Abstract
Background
Vitamin D exerts profound effects on both adaptive and innate immune functions involved in the development and course of autoimmune and inflammatory diseases. As the incidence of vitamin D insufficiency is surprisingly high in the general population, experimental studies have started to investigate whether vitamin D levels (measured as serum 25 hydroxy vitamin D-25[OH]D) are correlated with immune cells and clinical parameters.
Purpose
The aim of the present research was to investigate serum vitamin D status in a case-control study in children with asthma and to study associations between vitamin D levels and certain immunological parameters.
Materials and methods
A case control study of thirty-nine children with clinically controlled asthma was enrolled to assess the relationship between serum vitamin D concentrations and disease activity. Vitamin D was assayed with a radioimmunoassay kit. We evaluated the relationship between vitamin D concentrations and forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and the FEV1/FVC ratio. Correlations between inflammatory mediators, Th1, Th2, Th17, and regulatory T cells (Treg) and vitamin D were investigated.
Results
Only 15.38% of our asthmatic children had a sufficient serum 25(OH)D (≥30 ng/mL) whereas 80% of healthy children expressed sufficient levels. Deficient values of vitamin D (<20 ng/mL) were observed in 17 (43.59%) asthmatic patients (14.40 ± 3.30 ng/mL; P = 0.0001). Deficiency was not observed in controls. Th1/Th2 ratio was significantly correlated to 25(OH) D level (r = 0.698; P = 0.0001). A significant negative correlation was observed between serum interleukin-17 and vitamin D levels in young asthmatics (r = −0.617; P = 0.001). A significant correlation was observed between CD25+Foxp3+ Treg cells and vitamin D values in asthmatics (r = 0.368; P = 0.021).
Conclusion
Even in a southern Mediterranean country, hypovitaminosis D is frequent in children with asthma. Our findings suggest that vitamin D is an important promoter of T cell regulation in vivo in young asthmatics.
Keywords:
Introduction
Vitamin D deficiency is currently associated with asthma.Citation1–Citation4 The protective effects of vitamin D in asthma could be attributable to its immunomodulatory properties.Citation5 Beyond a central role in calcium and bone physiology, vitamin D metabolism, specifically the conversion of 25(OH)D to the active form of vitamin D (1,25[OH]2D), has effects on epithelial cell, T-cell, B-cell, and dendritic cell functions, which are important for innate and adaptive immunity.Citation6,Citation7 Among the vitamin D-stimulated processes that contribute to tolerance are induction of tolerogenic dendritic cells,Citation7,Citation8 development of CD4+CD25+Foxp3+ regulatory T (Treg) cells,Citation7 activation of T-cell and antigen receptor signalling,Citation8 and elaboration of anti-inflammatory cytokines, including interleukin-10 (IL-10).Citation9 Gene expression profiles of dendritic cells have identified many 1,25(OH)2D-regulated transcripts that are central to dendritic cell function.Citation7 The observation that 1,25(OH)2D-treated human dendritic cells have the capacity to convert CD4 T cells into IL-10-secreting Treg cells and suppress the proliferation of T cells is particularly provocative in light of the clinical importance of CD4+CD25+Foxp3+ Treg cells.Citation10 Vitamin D has the ability to establish homeostasis between regulatory and suppressor T cell functions to modulate inflammatory process as reported by Chambers and Hawrylowicz.Citation11 The active form of vitamin D, ie, 1,25(OH)2D3, mediates a shift of antigen-presenting cells (APC) and CD4+ T cells to a less inflammatory profile.Citation6 Recently, it was shown that 1,25(OH)2D3 significantly increased the secretion of IL-10 and decreased the secretion of IL-6 and IL-17 by immune cells. 1,25(OH)2D3 also increased the expression and biological activity of indoleamine 2,3-dioxygenase, mediating a significant increase in the number of CD4+CD25+ Treg cells.Citation11 The transcriptional characteristics of CD4+ T cells in young asthmatics indicate that Foxp3 (Treg cells) and RORC (Th17) mRNA expression could be associated with the sustained inflammatory process in asthmatics.Citation12
The aim of our study was to correlate serum vitamin D levels with asthma severity in children, and to correlate vitamin D levels with Th17 and Treg cells.
Materials and methods
Study subjects
Thirty-nine children with asthma (aged 6–16 years), who were diagnosed and classified according to Global Initiative for Asthma (GINA) recommendationsCitation13 and attending the Department of Pediatric Respiratory Disease (Unit Research: Homeostasis and Cell Dysfunction [UR/99/08-40]), A Mami Hospital, Ariana Hospital Tunisia between July 1, 2010 and August 31, 2010, were enrolled in the study during a planned visit. Severity was assessed before treatment. All patients were on regular treatment with inhaled steroids. Control was assessed on clinical and functional criteria. Patients presenting an asthma exacerbation or suffering from comorbidities were excluded from the study. Thirty age and sex-matched children attending pediatric emergency for acute extra respiratory symptoms served as healthy controls (aged 6–16 years, mean 9.2; 20 males). Asthmatic patients were excluded. Children suffering with other chronic diseases were excluded. Selection was made according to clinical criteria. The hospital ethical committee approved the study according to Global Initiative for Asthma guidelines as we recently reported, and the parents and children gave their informed consent.Citation12,Citation13
Vitamin D status definitions
Blood withdrawal was performed between July and August, 2010. Immune tests and serum for vitamin D measurement were retrieved at the same time, and cellular assays were performed on the day of blood collection. The collected serum was immediately shielded from direct light and stored at −20°C. At the end of the study, all samples were analyzed simultaneously. Serum concentrations of 25(OH) D were assayed with a radioimmunoassay kit (Dia-Sorin, Stillwater, MN). Vitamin D level values were used as a continuous variable and were categorized in descriptive analyses as desirable (or sufficient) when scores were at least 30 to 40 ng/mL (75 to 100 nmol/L), insufficient between 20 and 30 ng/mL (50 and 75 nmol/L) and deficient when <20 ng/mL, as previously recommended.Citation3,Citation14
Lymphocytes subsets T cell phenotyping
Lymphocytes were isolated from peripheral blood drawn from 39 asthmatic patients and 30 healthy children. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Hypaque gradient centrifugation (Histopaque®; Sigma Aldrich, Zwijndrecht, The Netherlands). CD4+ T cells were selectively isolated with RosetteSep® (Stem Cell Technologies, Grenoble, France). The method for intracellular staining of CD4+ T-cell subsets has been described in detail previously.Citation15,Citation16 The following monoclonal antibodies were used: fluorescein isothiocyanate (FITC)-labeled anti-interferon-gamma (anti-IFN-γ), phycoerythrin (PE)-labeled anti-IL-4, PE-conjugated anti-IL-10 (all from BD Biosciences, San Jose, CA) or PE-labeled anti-IL-17 (R&D Systems, Minneapolis, MN). Based on intracytoplasmic staining, the phenotypes within CD4+ cells were determined as follows: Th1 cells, CD4+IFN-γ +IL-4−; Th2 cells, CD4+IFN-γ −IL-4+; Tr1 (Type 1 regulatory T) cells, CD4+IL10+; and Th17 cells, CD4+IL17+. Cell surface (CD4, CD25) and intracellular (Foxp3) staining was carried out on freshly isolated PBMCs from heparinized blood, as we reported recently.Citation17 Lymphocytes were gated on the basis of their forward and side scatter properties by using FACSCalibur flow cytometry (Becton Dickinson, San Jose, CA). The data were analyzed using CellQuest software (Becton Dickinson). The following reagents were used: Ficoll and CD4-FITC monoclonal antibody (Sigma Aldrich, St Louis, MO); CD25-PC5 (Immunotech, Marseille, France); Foxp3-PE, clone: PCH101 (eBioscience, San Diego, CA); and an intracellular staining kit (eBioscience).
Plasma cytokines
Plasma IL-17, IL-6, and IL-10 concentrations were measured by enzyme-linked immunosorbent assay (using reagent kits from R&D Systems).
Statistical analysis
Since plasma cytokine concentrations were not in a Gaussian distribution, the Mann–Whitney rank sum test was used to assess the differences in cytokine concentrations in asthmatic patients and control subjects. The Pearson correlation test was used to ascertain the correlation among plasma cytokine concentrations. All analyses were performed using SPSS statistical software for Windows (version 9.0; SPSS Inc, Chicago, IL). A probability of P < 0.05 was considered as significant. Unless otherwise specified, results in the figures are expressed as median (interquartile range: 25th, 75th, 10th and 90th percentiles; the 50th percentile represents the median).
Results
Demographic data on patients and healthy controls
Data of asthmatic patients are depicted in . The severity of asthma in this group of patients was presented according to GINA guidelines. The severity of asthma was depicted as mild in 60.5%, moderate in 36.8%, and severe in 2.7%. Considering the low number of severe asthmatics, they were included with moderate asthmatics in the study. No difference was observed in the body weight between asthmatic children (32.7 kg; range: 26–45) and the healthy control group (36.8 kg; range: 29–47).
Table 1 Patients’ characteristics
Vitamin D levels in asthmatic patients and healthy controls
The healthy subjects and asthmatic children do not differ in sex and age. There was no significant difference in mean vitamin D serum levels between healthy controls (23.30 ± 4.57 ng/mL) and patients (20.89 ± 7.51 ng/mL; P = 0.12). However, when patients and controls were divided according to vitamin D classes, significant differences were observed (). Sufficient vitamin D levels (33.83 ± 3.31 ng/mL; range: 30–38 ng/mL) were observed in six asthmatics (15.38%). Insufficient levels were found in 16 (41%) patients (22.94 ± 2.94% ng/mL; range: 20–29.2 ng/mL), and deficient values (14.40 ± 3.30 ng/mL; range: 9.7–19.7 ng/mL) were observed in 17 patients (43.59%). Healthy controls (n = 30) were either sufficient (80%) (31.83 ± 2.64 ng/mL; range: 30–37 ng/mL) or insufficient (20%) (21.20 ± 1.12 ng/mL; range: 20.8–29.8 ng/mL). No deficiency was observed in the controls.
Table 2 Comparison of vitamin D levels in asthmatic patients and controls
Vitamin D serum concentrations depending on clinical data
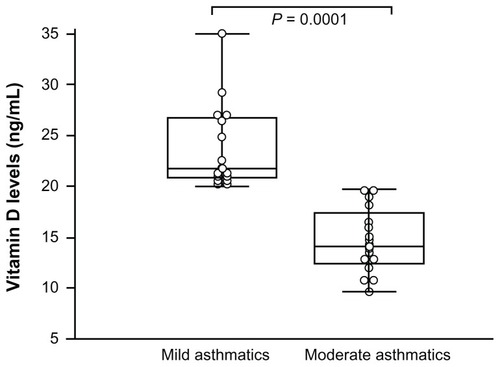
Vitamin D level in asthmatics (sufficient, insufficient, and deficient) was studied according to the disease severity (mild and moderate/severe asthma). Patients with moderate asthma expressed lower vitamin D levels (14.67 ± 3.20 ng/mL) than mild asthmatics did (26.10 ± 5.85 ng/mL; P = 0.0001) ().
Figure 1 Vitamin D concentrations of asthmatic patients.
Serum levels of 25(OH)D were slightly associated with forced vital capacity (FVC) percent predicted (r = 0.358; P = 0.025). Nonsignificant correlation was observed between percentage of forced expiratory volume in 1 second (FEV1) predicted and serum levels of vitamin D (r = 0.319; P = 0.0507). No correlation was observed between FEV1/FVC and vitamin D. No correlation was found between weight status and vitamin D level.
Serum concentrations of proinflammatory cytokines
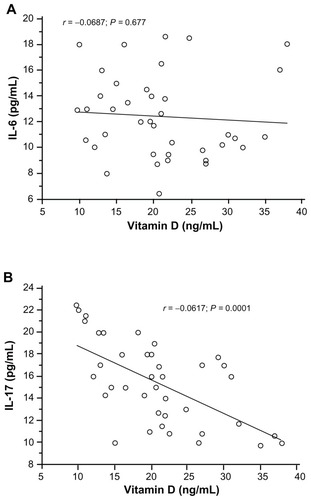
Asthmatic patients showed higher serum IL-17 (18.5 pg/mL; range: 9.75–22.46) and IL-6 (14.72 pg/mL; range: 10.56–18.62) concentrations than healthy controls (IL-17: 10.5 pg/mL; range: 0.08–14.82; IL-6: 2.75 pg/mL; range: 0.02–8.43). The differences were statistically significant (IL-17: P = 0.001; IL-6: P = 0.002). There was no correlation between serum concentrations of IL-6 and vitamin D levels (). A significant negative correlation was observed between IL-17 and vitamin D levels in young asthmatics (r = −0.617; P = 0.001) ().
Figure 2 Correlation between vitamin D level and serum IL-6 (A) and serum IL-17 (B) using, Pearson’s correlation coefficient.
Abbreviation: IL, interleukin.
Intracellular cytokines Th1 and Th2 in peripheral T helper cells
The percentage of IFN-γ-producing CD4+ Th1 cells was found to be significantly higher in healthy children (20.37% ± 4.4%; range: 10.5%–27%) than in asthmatic patients (6.62% ± 3.46%; range: 2.0%–13.8%; P = 0.0001) (). A significant difference was observed in the percentage of IL-4-producing CD4+ Th2 cells between healthy controls (0.92% ± 0.59%; range: 0.33%–1.9%) and asthmatics (0.617% ± 0.35%; range: 0.21%–1.7%; P = 0.0035) (). The Th1/Th2 ratios of normal controls were significantly higher (27.26% ± 14.35%; range: 9.5%–27.3%) than those of asthmatic children (13.48% ± 8.55%; range: 2.15%–39.4%; P = 0.0001) (). A significant positive correlation was observed between 25(0H)D levels and Th1/Th2 ratios in asthmatic children (r = 0.698; P = 0.0001) ().
Figure 3 Intracellular Th1 and Th2 cytokines. (A–D) Box and whiskers plot of (A) percentage of interferon-gamma (IFN-γ) producing cells; (B) percentage of IL-4 producing cells; and (C) the ratio of Th1/Th2 cytokine (interferon-gamma [IFN-γ] and IL-4) determined by Fast Immune Cytokine System for normal controls (n = 30) and asthmatic patients (n = 39). The medians are indicated by a line inside each box, the 25th and 75th percentiles are indicated by the box limits, and the lower and upper error bars represent the 10th and 90th percentiles, respectively. The mean values were compared and the P values are indicated at the figure. (D) Correlation between Th1/Th2 ratio and vitamin D levels in asthmatic patients using Pearson’s correlation coefficient.
Abbreviation: IL, interleukin.
![Figure 3 Intracellular Th1 and Th2 cytokines. (A–D) Box and whiskers plot of (A) percentage of interferon-gamma (IFN-γ) producing cells; (B) percentage of IL-4 producing cells; and (C) the ratio of Th1/Th2 cytokine (interferon-gamma [IFN-γ] and IL-4) determined by Fast Immune Cytokine System for normal controls (n = 30) and asthmatic patients (n = 39). The medians are indicated by a line inside each box, the 25th and 75th percentiles are indicated by the box limits, and the lower and upper error bars represent the 10th and 90th percentiles, respectively. The mean values were compared and the P values are indicated at the figure. (D) Correlation between Th1/Th2 ratio and vitamin D levels in asthmatic patients using Pearson’s correlation coefficient.](/cms/asset/ce5c32f9-44fa-4b4b-8e1d-0829ff868718/djaa_a_29566_f0003_b.jpg)
Intracellular cytokines Tr1 and Th17, in peripheral T helper cells
Th17 (IL-17) and Tr1 (IL-10) by CD4+ T cells were assessed depending on intracellular cytokine expression. In asthmatic patients, the median percentage of IL-10+ cells within the CD4+ T cell compartment (0.50% ± 0.21%; range: 0.2%–0.95%) and the percentage of IL-17+ cells were decreased (1.048% ± 0.48%; range: 0.23%–1.8%) compared to healthy controls (IL-10+ cells, 1.39% ± 0.85%, range: 0.5%–3.4%; IL-17+ cells, 0.44% ± 0.20%; range: 0.21%–0.99%; P = 0.0001).
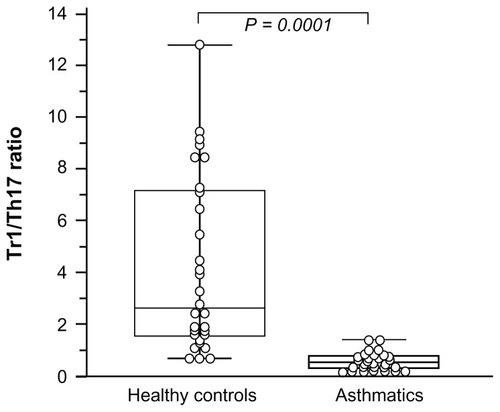
A significant positive correlation was observed between serum 25(OH)D levels and the IL-10+CD4+ T cells (r = 0.428; P = 0.0081). No correlation was found between IL-17+CD4+ cells and vitamin D levels (r = 0.123; P = 0.23). Tr1/Th17 (IL-10 producing CD4+ cells/IL-17 producing CD4+ cells) ratio was significantly decreased in the asthmatic children compared with the healthy controls ().
Figure 4 Intracellular Type 1 regulatory T (Tr1) cells (IL-10) and Th17 (IL-17) cytokines CD4+ production.
Regulatory T cells correlate with vitamin D level
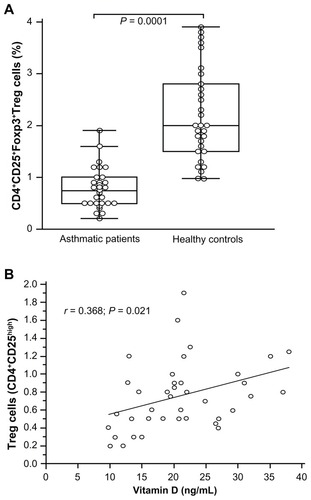
We have recently reported that children with asthma expressed low percentages of Treg cells.Citation17 Patients studied for their serum vitamin D levels were investigated for their CD4+ T cells expressing Treg cells. Treg cells were evaluated in the peripheral circulation of 39 asthmatic patients. Regulatory cells within the CD4+ T cell compartment were defined as CD25highFoxp3+ cells. The median percentage of CD25highFoxp3+ Treg in the CD4+ T cell compartment was decreased (0.75% ± 0.37%; range: 0.2%–1.9%) compared to healthy children (2.20% ± 0.88%; range: 0.98%–3.9%; P = 0.0001) (). A significant correlation was observed between the percentage of CD25highFoxp3+ Treg cells and vitamin D values in asthmatics (r = 0.368; P = 0.021) (). The asthmatic children, whatever their vitamin D value, expressed decreased Treg cells compared with the healthy children (2.64% ± 0.28%; P = 0.0028). A significant positive correlation was observed between vitamin D and IL-10 levels in asthmatics (r = 0.428; P = 0.008).
Figure 5 CD25+Foxp3+ Treg in the CD4+ T cell compartment. Box and whiskers plot of percentage of CD25+Foxp3+ Treg in the CD4+ T cells (A). The medians are indicated by a line inside each box, the 25th and 75th percentiles are indicated by the box limits, and the lower and upper error bars represent the 10th and 90th percentiles, respectively. Correlation between CD25+Foxp3+ Treg cells and vitamin D levels in asthmatic patients using Pearson’s correlation coefficient (B).
Discussion
Contrasting levels of vitamin D have been found in asthmatics. In our report, 43% of asthmatics were deficient, and 41% have insufficient vitamin D levels. The decrease in vitamin level was associated with the severity of the disease.
In agreement with a previous observation of Black and Scragg,Citation18 the data from our study confirm that serum 25-OH vitamin D is significantly and positively correlated with FVC. No correlations were observed with FEV1 or FEV1/FVC. Recently, Zosky et al reported that vitamin D deficiency decreased lung functions.Citation19 Similarly, Li et al reported that vitamin D deficiency was highly prevalent in Chinese asthma patients, and vitamin D status was correlated with lung function.Citation1
The results for the asthmatic subjects in this study were in accord with those reported by Chinellato et al, who found that hypovitaminosis D is frequent in children with asthma who live in a Mediterranean country and that lower levels of vitamin D are associated with reduced asthma control.Citation2 Vitamin D insufficiency was also reported to be common in a population of North American children with mild-to-moderate persistent asthma and is associated with higher odds of severe exacerbation over a 4-year period.Citation4
This is the first study to investigate the correlation between vitamin D status and the composition of the T cell compartment in vivo in asthmatic patients. Treg cells in the peripheral blood of asthmatic patients were significantly correlated with vitamin D status. The decreased Th1/Th2 ratio reflected a skewing of the IFN-γ/IL-4 balance. Our data indicate that low serum 25(OH)D levels are associated with elevated inflammatory mediators and less regulated T cell compartments in asthmatic patients.
Several studies suggested that vitamin D deficiency could lead to immune malfunctioning.Citation19,Citation20 Although the exact mechanisms of lower vitamin D levels in chronic inflammatory states are not yet elucidated in asthma, this deficit probably interferes with immunoregulatory functions of vitamin D. Immune cells (T and B lymphocytes, macrophages and dendritic cells) express vitamin D receptors (VDR) and are affected by vitamin D deficiency during their maturation process.
Disturbances in vitamin D homoeostasis may contribute to the inflammatory process in asthma. Th1, Th2, and Treg cells have been shown to express the vitamin D receptor and to be vitamin D targets.Citation21–Citation23 The development of certain immune cells requires the expression of VDR both intrinsically and extrinsically as reported by Yu and Cantorna and Maruotti and Cantatore.Citation24,Citation25 Recently, the effect of vitamin D on innate immunity-mediated inflammation, enhancing the antimicrobial properties of immune cells, such as monocytes and macrophages, has been demonstrated.Citation24,Citation25 In addition, vitamin D directly affects T cell responses by inhibiting the production of Th1 cytokines (IL-2 and IFN-γ), Th17 cytokines (IL-17), and by stimulating Th2 cytokine production (IL-4). Moreover, vitamin D influences the maturation of dendritic cells.Citation22 In asthma, vitamin D could be considered an important mediator, in which fluctuation is correlated to the inflammatory state of the disease.
VDR may function as a regulator of asthma and atopy susceptibility.Citation26 A strong association between genetic variants at the VDR locus and asthma/atopy in a Quebec cohort was reported.Citation27 Along with other known asthma risk genes such as ADAM33,Citation27 TLR,Citation28 and RANTES,Citation29 the addition of VDR involvement in the understanding of asthma/atopy pathogenesis will shed light on better control and treatment. VDR- deficient mice failed to develop experimental allergic asthma, suggesting a role for vitamin D in driving Th2 inflammation in the airways.Citation30
In asthmatic patients, T cells and particularly CD4+ lymphocytes are stimulated and switch from naïve to memory CD4+ T cells.Citation7 The decreased level of vitamin D in asthma could be explained in part by CD4 cells consuming intrinsic vitamin D during their activation, as VDR expression on CD4+ T lymphocytes increases drastically after cell activation.Citation23 Addition of 1,25(OH)2D3 leads to decreased secretion of IL-2 and IFN-γ by CD4 T cells and promotes IL-5 and IL-10 production, which further shifts the T cell response towards Th2 dominance.Citation23,Citation24 Importantly, immune cells are able to activate vitamin D locally, which indicates an autocrine or paracrine role for this vitamin within the immune system.
Lower levels of vitamin D in children were associated with increased markers of asthma severity.Citation4 Several studies have reported associations of low vitamin D status with poor pulmonary function and increased incidence of respiratory infections, asthma, and chronic obstructive pulmonary disease.Citation6 Mechanistic studies suggested that 1,25-(OH)2D3 influenced downregulation of the glucocorticoid receptor by its ligand.Citation31,Citation32 These studies suggest that vitamin D may have therapeutic potential in severe asthma patients as a steroid-enhancing agent. Emerging studies show an association between low vitamin D status and poor clinical responsiveness to glucocorticoids in asthma patients.Citation33 If a steroid-enhancing role for vitamin D is proven, it is likely to be applicable to other chronic illness in which steroids represent a primary treatment, as also suggested by data on animal models.Citation32 Nevertheless, considerable interest remains in the therapeutic application of vitamin D to asthma, and examples of beneficial effects exist.
The fact that Th1/Th2 ratio and Th17 were correlated to serum vitamin D raises a question about the origin of vitamin D deficiency in asthma. More studies are needed to clarify the mechanisms by which vitamin D regulates cellular immunity. Similarly, the genetic factors influencing 1,25(OH)2D3 production and vitamin D receptor expression should be explored. The microbial pattern-recognition receptor known as Toll-like receptor 9 (TLR9) was described as a biomarker of vitamin D-induced IL-10+ Tregs. Ligation of TLR9 with its agonist CpG (cytosine-phosphate-guanine) oligonucleotide turned off IL-10 production, suggesting a control mechanism abrogating Treg.Citation33,Citation34 The active form of vitamin D influences innate and adaptive immunity. It acts on APCs and T cells to promote peripheral tolerance through the inhibition of inflammatory responses and the induction of Treg cells. Recently, Wong et al reported that a coculture of bronchial epithelial cells and basophils could significantly induce the release of IL-6, an epithelial inflammatory cytokine as well as CCL2, a chemokine for basophils, eosinophils, and monocytes.Citation35 This induction was synergistically enhanced by IL-17A. The interaction of basophils and bronchial epithelial cells was under IL-17A stimulation. It was differentially regulated by extracellular signal-regulated kinase, c-Jun N-terminal protein kinase, p38 mitogen-activated protein kinase, and nuclear factor-κB pathways. These findings suggest a new immunopathological role of Th17 cells and basophils in allergic asthma.Citation35 Recently, Baeke et al reported that TX527 (hypocalcemic vitamin D analogs) directly affects human T cell function, thereby inhibiting effector T cell reactivity while inducing regulatory T cell characteristics, imprinting them with a specific homing signature favoring migration to sites of inflammation.Citation36 These findings confirmed that vitamin D is an important regulator of immune function. T cells express the vitamin D receptor and have been shown to be direct and indirect vitamin D targets.Citation37 CantornaCitation37 suggested that the expression of VDR is required for the development of two cell types, NKT cells and CD8αα T cells, which inhibit autoimmunity. In addition, effector T cell cytokine production is regulated by vitamin D. The available evidence suggests that NKT and CD8αα T cells express VDR as part of the selection process to protect against the generation of autoimmunity.Citation37
Conclusion
Our study provides important information on vitamin D levels in asthmatic children in the Mediterranean region. We were able to show that vitamin D deficiency in asthmatic patients is common, and it is similar to that seen in the autoimmune diseases. We found significant correlations between several inflammatory and immunological markers and vitamin D levels. These findings should be confirmed prospectively through the generation of an efficient multivariate model allowing further research about the use of vitamin D supplementation in patients with asthma.
Acknowledgments
Maalmi H, Berraïes A, Hamzaoui K, and Hamzaoui A contributed substantially to conception and design, or acquisition of data, or analysis and interpretation of data; Maalmi H, Berraïes A, Hamzaoui K, and Hamzaoui A drafted the article or revised it critically for intellectual content; and Maalmi H, Berraïes A, Ammar J, Hamzaoui K, and Hamzaoui A had final approval of the version to be published.
Disclosure
The authors report no conflicts of interest in this work.
References
- LiFPengMJiangLVitamin D deficiency is associated with decreased lung function in Chinese adults with asthmaRespiration201181646947521124013
- ChinellatoIPiazzaMSandriMPeroniDPiacentiniGBonerALVitamin D serum levels and markers of asthma control in Italian childrenJ Pediatr2011158343744120870246
- ChinellatoIPiazzaMSandriMSerum vitamin D levels and exercise-induced bronchoconstriction in children with asthmaEur Respir J20113761366137021071468
- BrehmJMSchuemannBFuhlbriggeALSerum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program studyJ Allergy Clin Immunol201012615258. e520538327
- LangeNELitonjuaAHawrylowiczCMWeissSVitamin D, the immune system and asthmaExpert Rev Clin Immunol20095669370220161622
- HolickMFVitamin D deficiencyN Engl J Med2007357326628117634462
- HewisonMVitamin D and the intracrinology of innate immunityMol Cell Endocrinol2010321210311120156523
- SzelesLKeresztesGTöröcsikD1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotypeJ Immunol200918242074208319201860
- von EssenMRKongsbakMSchjerlingPOlgaardKOdumNGeislerCVitamin D controls T cell antigen receptor signalling and activation of human T cellsNat Immunol201011434434920208539
- MoraJRIwataMvon AndrianUHVitamin effects on the immune system: vitamins A and D take centre stageNat Rev Immunol20088968569819172691
- ChambersESHawrylowiczCMThe impact of vitamin D on regulatory T cellsCurr Allergy Asthma Rep2011111293621104171
- HamzaouiAMaalmiHBerraïesAAbidHAmmarJHamzaouiKTranscriptional characteristics of CD4+ T cells in young asthmatic children: RORC and FOXP3 axisJ Inflamm Res2011413914622259252
- Global strategy for asthma management and prevention: GINA executive summaryEur Respir J200831114317818166595
- Bischoff-FerrariHAGiovannucciEWillettWCDietrichTDawson-HughesBl. Estimation of optimal serum concentrations of 25-hydroxy vitamin D for multiple health outcomesAm J Clin Nutr2006841182816825677
- HamzaouiKHamzaouiAGuemiraFBessioudMHamzaMAyedKCytokine profile in Behçet’s disease patients. Relationship with disease activityScand J Rheumatol200231420521012369651
- HamzaouiKHoumanHHamzaouiARegulatory T cells in cerebrospinal fluid from Behçet’s disease with neurological manifestationsJ Neuroimmunol20071871–220120417559948
- HamzaouiAAmmarJHamzaouiKRegulatory T cells in induced sputum of asthmatic children: association with inflammatory cytokinesMultidiscip Resp Med2010512230
- BlackPNScraggRRelationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination surveyChest200512863792379816354847
- ZoskyGRBerryLJElliotJGJamesALGormanSHartPHVitamin D deficiency causes deficits in lung function and alters lung structureAm J Respir Crit Care Med2011183101336134321297070
- CannellJJHollisBWZasloffMHeaneyRPDiagnosis and treatment of vitamin D deficiencyExpert Opin Pharmacother20089110711818076342
- PappaHMGrandRJGordonCMReport on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and diseaseInflamm Bowel Dis200612121162117417119391
- CantornaMTMahonBDMounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalenceExp Biol Med200422911361142
- BaekeFKorfHOverberghLHuman T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune systemJ Steroid Biochem Mol Biol20101211–222122720302932
- YuSCantornaMTThe vitamin D receptor is required for iNKT cell developmentProc Natl Acad Sci U S A20081055207521218364394
- MaruottiNCantatoreFPVitamin D and the immune systemJ Rheumatol201037349149520080911
- PoonAHLapriseCLemireMAssociation of vitamin D receptor genetic variants with susceptibility to asthma and atopyAm J Respir Crit Care Med2004170996797315282199
- Van EerdeweghPLittleRDDupuisJAssociation of the ADAM33 gene with asthma and bronchial hyperresponsivenessNature2002418689642643012110844
- LachhebJChelbiHHamzaouiKHamzaouiAToll-like receptors and CD14 genes polymorphisms and susceptibility to asthma in Tunisian childrenTissue Antigens200871541742518312481
- LachhebJChelbiHHamzaouiKHamzaouiAAssociation between RANTES polymorphisms and asthma severity among Tunisian childrenHum Immunol200768867568017678722
- WittkeAWeaverVMahonBDAugustACantornaMTVitamin D receptor-deficient mice fail to develop experimental allergic asthmaJ Immunol200417353432343615322208
- LloydCMHawrylowiczCMRegulatory T cells in asthmaImmunity200931343844919766086
- XystrakisEKusumakarSBoswellSReversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patientsJ Clin Invest2006116114615516341266
- SearingDASearingDAZhangYDecreased serum vitamin D levels in children with asthma are associated with increased corticosteroid useJ Allergy Clin Immunol20101255995100020381849
- DanielCSartoryNAZahnNRadekeHHSteinJMImmune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th)1/Th17 to a Th2 and regulatory T cell profileJ Pharmacol Exp Ther20083241233317911375
- WongCKCaoJYinYBLamCWInterleukin-17A activation on bronchial epithelium and basophils: a novel inflammatory mechanismEur Respir J201035488389319741026
- BaekeFKorfHOverberghLThe vitamin D analog, TX527, promotes a human CD4+CD25highCD127low regulatory T cell profile and induces a migratory signature specific for homing to sites of inflammationJ Immunol2011186113214221131424
- CantornaMTWhy do T cells express the vitamin D receptor?Ann N Y Acad Sci20111217778221114675