Abstract
Rationale
Asthma, obesity and physical activity (PA) are interrelated. However, longitudinal data with objective PA measures and direct assessment of body composition are still lacking.
Objective
To study the impact of symptom control on PA and body composition.
Methods
In a longitudinal cohort study of the German Center for Lung Research, we assessed the body composition of 233 asthma patients and 84 healthy controls using bioelectrical impedance analysis. PA (ie average daily steps and time of at least moderate activity, steps/min) was measured by accelerometry for one week. Asthma control was assessed by ACT score, ACQ-5 score and history of severe exacerbations. After two years of follow-up, we studied changes in physical activity and body composition in relation to asthma control.
Results
Patients with uncontrolled asthma had increased fat mass and decreased muscle mass compared to patients with controlled asthma or healthy controls. Both fat mass and muscle mass correlated better with asthma control than the body mass index (BMI). In multivariate regressions adjusted for age and sex, asthma control and physical activity were independent predictors of body composition (R2 = 0.61, p < 0.001). Persistent uncontrolled asthma patients (n=64) had lower physical activity at both baseline (6614 steps/118 min) and follow-up (6195/115). Despite having stable BMI, they also had significant muscle loss (−1.2%, −0.88 kg, p<0.01) and fat accumulation (+1%, +1.1 kg, p<0.01). By contrast, temporarily uncontrolled or controlled asthma patients had higher physical activity at baseline (8670/156) and follow -up (9058/153) with almost unchanged body composition.
Conclusion
Persistent uncontrolled asthma is associated with sustained physical inactivity and adverse changes in body composition that might be overlooked by relying solely on BMI. Physical activity is an independent predictor of body composition and reliable long-term marker of symptom control.
Background
Asthma is a common worldwide health problem affecting patients from all age groups.Citation1 In most patients, a proper therapy allows adequate symptom control. However, a subset of severe asthma patients remains persistently uncontrolled.Citation2 Uncontrolled asthma is often associated with obesityCitation3 and physical inactivityCitation4,Citation5 in addition to corticosteroid induced comorbidities.Citation6 While the relationship between asthma and obesity has been studied extensively, most of the studies have relied on the body mass index (BMI) as surrogate for body composition rather than the direct measures of fat mass and muscle mass. Besides, the role of physical inactivity in this important association still remains uncertain, particularly, the long-term association of asthma control with physical activity and body composition. It has been suggested that functional impairments and physical inactivity in people with chronic lung diseases might adversely affect their body composition.Citation7 For instance, physical inactivity and the associated muscle depletion are well established disease features of severe COPD.Citation8 However, corresponding data in severe uncontrolled asthma are still lacking. Understanding the long-term consequences of asthma on physical activity and body composition is important as both might affect the overall health outcomes and are relevant for patients’ quality of life.Citation9,Citation10
In this context, the aims of this study were: 1) to study the association of asthma control with body composition, 2) to determine potential predictors of body composition in asthma, particularly, the role of physical activity and 3) to study the longitudinal changes in body composition and physical activity in patients with persistent uncontrolled asthma compared to asthma patients with better symptom control.
Methods
Study Design
The study subjects were adults with asthma and healthy controls who were recruited to the multicenter prospective longitudinal All Age Asthma Cohort (ALLIANCE), a cohort of pediatric and adult patients with asthma in Germany, initiated by the German Centre for Lung Research (DZL). The study was approved by the ethics committee at the university of Luebeck (Az.21–215) according with the Declaration of Helsinki and is registered at clinicaltrials.gov (adult arm: NCT02419274). Verbal and written informed consents were obtained from all subjects prior to enrollment.
The adult arm of the ALLIANCE cohort recruits patients with mild to severe asthma and healthy controls. Detailed information regarding recruitment, inclusion and exclusion criteria was described previously.Citation11 Patients had to have stable disease without acute exacerbations or respiratory tract infections within four weeks prior to study visit. In this analysis, we studied data from baseline and follow-up visits that took place 2 years later. An overview of the selection process for this analysis is given in (Online Supplementary 1 and 2).
Spirometry and Symptom Control
We measured forced expiratory volume in the first second (FEV1), forced vital capacity (FVC) and peak expiratory flow (PEF) from spirometry according to ERS standardizations.Citation12
We defined asthma control based on European Respiratory Society/American Thoracic Society guidelines.Citation2 Asthma control was assessed by the asthma control test (ACT), asthma control questionnaire (ACQ5) and the annualized number of severe exacerbations 12 months prior to study visit, defined as a burst of systemic corticosteroids for ≥3 days. Uncontrolled asthma was defined as either ACQ ≥ 1.5 or ACT <20, or two or more severe exacerbations or one serious exacerbation with hospitalization, ICU stay or mechanical ventilation in the previous year.Citation2
To study the long-term changes in body composition and its relation to asthma control, we compared the changes in body composition between patients with persistent uncontrolled asthma and patients with controlled or temporarily uncontrolled asthma. Persistent uncontrolled defines patients who had uncontrolled symptoms at baseline and at follow-up, while the rest of the patients had controlled asthma at both study visits or had uncontrolled symptoms at only one of the study visits.
Skeletal Muscle Mass and Body Composition
We performed whole body bioelectrical impedance analysis (BIA) using Nutribox analyzer (Data Input, Pöcking, Germany). BIA has shown to be a feasible tool that enables simple assessment of body composition in many clinical conditions.Citation13 An alternating current was used at 50 kHz. We calculated the skeletal muscle mass (SM, [kg]) and its ratio to total body mass was expressed in percentage according to Janssen and colleagues.Citation14,Citation15 To study the relationship between SM, asthma control and physical activity, we classified asthma patients based on the SM quartiles into four groups for each gender. Further body composition measures were the fat mass (FM, [kg]) also in percent of the total body mass and the body mass index (BMI; [kg/m2]).
Physical Activity
Physical activity was measured over a period of one week by a multisensory activity monitor (SenseWear Pro Armband; BodyMedia, Pittsburgh, PA, USA) as previously described.Citation16,Citation17 Accelerometer outputs were: average daily steps and the average daily minutes of at least moderate activity (any energy expenditure above 3 metabolic equivalents (METs)). A threshold of 94% of wearing time (22.5 h) for at least five days was set to identify valid analyses.Citation17
Statistical Analysis
We used one-way analysis of variance ANOVA, or Fisher exact test to identify the significance of the differences of the measured clinical variables between asthma patients and healthy controls as well as between asthma patients in the different SM quartiles. For pairwise comparison, a post hoc analysis with Tukey’s test was done. We used Spearman correlation to evaluate the association between measures of body composition and asthma control scores. We conducted multivariate regression analyses to study the association of muscle mass and fat mass with their potential predictors ie: sex, age, asthma control, corticosteroid therapy and physical activity. The statistical significance of the longitudinal changes in clinical variables was determined using paired Wilcoxon signed-rank test.
Statistical analyses were performed using R (version 3.6.2; R Foundation, Vienna, Austria). An alpha error of less than 5% was considered statistically significant.
Results
Body Composition in Asthma Patients versus Healthy Controls
For the cross-sectional analysis of baseline data, we included 106 patients with controlled asthma, 127 patients with uncontrolled asthma and 84 healthy controls. Detailed clinical characteristics and measures of physical activity and body composition are presented according to asthma control in . The post hoc analysis revealed that the age difference was only significant between uncontrolled asthma and healthy controls (p=0.012), but neither between controlled and uncontrolled asthma patients (p=0.75), nor between controlled asthma and healthy controls (p=0.088). Expectedly, uncontrolled asthma patients had frequent daily symptoms, more severe exacerbations, worse lung function, higher demand for OCS and higher doses of ICS. Accordingly, uncontrolled asthma patients engaged in lower physical activity and were heavier, had increased fat mass and decreased muscle mass compared to controlled asthma patients or healthy controls. Although that both controlled asthma patients and healthy controls had similar physical activity levels, controlled asthma patients were heavier, had lower muscle mass (p=0.039) and a tendency of fat mass accumulation (p=0.061) compared to healthy controls. Further, an age-matched analysis that included subjects who aged 40–64 years only, confirmed that uncontrolled asthma patients had significant decreased physical activity, increased fat mass and decreased muscle mass compared to controlled asthma patients or healthy control, (Online Supplementary 3).
Table 1 Baseline Clinical Characteristics of Patients with Asthma and Healthy Controls
Predictors of Body Composition in Asthma
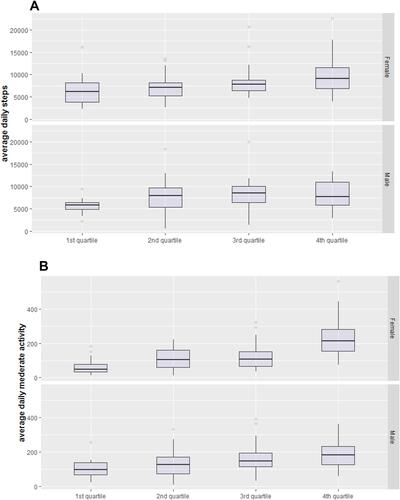
The classification of asthma patients based on SM quartiles and gender is demonstrated in . The first quartile represents the lowest SM values. Pairwise comparisons between all quartiles from both genders also revealed statistically significant differences in BMI and FM except for the BMI which was similar in the second and third male quartiles. Compared to other quartiles, the first quartiles in both males and females showed higher BMI, higher FM, higher ICS doses and higher proportion of female patients who were on regular OCS therapy (). We also found that the first quartiles are characterized with poorer symptom control compared to the other quartiles (). Interestingly, we observed that the correlations of the fat mass with ACT score (R=−0.29) and ACQ score (R= 0.28), and between the muscle mass with ACT score (R=0.26) and ACQ score (R= −0.28) were better than the correlation of BMI with ACT score (R=−0.19) and ACQ score (R=0.18), (all p-values <0.05). Further, we found no statistically significant differences in lung function measures across all SM quartiles. In addition, measures of physical activity were closely associated with the SM as demonstrated in . First quartiles of both genders had a relatively low physical activity compared to the other quartiles. In female patients, the mean average daily steps and average daily time of at least moderate activity (steps/minutes) ranged from 6300/61 in the first quartile to 9653/226 in the fourth quartile (). Likewise, in male patients the means of daily steps and time in at least moderate activity ranged from 5671/102 (steps/minutes) in the first quartile to 8349/186 (steps/minutes) in the fourth quartile.
Table 2 Clinical Characteristics of Asthma Patients Based on Skeletal Muscle Mass and Gender
Figure 1 Physical activity in asthma patients in relation to skeletal muscle mass quartiles and gender: (A) the distribution of average daily steps in muscle mass quartiles. (B) the distribution of at least daily time in moderate activity according to muscle mass quartiles. In females, the post hoc analysis showed statistically significant differences in means of average daily steps between the first and fourth quartiles (p<0.0001), first and third quartiles (p=0.005), and second and fourth quartiles (p=0.01), (A, upper panel). The average daily time of at least moderate activity was significantly different between all quartile (p<0.001) except between the second and third quartiles (p=0.08) (B, upper panel). In males, average daily steps were only significantly different between the first and fourth quartiles (p=0.031), and between the second and fourth quartiles (p=0.002) (A, lower panel). Likewise, the average daily time of at least moderate activity was also significantly different between the first and fourth (p=0.031), and between the second and fourth quartiles (p=0.026) (B, lower panel).
Factors that correlated with body composition were integrated in multivariate regressions. The first regression (R2=0.61, p< 0.0001) showed that main predictors of muscle mass were sex, followed by asthma control; age and physical activity, respectively ().The second regression showed that predictors of fat mass were sex, asthma control and physical activity (). In a further multivariate regression analysis, we did not included asthma control as predictor of body composition. This additional analysis revealed that the OCS use and ICS dose were also predictors of body composition that were exchangeable with asthma control and might be reflecting the demand of intensified corticosteroid therapy in uncontrolled asthma patients (Online Supplementary 4).
Table 3 Predictors of Body Composition in Asthma
Longitudinal Change of Body Composition in Persistent Uncontrolled Asthma
From 161 patients who attended their follow-up visit after two years, we identified 64 patients who had persistent uncontrolled asthma. While age and gender distribution of patients with persistent uncontrolled asthma were comparable to the remaining patients (56 ±11 vs 54 ±13, years, p=0.16) and (45% vs 49, %male, p=0.63), the proportion of patients with regular OCS intake (20% vs 7%, p=0.02) and the ICS doses (795 µg vs 530 µg, p<0.01), were significantly higher in persistent uncontrolled asthma at follow-up.
Despite having unchanged BMI, patients with persistent uncontrolled asthma had a statistically significant loss of muscle mass with a mean loss of 1.2% (0.88 kg) over two years. In contrast, patients with controlled or temporarily uncontrolled asthma had almost unchanged muscle mass with a reduction of only 0.3% (0.1 kg), ( and ). In addition to the decrease in muscle mass, there was a significant increase in fat mass in persistent uncontrolled asthma whereas patients with controlled or temporarily uncontrolled asthma showed no statistically significant changes in body composition ().
Table 4 Measures of Body Composition, Physical Activity and Symptom Control at Baseline and Follow-Up
Figure 2 Longitudinal differences in muscle mass (A) average daily steps (B) and time of at least moderate activity (C) between persistent uncontrolled and controlled asthma patients at baseline and follow-up. Patients with persistent uncontrolled asthma had lower muscle mass (A), lower average daily steps (B) and time in at least moderate activity (C) at baseline and at follow-up p<0.01 compared to the rest of patients.
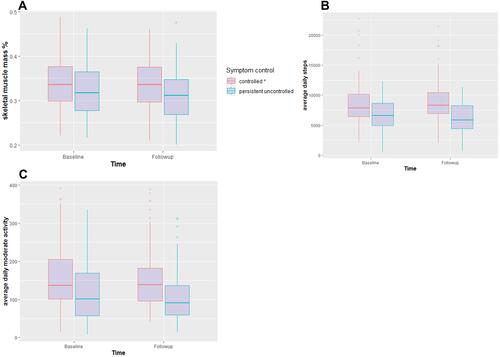
At follow-up, levels of physical activity of both asthma groups remained relatively unchanged. However, we observed a constantly reduced physical activity in persistent uncontrolled asthma patients ( and ). They also had a numeric reduction in the average daily steps by −445/day compared to a slight numeric increase of step counts (+195 steps/day) in the rest of patients at follow-up. Minutes of physical activity did not differ between baseline and follow-up in either asthma group.
Discussion
Our main study findings were: first, uncontrolled asthma patients have a reduced skeletal muscle mass and increased fat mass compared to controlled asthma patients or healthy controls. Second, asthma control and physical activity were independent predictors of body composition irrespective of age and gender. Third, persistent uncontrolled asthma results in sustained physical inactivity which might lead to further unfavorable changes in body composition on the long-term.
So far, studying the relationship between asthma and obesity has mainly relied upon the BMI. Several studies have shown a modest overall increase in asthma incidence and prevalence in obese individuals,Citation18 in addition to the negative impact of obesity on asthma outcomes.Citation19 Interestingly, our data revealed that significant changes in body composition including the increase in fat mass can be observed in patients with persistent uncontrolled asthma despite having stable BMI. Our findings indicate that the measurement of body composition by BIA provides additional information on the relation between asthma control and body composition that cannot be obtained by the crude measure of BMI.
Unlike in asthma, the impact of COPD on physical activity and body composition has been studied extensively, particularly the progressive loss of muscle mass as important marker of morbidity and mortality.Citation20,Citation21 Furthermore, these studies also demonstrated an association between sustained physical inactivity and the accelerated loss of muscle mass.Citation22 Here, we report for the first-time longitudinal changes in body composition in asthma patients considering the long-term status of symptom control and the objectively measured physical activity. Persistent uncontrolled asthma was associated with sustained physical inactivity which appears to be an important contributor to the long-term adverse changes in body composition including the loss of muscle mass.
Furthermore, our data indicate that physical activity is a reliable long-term marker of asthma control. We found that persistent uncontrolled asthma patients had mean daily steps of (6614 and 6195) versus (8670 and 9058) in controlled asthma patients at baseline and follow-up, respectively. Interestingly, we observed a numeric decline of only 445 daily steps over two years in persistent uncontrolled asthma, however, with a sustained difference of at least 2000 daily steps between both groups. In line with the difference in step counts, the time in at least moderate activity remained constantly lower in patients with persistent uncontrolled asthma with a difference of 38 minutes at both baseline and follow-up. Therefore, our findings also indicate that the long-term changes of physical activity in persistent uncontrolled asthma are modest compared to the significant decline of physical activity in severe progressive COPD where we showed a decline of nearly 400 steps per year.Citation22 Accordingly, in contrast to severe COPD, persistent uncontrolled asthma is characterized by sustained low rather than continuous declining physical activity. Furthermore, our findings regarding the longitudinal association between asthma control and physical activity confirm previous comparable findings of cross-sectional studies from Holland, Australia and Brazil.Citation4,Citation5
The association of physical activity with asthma outcomes, ie symptom control and body composition, supports the potential role of physical activity in asthma management. In COPD, the benefits of physical activity on disease outcomes have been addressed adequately.Citation23,Citation24 In asthma however, corresponding data are scarce. Dogra and co-workers demonstrated improvements in symptom control and quality of life of 21 asthma patients who were enrolled in a 12-week supervised exercise intervention.Citation25 Indeed, further prospective studies with larger samples are needed to establish the role of physical activity in asthma outcome.
Interestingly, we also found that both high dose ICS and long-term OCS therapy are predictors of increased fat mass and low muscle mass in asthma. However, the impact of corticosteroid therapy as predictor of body composition was exchangeable with asthma control in multivariate regressions. Therefore, based on our multivariate regression analysis, it is hard to determine whether the impact of corticosteroid therapy on body composition is to explain by the effect of corticosteroid only or whether it is attributable to other factors associated with uncontrolled asthma. While systemic corticosteroids are known to cause muscle mass depletion and fat accumulation,Citation26,Citation27 less is known about the impact of higher doses of ICS on body composition. Nevertheless, it is unlikely that the longitudinal changes in body composition are caused by systemic corticosteroid therapy only, as we observed an overall decline in the proportion of patients who were on OCS from baseline (46%) to follow-up (27%).
Our study has limitations. First, we have no follow-up data from healthy controls which might have helped demonstrating the longitudinal changes in body composition in comparison to asthma patients. However, we were able to demonstrate long-term differences of body composition among asthma patients based on asthma severity and symptom control. Second, we used BIA to study muscle mass. BIA-measured muscle mass is dependent on the relations between body composition, body water and BMI. Despite of that, BIA is a simple, feasible tool that was found to be reasonably valid compared to other standardized methods used in the assessment of body composition such like the dual X-ray absorptiometry.Citation28 Finally, we did not asses the muscle function, which might have emphasized the impact of severe uncontrolled asthma on muscle dysfunction that known to be associated with muscle depletion in COPD.Citation26 However, we used the objectively measured physical activity to correlate skeletal muscle mass with the physical performance. Further, for all we know, this is the first study to demonstrate the longitudinal association between symptom control, physical activity and body composition.
In summary, persistent uncontrolled asthma is associated with adverse changes in the body composition. Direct measures of body composition appear to be more appropriate for studying the relationship between asthma and body composition rather than the crude BMI measure. Moreover, persistent uncontrolled asthma is closely associated with sustained physical inactivity. Physical activity appears to be an independent predictor of body composition and reliable long-term marker of symptom control.
Abbreviations
ACT, asthma control test; ACQ, asthma control questionnaire; BIA, bioelectrical impedance analysis; BMI, body mass index; SM, skeletal muscle mass; FM, fat mass.
Data Sharing Statement
The datasets used during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The study is a part from the prospective longitudinal All Age Asthma Cohort (ALLIANCE). The study was approved according with the Declaration of Helsinki by the ethics committee at Medical School Luebeck, Germany, and is registered at clinicaltrials.gov (pediatric arm: NCT02496468; adult arm: NCT02419274).
Acknowledgments
We thank Regine Wieland, Petra Hundack-Winter, Margret Gleiniger, Zaklina Hinz, Susann Prange, Corinna Derwort (LungClinic Grosshansdorf, Grosshansdorf, Germany) for their excellent support in study logistics.
Disclosure
Mustafa Abdo reports no conflict of interest. Frederik Trinkmann received travel support from Actelion, Berlin Chemie, Boehringer Ingelheim, Chiesi, Novartis, Mundipharma and TEVA as well as speaker or consultation fees from AstraZeneca, Berlin Chemie, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, GlaxoSmithKline, Novartis and Roche, Sanofi aventis, all outside the submitted work. Anne-Marie Kirsten, Heike biller, Frauke Pedersen and Christian Herzmann report no conflict of interest. Erika von Mutius reports personal fees from Pharmaventures, personal fees from OM Pharma S. A., personal fees from Springer-Verlag GmbH, personal fees from Elsevier GmbH and Elsevier Ltd., personal fees from Peptinnovate Ltd., personal fees from Turun Yliopisto, personal fees from Tampereen Yliopisto, personal fees from Helsingin Yliopisto, personal fees from European Respiratory Society, personal fees from Deutsche Pharmazeutische Gesellschaft e. V., personal fees from Massachusetts Medical Society, personal fees from Chinese University of Hongkong, personal fees from European Commission, personal fees from Böhringer Ingelheim International GmbH, personal fees from Universiteit Utrecht, Faculteit Diergeneeskunde, personal fees from Universität Salzburg, personal fees from Georg Thieme Verlag, personal fees from Japanese Society of Pediatric Allergy and Clinical Immunology (JSPACI), outside the submitted work; In addition, Dr. von Mutius has a patent LU101064 - Barn dust extract for the prevention and treatment of diseases pending, a patent EP2361632: Specific environmental bacteria for the protection from and/or the treatment of allergic, chronic inflammatory and/or autoimmune disorders with royalties paid to ProtectImmun GmbH, a patent EP 1411977: Composition containing bacterial antigens used for the prophylaxis and the treatment of allergic diseases licensed to ProtectImmun GmbH, a patent number EP1637147: Stable dust extract for allergy protection licensed to ProtectImmun GmbH, and a patent EP 1964570: Pharmaceutical compound to protect against allergies and inflammatory diseases licensed to ProtectImmun GmbH. Matthias V. Kopp, Gesine Hansen, Benjamin Waschki, Klaus F. Rabe and Henrik Watz reports no relevant conflict of interest. Thomas Bahmer reports grants from BMBF: Unrestricted research grant for the German Center for Lung Research (DZL), during the conduct of the study; personal fees from AstraZeneca, personal fees from GlaxoSmithKline, personal fees from Novartis, and personal fees from Roche, outside the submitted work. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2020. Available from: ginasthma.org. Accessed February 26, 2021.
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169–1179. doi:10.1016/j.jaci.2018.02.004
- van ‘T Hul AJ, Frouws S, van den Akker E. Decreased physical activity in adults with bronchial asthma. Respir Med. 2016;114:72–77. doi:10.1016/j.rmed.2016.03.016
- Freitas PD, Xavier RF, McDonald VM, et al. Identification of asthma phenotypes based on extrapulmonary treatable traits. Eur Respir J. 2020.
- Taube C, Bramlage P, Hofer A, Anderson D. Prevalence of oral corticosteroid use in the German severe asthma population. ERJ Open Res. 2019;5(4):00092–2019. doi:10.1183/23120541.00092-2019
- Zanella PB, Àvila CC, Chaves FC. et al. Phase angle evaluation of lung disease patients and its relationship with nutritional and functional parameters. J Am Coll Nutr. 2020. 1–6. doi:10.1080/07315724.2020.1801535
- Waschki B, Kirsten A, Holz O, Müller K-C, Schaper M. The role of sustained physical inactivity in the progression of exercise intolerance and muscle depletion in COPD. In: 1.2 Rehabilitation and Chronic Care. European Respiratory Society; 2015:PA3550.
- Morley JE, Abbatecola AM, Argiles JM, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12(6):403–409. doi:10.1016/j.jamda.2011.04.014
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: european consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
- Fuchs O, Bahmer T, Weckmann M, et al. The all age asthma cohort (ALLIANCE) - from early beginnings to chronic disease: a longitudinal cohort study. BMC Pulm Med. 2018;18(1):140. doi:10.1186/s12890-018-0705-6
- Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
- Kyle UG, Bosaeus I, De Lorenzo AD. Bioelectrical impedance analysis-part II: utilization in clinical practice. Clin Nutr. 2004;23(6):1430–1453. doi:10.1016/j.clnu.2004.09.012
- Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89(1):81–88. doi:10.1152/jappl.2000.89.1.81
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889–896. doi:10.1046/j.1532-5415.2002.50216.x
- Bahmer T, Waschki B, Schatz F, Herzmann C, Zabel P. Physical activity, airway resistance and small airway dysfunction in severe asthma. Eur Respir J. 2017;49(1):1601827. doi:10.1183/13993003.01827-2016
- Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–272. doi:10.1183/09031936.00024608
- Beuther DA, Weiss ST, Sutherland ER. Obesity and asthma. Am J Respir Crit Care Med. 2006;174(2):112–119. doi:10.1164/rccm.200602-231PP
- Dias-Júnior SA, Reis M, de Carvalho-pinto RM, Stelmach R, Halpern A, Cukier A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014;43(5):1368–1377. doi:10.1183/09031936.00053413
- Jagoe RT, Engelen MPKJ. Muscle wasting and changes in muscle protein metabolism in chronic obstructive pulmonary disease. Eur Respir J. 2003;46:52s–63s. doi:10.1183/09031936.03.00004608
- Marquis K, Debigaré R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. doi:10.1164/rccm.2107031
- Waschki B, Kirsten AM, Holz O, Mueller K-C, Schaper M. Disease progression and changes in physical activity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(3):295–306. doi:10.1164/rccm.201501-0081OC
- Watz H, Pitta F, Rochester CL, et al. An official European respiratory society statement on physical activity in COPD. Eur Respir J. 2014;44(6):1521–1537. doi:10.1183/09031936.00046814
- Spruit MA, Singh SJ, Garvey C, et al. An official American thoracic society/European respiratory society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188(8):e13–64.
- Dogra S, Kuk JL, Baker J, Jamnik V. Exercise is associated with improved asthma control in adults. Eur Respir J. 2011;37(2):318–323. doi:10.1183/09031936.00182209
- Maltais F, Decramer M, Casaburi R, et al. An official American thoracic society/European respiratory society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189(9):e15–62.
- Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity - definition, etiology and consequences. Curr Opin Clin Nutr Metab Care. 2008;11(6):693–700. doi:10.1097/MCO.0b013e328312c37d
- Jaffrin MY. Body composition determination by bioimpedance: an update. Curr Opin Clin Nutr Metab Care. 2009;12(5):482–486. doi:10.1097/MCO.0b013e32832da22c
