluated asthma-specific outcomes before and after nasal surgery. Additionally, studies investigating patient-reported outcomes are prone to self-report bias, including changes in internal standards, priorities, and interpretation of a given instrument. These biases may have been inherent in our findings, although the ACT and SNOT-22 questionnaires to quantify changes in symptoms and dysfunction have been validated in previous studies.
Conclusion
We found that nasal symptom severity was closely associated with asthma control level in asthmatic patients with CR and nasal obstruction. Assessment of CR and nasal obstruction in patients with uncontrolled asthma should be considered an essential approach to improve the response to treatment and patient quality of life.
Terms and Definitions
Chronic rhinitis: Inflammation of the mucous membrane inside the nose lasting for more than four consecutive weeks.
Hypertrophied turbinates: Descriptions of the turbinate tissue on the lateral walls of the nose are enlarged and causing nasal obstruction.
Nasal septal deviation: Descriptions of a displacement of the nasal septum from the midline where it is supposed to be. It tends to cause airway obstruction.
Inferior turbinoplasty: Encompasses a variety of surgical techniques for volumetrically reducing the inferior turbinates to restore nasal patency.
Septoplasty: a corrective surgical procedure for straightening a deviated nasal septum.
Abbreviations
ACT, Asthma Control Test; CR, chronic rhinitis; CRS, chronic rhinosinusitis; CT, computed tomography; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; GINA, Global Initiative for Asthma; IgE, immunoglobulin E; INS, intranasal corticosteroid; SNOT-22, Sino-Nasal Outcome Test-22.
Acknowledgments
The authors thank Ms. Meng-Chieh Tsai for her help in the collection of clinical data. The authors received a research grant from the Chang Gung Memorial Hospital (CMRPG3J1861, CMRPG2K0161).
Disclosure
The authors report no conflicts of interest in this work.
References
- Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy. 2012;26(3):187–190.22643942
- Cruz AA, Popov T, Pawankar R, et al. Common characteristics of upper and lower airways in rhinitis and asthma: ARIA update, in collaboration with GA(2)LEN. Allergy. 2007;62(Suppl 84):1–41. doi:10.1111/j.1398-9995.2007.01551.x
- Rosati MG, Peters AT. Relationships among allergic rhinitis, asthma, and chronic rhinosinusitis. Am J Rhinol Allergy. 2016;30(1):44–47.26867529
- Yii ACA, Tay TR, Choo XN, Koh MSY, Tee AKH, Wang DY. Precision medicine in united airways disease: a “treatable traits” approach. Allergy. 2018;73(10):1964–1978. doi:10.1111/all.1349629869791
- Crystal-Peters J, Neslusan C, Crown WH, Torres A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol. 2002;109(1):57–62. doi:10.1067/mai.2002.12055411799366
- Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;1:CD003559.
- Tsabouri S, Tseretopoulou X, Priftis K, Ntzani EE. Omalizumab for the treatment of inadequately controlled allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. J Allergy Clin Immunol Pract. 2014;2:332–340. doi:10.1016/j.jaip.2014.02.00124811026
- Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta-analysis. Allergy. 2017;72:1597–1631.28493631
- Dhami S, Kakourou A, Asamoah F, et al. Allergen immunotherapy for allergic asthma: a systematic review and meta-analysis. Allergy. 2017;72:1825–1848. doi:10.1111/all.1320828543086
- Corren J, Manning BE, Thompson SF, Hennessy S, Strom BL. Rhinitis therapy and the prevention of hospital care for asthma: a case-control study. J Allergy Clin Immunol. 2004;113:415–419. doi:10.1016/j.jaci.2003.11.03415007339
- Bousquet J, Schünemann HJ, Samolinski B, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130(5):1049–1062. doi:10.1016/j.jaci.2012.07.05323040884
- Jose J, Coatesworth AP. Inferior turbinate surgery for nasal obstruction in allergic rhinitis after failed medical treatment. Cochrane Database Syst Rev. 2010;12:CD005235.
- From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA); 2016 Available from: http://www.ginasthma.org/. Accessed 520, 2018.
- Marks GB. Are reference equations for spirometry an appropriate criterion for diagnosing disease and predicting prognosis? Thorax. 2012;67(1):85–87. doi:10.1136/thoraxjnl-2011-20058421825082
- Tu YL, Chang SW, Tsai HJ, et al. Total serum IgE in a population-based study of Asian children in Taiwan: reference value and significance in the diagnosis of allergy. PLoS One. 2013;8:e80996. doi:10.1371/journal.pone.008099624278361
- Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi:10.1016/j.jaci.2006.01.01116522452
- Toma S, Hopkins C. Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhinology. 2016;54(2):129–133. doi:10.4193/Rhin15.07227017484
- DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014;140:712–719. doi:10.1001/jamaoto.2014.104525074504
- Fonseca JA, Nogueira-Silva L, Morais-Almeida M, et al. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy. 2010;65(8):1042–1048. doi:10.1111/j.1398-9995.2009.02310.x20121755
- Passalacqua G, Ciprandi G, Canonica GW. The nose-lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001;1:7–13.11964663
- Passalacqua G, Canonica GW. Impact of rhinitis on airway inflammation: biological and therapeutic implications. Respir Res. 2001;2(6):320–323. doi:10.1186/rr8011737929
- Chen FH, Zuo KJ, Guo YB, et al. Long-term results of endoscopic sinus surgery-oriented treatment for chronic rhinosinusitis with asthma. Laryngoscope. 2014;124:24–28. doi:10.1002/lary.2419623686815
- Schlosser RJ, Smith TL, Mace J, Soler ZM. Asthma quality of life and control after sinus surgery in patients with chronic rhinosinusitis. Allergy. 2017;72(3):483–491.27638398
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16:45–56. doi:10.1038/ni.304925521684
- Fuhlbrigge A, Peden D, Apter AJ, et al. Asthma outcomes: exacerbations. J Allergy Clin Immunol. 2012;129(3 Suppl):S34–S48. doi:10.1016/j.jaci.2011.12.98322386508
