Abstract
The respiratory epithelium constitutes the physical barrier between the human body and the environment, thus providing functional and immunological protection. It is often exposed to allergens, microbial substances, pathogens, pollutants, and environmental toxins, which lead to dysregulation of the epithelial barrier and result in the chronic inflammation seen in allergic diseases and asthma. This epithelial barrier dysfunction results from the disturbed tight junction formation, which are multi-protein subunits that promote cell–cell adhesion and barrier integrity. The increasing interest and evidence of the role of impaired epithelial barrier function in allergy and asthma highlight the need for innovative approaches that can provide new knowledge in this area. Here, we review and discuss the current role and mechanism of epithelial barrier dysfunction in developing allergic diseases and the effect of current allergy therapies on epithelial barrier restoration.
Introduction
The human respiratory system consists of the nasal cavity, trachea, respiratory bronchioles, and distal alveoli, and it is linked together with the cardiovascular system to accomplish gas exchange.Citation1,Citation2 The integral component in maintaining this process is a continuous layer of epithelial cells, which has a central role in defending the lungs against inhaled environmental factors. Airway epithelial cells are continuously exposed to several environmental factors and allergens, which are then cleared by the immune system. Mucociliary clearance is mediated by the actions of diverse conducting airway and secretory cells, such as goblet cells, and the mucous and serous cells in the submucosal glands. They secrete fluids, electrolytes, antimicrobial and anti-inflammatory proteins, and mucus onto airway surfaces and therefore play a critical role in protecting the lungs during an acute injury.Citation3 The continuous exposure of bronchial epithelium to external and internal factors causes structural, protein and genetic changes, which contribute to the development of allergy and asthma. Several treatments for asthma and allergy symptoms are currently available for patients but there are also novel possible therapies and studies, that are aimed at improving the impaired epithelial barrier.
Structure of Bronchial Epithelial Cells
The airway epithelium is pseudostratified in the large airways and becomes columnar and cuboidal in the small airways. It consists of the predominant ciliated epithelial cells, mucous-secreting goblet cells, club cells, airway basal, suprabasal cells and rare cell types such as neuroendocrine cells, ionocytes, Hillock cells, and tuft cells (, ).Citation4–Citation6 Epithelial cells form a barrier between neighboring cells via junctional complexes which consist of apical tight junctions (TJs), adherens junctions (AJs), and desmosomes ().Citation7,Citation8 TJs form a border between the apical and basolateral plasma-membrane domains, which controls cell polarization, transcription, growth, and differentiation. They are critical regulators of paracellular permeability and limit the transport of macromolecules.Citation9–Citation11 Approximately 40 different proteins have been identified as TJ components, and these include the main transmembrane proteins belonging to the claudin family (26 members in humans and 27 in mice) and the three junctional MARVEL (MAL and related proteins for vesicle trafficking and membrane link) domain proteins: occludin, tricellulin and MARVELD3 that regulate the recruitment of signaling complex proteins to TJs.Citation8,Citation12–Citation14 Other transmembrane TJs include junctional adhesion molecules (JAMs), coxsackievirus and adenovirus receptor and angulins (also known as lipolysis-stimulated lipoprotein receptors).Citation8,Citation15,Citation16 The zonula occludens (ZO)-1, ZO-2, and ZO-3 cytoplasmic molecules bind directly to occludin and claudin on one end while also linking to actin fibers on the other end, which is essential for the epithelial barrier function. Several other proteins are located in the cytoplasm, such as multi-PDZ domain protein-1 (MUPP1), cell polarity molecules ASIP/PAR-3, PAR-6, PALS-1, and PALS-1-associated tight junction (PATJ); and non-PDZ proteins, cingulin, symplekin, ZONAB, GEF-H1, aPKC, PP2A, Rab3b, Rab13, PTEN, and 7H6.Citation17,Citation18 Multiple protein interactions couple the extra- and intracellular signaling that allows the complexity and plasticity of TJ function.Citation13,Citation19
Table 1 Types of Bronchial Epithelial Cells
Figure 1 Bronchial epithelial cells repertoire. Common cell types: basal cells, suprabasal cells, goblet cells, club cells (Clara cells) and ciliated cells. Rare cell types: neuroendocrine cells, ionocytes, Hillock cells and Tuft cells (brush cells). Created with affinity.serif.com.
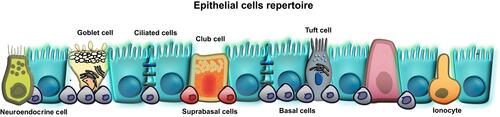
Figure 2 The junctional complex of bronchial epithelial cells. Tight junctions, adherens junction, gap junctions and desmosomes are intracellular junctions which regulate the transport of ions, water and macromolecules between tissue and lumen. TJs consist of claudins, occludin, tricellulin, and JAMs, located directly between neighboring bronchial epithelial cells. They directly interact with cytoplasmic TJs such as cingulin, MUPP1, MAGIs, non-PDZ proteins, and ZO-1, ZO-2, ZO-3 which bind directly to occludin and claudin on one end while also linking to actin fibers on the other end. Created with affinity.serif.com.
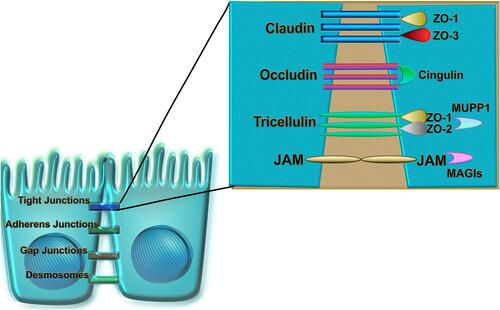
AJs are cadherin-catenin adhesion complexes located below TJs and have an important role in tissue homeostasis, stabilization, and transcriptional and intracellular signaling.Citation20 Cadherin adhesion molecules are core AJ components.Citation21 The cytoplasmic tail of classic cadherin binds to the catenins, which allows for links to cytoskeletal networks as well as to the exocytotic and endocytic machinery. Crosstalk between cadherin–catenin clusters and actin regulators controls AJ assembly from initial cell–cell contacts.Citation20 Gap junction proteins (GJs), connexins, which are expressed in different types of cells in the lung tissue, coordinate ciliary beat frequency, enable the direct flow of signaling molecules and metabolites between cells, and regulate inflammation.Citation22 Desmosomes are specialized adhesive protein complexes responsible for maintaining the mechanical integrity of tissues.Citation24 They may also act as signaling centers, regulating the availability of signaling molecules and participating in fundamental processes such as cell proliferation, differentiation, and morphogenesis.Citation25 Desmosome composition and size vary depending on tissue-specific expression and differentiation state. Their constituent proteins are highly regulated by post-translational modifications that control their function in the desmosome itself and regulate many desmosome-independent functions.Citation26
All these components of airway epithelium, besides their specific functions, closely interact with each other to form and maintain the epithelial cells’ polarity from the apical and basolateral sides.Citation19 It was shown that TJs proteins like ZO-1, which are distributed in AJs and GJs, interact with their proteins like E-cadherin (AJs) and certain connexins (GJs) Such interactions are important for transmitting signals between intracellular junctions and inner cells.Citation19,Citation27–Citation29 Furthermore, the association between a cadherin and plakoglobin, the only known component of desmosomes and AJs, is essential for desmosomes formations.Citation30 The flexibility of cadherin molecules was shown to have an impact on desmosomes plasticity on strong calcium-independent hyper adhesion in adult tissues and on weaker calcium-dependent adhesion in wounds.Citation31 The proper function and homeostasis of airway epithelial cells works through the cooperation of junctional complex molecules.
What Causes Epithelial Barrier Damage?
Airway epithelial cells are an essential part of the innate immune system in the lung. They are susceptible to damage due to exposure to allergens with complex proteolytic activity like house dust mite (HDM) (Der p 1, Der p 3, Der p 6, Der p 9), pollen (Ragweed pollen, Amb a, Birch pollen, Bet v), fungi (Aspergillus fumigatus and Aspergillus oryzae, Asp f 5, Asp f 6, Asp f 11), cockroaches (Bla g) and also animal dander and pathogens.Citation32–Citation34 Allergens with a protease activity were shown to injure the airway epithelial cells and help with initiation of allergen uptake by mucosal dendritic cells (DC) and antigen presentation with major histocompatibility class II to naïve T cells.Citation33,Citation35 In mice, the intraepithelial DC expressing TJs claudin-1, claudin-7 and ZO-2, and the interaction with E-cadherin expressed by epithelial cells is used to uptake allergens by dendritic extensions between epithelial cells.Citation32,Citation35 Infection of human bronchial epithelial cells (HBECs) with human rhinovirus increased their permeability and altered their TJs expression.Citation36 Whereas, respiratory syncytial virus infection in mice was seen only in lung parenchyma with decreased mRNA expression of claudin-1 and occludin observed in whole lungs. This observation was not seen in asthmatic HBECs.Citation37
Increasing numbers of diseases with epithelial barrier damage are caused by lifestyle changes due to urbanization and modernization, and as a result more environmental toxins such as air pollutants, cigarette smoke and ozone are released, which affect more than one billion people worldwide.Citation38–Citation45 The cadmium present in air pollutants and cigarette smoke disrupts epithelial integrity in in vitro human air-liquid interface (ALI) cultures through both occludin hyperphosphorylation via kinase activation and by direct disruption of the junction-interacting complex.Citation46 Recent studies have also revealed that nanoparticles, macroparticles, and toxins contained in laundry, dishwashing, and household cleaning agents can cause epithelial barrier disturbance in human keratinocytes and human bronchial epithelial cells.Citation47–Citation50 Disturbance to the homeostatic balance in the epithelium including loss of differentiation, impairment of junctional complexes or insufficient innate immune response define the epithelial barrier dysfunction.Citation51 The disruption of the basic functions of the epithelium manifested in inability to rebuild causes the penetration of inflammatory cells.Citation5 This leads to chronic inflammatory airway diseases like asthma and chronic rhinosinusitis, which are heterogeneous diseases with complex etiology.Citation52 It was shown that the airway epithelium in asthmatic patients and in vitro ALI cultures is less differentiated, has elevated numbers of basal cells, and has increased phosphorylation of p38 mitogen-activated protein kinase.Citation53 The epithelial permeability was higher in asthma, specifically severe asthma, compared to mild asthma, and in biopsy specimens from patients with chronic rhinosinusitis with nasal polyps.Citation53–Citation57 This decreased integrity of epithelial barrier was associated with decreased expression of the TJs molecules occludin and ZO-1. In in vitro study of asthma and HDM-induced allergic rhinitis, claudin-18 was shown in epithelial brushings in asthma patients and healthy controls. AJ like E-cadherin and β-catenin in patients with atopic asthma, were also shown to contribute to the disease development.Citation56,Citation58–Citation61 E-cadherin plays an important role in the epithelial-to-mesenchymal transition, a cellular process where epithelial cells acquire mesenchymal phenotypes and behavior following the downregulation of epithelial features. The epithelial cells lose their cell polarity and cell–cell adhesion, then display fibroblast-like morphology and cytoarchitecture, and gain migratory and invasive properties.Citation62,Citation63 The Wnt/β-catenin pathway was shown to be involved in the remodeling process of fibrosis and allergic inflammation in a genetically modified mouse model.Citation64 Blocking of β-catenin pathway could be a promising therapeutic target in asthma because it can reduce allergic airway inflammation in mouse models.Citation65,Citation66
Disruption of the complex lung epithelium structure by the external components of the environment initiates the immune response, which could enhance the disease development and lead to a chronic stage.
Epithelial Cell Response to Danger
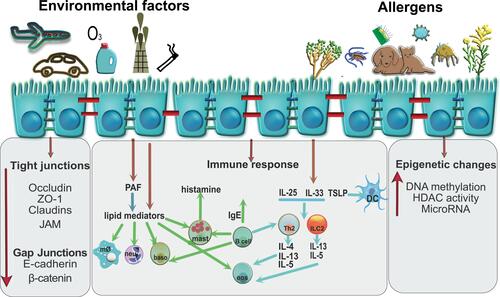
The response of bronchial epithelium to danger is manifested by elevated serum IgE, increased smooth muscle mass, subepithelial fibrosis, epithelial desquamation, eosinophilic airway inflammation, and goblet cell hyperplasia.Citation67 The airway epithelium acts as a chemical barrier against environmental insults by secreting, for example, antimicrobial peptides, anti-proteases, and antioxidants.Citation52 The epithelial cells recognize pathogen-associated molecular patterns on inhaled microbes, parasites, and allergens as well as alarmins/damage-associated molecular patterns released from dying or damaged cells by expressing pattern recognition receptors like toll-like receptors, retinoic acid-inducible gene like receptors, nucleotide-binding oligomerization domain like receptors, C-type lectin receptors, protease activated receptor 2 and purinergic receptors.Citation68,Citation69 Upon activation, epithelial cells produce and release chemokines, growth factors, lipid mediators, pro-inflammatory cytokines such as interleukin (IL)-6, IL-8, IL-25, IL-33, CCL20, CCL17, thymic stromal lymphopoietin (TSLP), and granulocyte-macrophage colony-stimulating factor (GM-CSF) which then attract and activate cells from the innate and adaptive immune system ().Citation70,Citation71 It has been shown that epithelial expression of the neutrophil chemoattractant IL‐8 and macrophage inflammatory protein 1 alpha is increased in the biopsies from severe asthma patients. Their presence correlates with increased epidermal growth factor receptor (EGFR) expression as a marker of epithelial damage.Citation72 Human and mouse studies have revealed another molecule secreted by the respiratory epithelial cells which is nitric oxide that plays a role in ion transport, modulation of inflammation, and wound repair processes after injury.Citation73–Citation75 Impairment of epithelial barrier induces deposition of extracellular matrix components and release of vascular endothelial growth factor (VEGF), which cause an increase in the size of airway wall vessels and promotes angiogenesis.Citation76,Citation77 The airway epithelium maintains an active physical and functional barrier, and responds to the danger with secretion of cytokines, chemokines and mediators therefore activating innate and adaptive immune cells.
Figure 3 Mechanisms involved in a bronchial epithelial cell response to environmental factors and allergens. Airway epithelial cells are susceptible to damage as a result of exposure to allergens (house dust mite, pollen, and animal dander), pathogens (viruses, bacteria), and environmental toxins (air pollutants, cigarette smoke, ozone, detergents). Disruption of bronchial epithelium, indicated by red cell junctions, decreases the barrier integrity as evidenced by lower expression of TJs (occludin, ZO-1, E-cadherin, β-catenin, JAM and EGFR). Consequently, epithelial cells respond by secretion of cytokines IL-25, IL-33, and TSLP, which then attract other inflammatory cells like Th2 (IL-4, IL-5, IL-13), ILC2 (IL-13, IL-5), B cells, and dendritic cells (DC). Additional manifestations of respiratory disease occur in response to lipid mediators. Epithelial cells can also produce PAF and eicosanoids which have been shown to be chemotactic for neutrophils (neu), basophils (baso) and macrophages (mØ), activate eosinophils (eos) and macrophages, and alter vascular and epithelial permeability. Chronic inflammation also causes epigenetic changes in the bronchial epithelial cells by increasing DNA methylation and activating HDACs. Created with affinity.serif.com.
The Influence of Cytokines on Epithelial Barrier Disorders
The main players driving the allergic disease pathology are T helper 2 (Th2) cells and their cytokines IL-4, IL-5 and IL-13.Citation78 During allergic airway inflammation, Wu et al observed elevated levels of IL-5 in mice in bronchial epithelial cells, which can impact the microenvironment of the lung by modifying pathologic and protective immune responses in the airways.Citation79 We have shown that Th2 cell numbers and the level of their cytokines, IL-4 and IL-13, decreased barrier integrity in ALI cultures of HBECs from control subjects. The HBECs from asthmatic patients had an initial low trans-epithelial resistance and reduced expression of ZO-1 and occludin, and the treatment with Th2 cells and cytokines IL-4 and IL-13 did not show any further changes. These cytokines induced a physical separation of the TJs of adjacent cells as seen in the immunofluorescence staining of the TJ molecules occludin and ZO-1.Citation55 Th2 cells and their cytokines (IL-4, IL-5, IL-13, IL-9) are necessary to initiate and propagate the inflammation associated with allergy. They induce class switching of B-cells to produce allergen-specific IgE, recruit mast cells (IL-9) and eosinophils (IL-5) to sites of allergic inflammation and induce goblet cell metaplasia (IL-4, IL-13).Citation80,Citation81 Type 2 innate lymphoid cells (ILC2) through IL-13 were also linked to asthma pathogenesis by reducing human and mice epithelial barrier integrity.Citation82 Similar results were observed in the analysis of TJs in bronchial biopsies from asthmatic subjects and in vitro cultures.Citation56 Mouse studies demonstrated decreased expression of ZO-1, ZO-2, occludin, and claudin-5-8-18 and −23 in three chronic HDM models of eosinophilic, neutrophilic and mixed granulocyte asthma.Citation83 In addition, prolonged interferon (IFN) production impairs lung epithelial regeneration during influenza recovery in mice.Citation84 IFNγ and tumor necrosis factor alpha (TNFα) synergistically or singly disrupt barrier function in ALI cultures associated with reduced ZO-1 and JAM expression.Citation54,Citation85,Citation86 Zabner et al showed that histamine, which is a crucial agonist released during the immediate response to an inhaled allergen, increases paracellular airway permeability and increases the susceptibility of airway epithelial cells to infection by adenovirus by interrupting E-cadherin adhesion ().Citation87,Citation88 During the acute inflammatory response to pathogens or tissue injury, respiratory epithelium produces and releases eicosanoids together with cytokines and chemokines, and mediators such as histamine ().Citation89 They induce the recruitment of neutrophils and other immune cells into the tissue to engulf and kill invading pathogens. The two classes of eicosanoids, leukotrienes and prostaglandins, were shown to be increased in the airways of asthmatic patients and could be involved in asthma pathogenesis.Citation90 They are metabolites of the cyclooxygenase (COX) and 5-lipoxygenase (5-LOX) pathways. Several studies have reported increased levels of COX pathway products, prostaglandin D (PGD2), prostaglandin F2 alpha and thromboxane B2 in the bronchoalveolar lavage fluid of allergic asthmatic.Citation91,Citation92 Specifically, PGD2 activities might contribute to asthma pathogenesis by vasodilation, increased capillary permeability, mucus production by lung epithelial cells, bronchoconstriction, and eosinophil recruitment.Citation93 PGD2 has also been implicated in the trafficking of T cells in allergic inflammation.Citation94 In addition, leukotriene B4 (LTB4) and the cysteinyl leukotrienes (Cys-LTs), products of the 5-LOX pathway, have been shown to be higher in exhaled breath condensate from asthmatic patients.Citation95 Cys-LTs were also reported to be higher in the induced sputum of asthmatic patients and their level correlated with disease severity.Citation96 LTB4 has also been shown to induce chemotaxis of effector T cells to the airways of mice immediately after exposure to an allergen.Citation97,Citation98 In addition to eicosanoids, platelet- activating factor (PAF), a phospholipid mediator, is prevalent in asthmatic airways. It is produced by various cells, including neutrophils, eosinophils, mast cells, fibroblasts, epithelial cells, and endothelial cells.Citation99 In the airways, PAF acts as a potent chemoattractant for neutrophils and eosinophils, promotes vascular permeability and edema and causes bronchoconstriction via acting on airway smooth muscle.Citation100
During the resolution phase in the lungs, specialized pro-resolving mediators (SPMs) are produced by leukocytes, platelets, bronchial epithelial cells, alveolar epithelial type II cells as well as alveolar macrophages.Citation101 Their actions include participation in epithelial cell restoring, inhibition neutrophils’ influx and activation, efferocytosis and phagocytosis of microorganisms, allergens and debris by macrophages as well as lymphocyte differentiation to effector cells that produce healing cytokines such as TGFβ.Citation102,Citation103
Severe asthma is resistant to current therapies and is marked by decreased lipoxin production in the airways due to the aberrant metabolism of AA.Citation104–Citation106 Reduced levels of lipoxins, specifically lipoxin A4 (LXA4), have been linked to more severe airway inflammation and a higher degree of airway obstruction.Citation106 In contrast, LXA4 inhalation by asthmatic patients has been shown to affect airway response by attenuating the leukotriene C4-triggered airway obstruction and improved lung function in asthmatic children.Citation107,Citation108 Infiltrating eosinophils during asthma pathogenesis are producing not only pro-inflammatory cytokines like IFNγCitation109 but also LXA4 which has been shown to suppress chemotaxis towards chemoattractants and inhibit the GM-CSF triggered secretion of IL-13 and eotaxin in vitro.Citation110 Eosinophils from severe asthmatic patients expressed lower levels of ALX the receptor for LXA4, compared to healthy humans.Citation111 Activation of ALX by LXA4 has protective benefits in the lung airway by promoting the proliferation and wound repair of human airway epithelial cells.Citation112 The activation of this receptor on natural killer cells from asthma patients also increased their triggered apoptosis of eosinophils.Citation113 Various studies reported the beneficial and pro-resolution effects of resolvin E1 in murine models of allergic airway inflammation, where it was shown to decrease eosinophil influx, airway hyperresponsiveness, and the secretion of IL-23, IL-17 and IL-6 in the lung while also increasing the production of LXA4.Citation114,Citation115 Resolvin D1 has also been shown to decrease allergic lung inflammation by stimulating the macrophages clearance of allergens.Citation116 Protectin D1 treatment after an allergen challenge in mouse lungs was associated with a faster resolution of airway inflammation.Citation117 Classically regarded as a pro-inflammatory mediator, PGE2 (prostaglandin E2) can promote resolution.Citation118 PGE2 by inhibiting the proliferation, activation, and secretion of cytokines by ILC2.Citation119 Studies in asthmatic patients reported an inverse correlation between the sputum levels of PGE2 and eosinophil numbers, thus suggesting it can play a role in reducing airway eosinophilia.Citation96,Citation120 Additionally, inhaled PGE2 was shown to have antiallergic effects by reducing the early and late bronchoconstrictor response to an allergen in asthmatic individuals.Citation121 PGI2 (prostacyclin) has been mostly studied in mice models of asthma where it was involved in decreasing allergic inflammation by signaling through its receptor IP, as well as reducing lung fibrosis and remodelling.Citation122–Citation124
Systemic inflammation coordinated by a large number of factors such as cytokines, chemokines and mediators produced by immune cells, interact with each other and consequently cause changes in the bronchial epithelium. Long-term changes in the TJs protein expression, which are important for the maintenance and proper function of epithelial cells, can have an impact on the chronic stage of diseases.
Genetic and Epigenetic Changes in Bronchial Epithelium
Under the influence of external factors and immune cell responses, bronchial epithelial cells undergo many changes in their DNA structure and post-translational genetic modifications. Several epithelial-derived genes have been identified in genome-wide association studies, such as metalloprotease 33 (ADAM33)Citation125 and protocadherin-1 (PCDH1),Citation126,Citation127 which are associated with epithelial barrier function, differentiation, and homeostasis. Cadherin-related family member 3 (CDHR3) as a receptor for rhinovirus C was associated with childhood asthma with severe exacerbations.Citation128,Citation129 β2-adrenergic receptor haplotype pair (2/4) was shown to be associated with severe asthma,Citation130 while serine peptidase inhibitor, Kazal type 5 (SPINK5), and TSLP were associated with childhood asthma.Citation131 A large study involving more than a hundred centers worldwide identified genes associated with asthma on chromosomes 2 (IL1RL1/IL18R1), 6 (HLA-DQ), 9 (IL33), 15 (SMAD3), 17 (ORMDL3/GSDMB), and 22 (IL2RB).Citation132 IL1RL1 encodes the ST2 receptor (ST2L) for IL-33, which promotes type 2 inflammation in some asthma patients. Soluble isoform, IL-1RL1-a or sST2, acts as a decoy receptor by sequestering IL-33, thereby inhibiting IL1RL1-b/IL-33 signaling, which could be used as a biomarker or target for pharmacological intervention.Citation133,Citation134 Orosomucoid- like 3 (ORMDL3), was shown to play an important role in regulating epithelial barrier function in allergic asthma,Citation135–Citation137 rhinovirus infectionCitation138,Citation139 and by inducing the p-ERK/MMP-9 pathway to promote pathological airway remodeling in patients with asthma.Citation140 SMAD3 is an essential signal transducer in TGF-β signaling, which is elevated in airway epithelial cells of some asthmaticsCitation141,Citation142 and is involved in the response of bronchial epithelial cells to viral infection.Citation143,Citation144 Deletion of P2Y13 in human airway epithelial cells and in a mouse model protects against asthma exacerbations.Citation145 A study in Der f 1 stimulated peripheral blood mononuclear cells from dust mite sensitized patients showed upregulation of IL9, IL5, and proteoglycan 2 (PRG2) expression with evidence for an interaction of IL9 polymorphisms with dust mite in childhood asthma.Citation146
Changes in bronchial epithelial cells also lead to epigenetic changes like DNA methylation, histone modification, and microRNA modifications, defined as heritable changes in gene activity without an alteration in the DNA sequence.Citation147–Citation149 Recent studies in epigenome-wide association studies have shown an association between epigenetic signatures and allergic diseases, including pediatric asthma.Citation150–Citation152 We showed a higher methylation level in bronchial epithelial cells from asthma donors following the changes in genes associated with cell growth, ion transport, and cytoskeletal remodeling. Additionally, higher methylation was observed in genes involved in the regulation of bronchial barrier integrity, eg, TJ family members: AMOTL1, CLDN11, CLDN18, MAGI1, TJP2, JAM3, actin protein: ACTB, a component of the cytoskeleton: TUBA1C, ROCK2, LLGL1. Interestingly ten-eleven translocation enzyme (TET1), which can reverse CpG methylation, was methylated in asthmatic HBEC.Citation153 Vermeulen et al reported differentially methylated regions between persistent asthma, remission, and healthy controls associated with ciliated epithelium genes.Citation154 Also, short-term exposure of bronchial epithelial cells to diesel exhaust, a significant contributor to air pollution, alters DNA methylation and could be implicated in pulmonary pathologies.Citation155 DNA-methyltransferases (Dnmts) play a crucial role in the methylation process. Qin et al showed that the bronchial epithelial Dnmt3b impairs the host defense during Pseudomonas-induced pneumonia, at least in part, by dampening the mucosal responses to flagellin.Citation156 Additionally, Dnmt1 deficiency disrupts epithelial-mesenchymal crosstalk and leads to an early-branching defect. It also causes a loss of epithelial polarity and proximal endodermal cell differentiation.Citation157 We showed that the inhibition of Dnmts restores leakiness in the bronchial epithelium in asthma.Citation153 Bronchial epithelium can also be influenced by histone acetyltransferases (HATs) and histone deacetylases (HDACs), that antagonistically control the overall balance of post-translational modification of DNA core histone proteins (). They play a crucial role in cell signaling, cell cycle control, and epigenetic gene transcription regulation. HDAC inhibitors can inhibit these enzymes, resulting in the increased acetylation of histones, thereby affecting gene expression.Citation158 The HDAC family consists of 11 members of HDACs and 7 silent information regulator genes. We have shown that human bronchial epithelial cells from asthma patients showed higher HDAC activity with higher expression of HDAC1 and HDAC9. Most HDACs were significantly upregulated in control subjects and asthmatic patients upon IL-4 and IL-13 stimulation.Citation55 Similarly, Steelant et alCitation159 observed increased HDACs activity in allergic rhinitis patients with high expression of HDAC5 and HDAC11 and decreased HDAC2 was reported in patients with chronic obstructive pulmonary disease.Citation160 In a mouse model of ovalbumin (OVA)-induced asthma, HDAC4 was upregulated in the lung tissue.Citation161 We and others have also shown that inhibition of endogenous HDAC activity reconstitutes the defective barrier by increasing TJ expression.Citation55,Citation159,Citation161–Citation163 Genetic and epigenetic changes in the bronchial epithelial cells are an important part of the complex changes observed upon epithelium injury and therefore could be a possible approach to improving the epithelial barrier.
The Effect of Treatment of Asthma and Allergy on Epithelial Barrier and Airway Remodeling
Epithelial barrier disruption, as a feature of airway remodeling which represents structural changes in bronchial wall encompassing wall thickening, basal membrane thickening, overgrowth of smooth muscle cell layer and enhanced angiogenesis, is observed in asthma patients. The effect of long-term asthma treatment on epithelial barrier and airway remodeling has been intensively studied (). Inhaled corticosteroids and β2-adrenoreceptor agonists are the first-line medications used in asthma treatment and are effective in most patients. Several data indicate that glucocorticoids (GCs) inhalation therapy, including budesonide, can improve epithelial barrier integrity and might contribute to the therapeutic effects of GCs for treating asthmaCitation164,Citation165 or chronic rhinosinusitis with nasal polyps.Citation166 Similarly, other GCs, including mometasone and fluticasone, were shown to be effective in restoring nasal epithelial barrier dysfunction in allergic rhinitis.Citation167 In animal models, budesonide was also proved to inhibit airway remodeling in the early stage of allergen-induced airway hyperresponsiveness (AHR), however it did not reverse established AHR.Citation168,Citation169 Interestingly, neither formoterol nor Montelukast, were shown to promote barrier integrity,Citation165 suggesting that β2-adrenoreceptor agonists and anti-leukotrienes themselves might not have any positive effect on epithelial barrier restoration. In severe asthma patients, the long-term oral corticosteroid (CS) therapy is associated with serious side effects.Citation170 Therefore, currently, several biologicals are used in severe asthma treatment as an alternative for systemic CS. Currently, they encompass omalizumab (anti-IgE), mepolizumab (anti-IL-5), benralizumab (anti-IL5R), dupilumab (anti-IL4/13R) and reslizumab (anti-IL-5) approved by the Food and Drug Administration.Citation171 The question is posed whether they may affect epithelial barrier disruption and related bronchial remodeling in asthma patients. As omalizumab has been used for more than 15 years, there are some data indicating that it may decrease unfavorable structural airway changes in allergic asthmatics, with respect to the fibronectin deposit, the increased thickness of the basal lamina and the bronchial wall thickness.Citation172–Citation174 Treatment with mepolizumab significantly reduced the expression of three extracellular matrix proteins: tenascin, lumican and procollagen III in the reticular basement membrane.Citation171 Benralizumab caused the consequent 29% relative reduction of airways smooth muscle mass and number of tissues myofibroblasts.Citation175 As IL-13 and IL-4 partly share the same receptor and signaling pathways and both are deeply involved in mucus secretion and airways remodeling dupilumab might exert a positive effect on airway remodelling.Citation176 Additionally, Anti-VEGF and TNF inhibition therapy was shown to be an effective treatment for remodeling in asthma with the significant restoration of the epithelial barrier.Citation177
Table 2 Summary of Current and Novel Biological Therapies to Treat Asthma and Allergic Diseases
Allergen-specific immunotherapy (AIT) represents the only curative treatment in which an allergic patient is incrementally exposed to increasing quantities of a specific antigen, such as pollen, fungi, HDM, or food allergens.Citation152 Successful AIT induces the reinstatement of tolerance toward allergens and represents a disease-modifying treatment.Citation178 Long-term efficacy with allergen immunotherapy is associated with decreases in IgE-dependent activation of mast cells, tissue eosinophilia, regulatory T cells induction and local and systemic IgG, IgG4, and IgA antibodies.Citation179,Citation180 In the mouse model of allergen specific immunotherapy (SIT), the restoration of the airway epithelial integrity was observed. Additionally, the use of 4-PBA, an inhibitor of endoplasmic reticulum (ER) stress, suppressed IL-25 induced airway epithelial ER stress and apoptosis triggered by Dermatophagoides farinae (Der f).Citation181 SIT has also been shown to affect HDM‐induced activation of lung structural cells including airway epithelium.Citation182 Sublingual Immunotherapy was also shown to have a beneficial impact on airway wall thickness and remodeling in allergic asthma.Citation183
Novel anti-inflammatory mediator, secretoglobin1A1, was shown as a long-term allergen-specific therapeutic intervention that can suppress pro-inflammatory epithelial gene expression.Citation184 Several recent studies have studied novel molecules which could be used as a new potential treatment for allergy and asthma. We have shown that the oral gavage of polyamines spermine or spermidine can modulate HDM-induced cell infiltration, cytokine secretion, and epithelial cell tight junction expression in murine models.Citation185 Additionally, a redox-sensitive transcription factor Nuclear erythroid 2-related factor 2 (Nrf2), a key regulator of oxidative and environmental stress, enhanced epithelial barrier function and increased localization of ZO-1 to the cell surface.Citation186 Furthermore, a study using the cannabinoid WIN55212‐2 illustrated an essential role of this chemical in restoring airway epithelial barrier during rhinovirus infection and in suppressing T cell-mediated inflammation in human tonsil cells.Citation187
Conclusion
The influence of external factors and immune cell responses, cytokines and mediators associated with allergic airway inflammation can disrupt the epithelial barrier by interfering with junctional complex assembly. Understanding all the changes occurring in the bronchial epithelium during injury is very important for developing future possible treatments for asthma and allergy diseases. It is still unclear whether, the increased airway epithelial permeability that enables transport of allergens, pathogens and other damaging factors is constant and predisposes to disease development. However, the changes in structure and function in bronchial epithelium asthma and allergic diseases are well documented and there are important indications that restoring the epithelial barrier could be a potential target for new treatments. Nevertheless, data on the effects of particular biological therapies on epithelial barrier and airway remodeling in allergy and asthma are currently incomplete and thus require further studies.
Disclosure
The authors report no conflicts of interest in this work.
References
- Davis JD, Wypych TP. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol. 2021;14(5):978–990. doi:10.1038/s41385-020-00370-7
- Basil MC, Katzen J, Engler AE, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26(4):482–502. doi:10.1016/j.stem.2020.03.009
- Whitsett JA. Airway epithelial differentiation and mucociliary clearance. Ann Am Thorac Soc. 2018;15(Suppl 3):S143–S148. doi:10.1513/AnnalsATS.201802-128AW
- Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5(7):772–777. doi:10.1513/pats.200805-041HR
- Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. 2020;145(6):1499–1509. doi:10.1016/j.jaci.2020.04.010
- Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8(4):432–446. doi:10.1046/j.1440-1843.2003.00493.x
- Cereijido M, Contreras RG, Shoshani L. Cell adhesion, polarity, and epithelia in the Dawn of metazoans. Physiol Rev. 2004;84(4):1229–1262. doi:10.1152/physrev.00001.2004
- Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):564–580. doi:10.1038/nrm.2016.80
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73:283–309. doi:10.1146/annurev-physiol-012110-142150
- Steelant B, Seys SF, Boeckxstaens G, Akdis CA, Ceuppens JL, Hellings PW. Restoring airway epithelial barrier dysfunction: a new therapeutic challenge in allergic airway disease. Rhinology. 2016;54(3):195–205. doi:10.4193/Rhino15.376
- Otani T, Furuse M. Tight junction structure and function revisited: (trends in cell biology 30, 805–817, 2020). Trends Cell Biol. 2020;30(12):1014. doi:10.1016/j.tcb.2020.10.001
- Raleigh DR, Marchiando AM, Zhang Y, et al. Tight junction-associated MARVEL proteins marveld3, tricellulin, and occludin have distinct but overlapping functions. Mol Biol Cell. 2010;21(7):1200–1213. doi:10.1091/mbc.e09-08-0734
- Vermette D, Hu P, Canarie MF, Funaro M, Glover J, Pierce RW. Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Med Exp. 2018;6(1):37. doi:10.1186/s40635-018-0203-4
- Kast JI, Wanke K, Soyka MB, et al. The broad spectrum of interepithelial junctions in skin and lung. J Allergy Clin Immunol. 2012;130(2):544–547 e544. doi:10.1016/j.jaci.2012.04.044
- Luissint AC, Nusrat A, Parkos CA. JAM-related proteins in mucosal homeostasis and inflammation. Semin Immunopathol. 2014;36(2):211–226. doi:10.1007/s00281-014-0421-0
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci U S A. 2001;98(26):15191–15196. doi:10.1073/pnas.261452898
- Yuksel H, Turkeli A. Airway epithelial barrier dysfunction in the pathogenesis and prognosis of respiratory tract diseases in childhood and adulthood. Tissue Barriers. 2017;5(4):e1367458. doi:10.1080/21688370.2017.1367458
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2(4):285–293. doi:10.1038/35067088
- Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4(3):225–236. doi:10.1038/nrm1055
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11(7):502–514. doi:10.1038/nrm2927
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251(5000):1451–1455. doi:10.1126/science.2006419
- Koval M. Sharing signals: connecting lung epithelial cells with gap junction channels. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L875–L893. doi:10.1152/ajplung.00078.2002
- Johnson LN, Koval M. Cross-talk between pulmonary injury, oxidant stress, and gap junctional communication. Antioxid Redox Signal. 2009;11(2):355–367. doi:10.1089/ars.2008.2183
- Dubash AD, Green KJ. Desmosomes. Curr Biol. 2011;21(14):R529–531. doi:10.1016/j.cub.2011.04.035
- Garrod D, Chidgey M. Desmosome structure, composition and function. Biochim Biophys Acta. 2008;1778(3):572–587. doi:10.1016/j.bbamem.2007.07.014
- Muller L, Hatzfeld M, Keil R. Desmosomes as signaling hubs in the regulation of cell behavior. Front Cell Dev Biol. 2021;9:745670. doi:10.3389/fcell.2021.745670
- Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132(3):451–463. doi:10.1083/jcb.132.3.451
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138(1):181–192. doi:10.1083/jcb.138.1.181
- Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem. 2000;275(13):9492–9500. doi:10.1074/jbc.275.13.9492
- Lewis JE, Wahl JK 3rd, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997;136(4):919–934. doi:10.1083/jcb.136.4.919
- Tariq H, Bella J, Jowitt TA, et al. Cadherin flexibility provides a key difference between desmosomes and adherens junctions. Proc Natl Acad Sci U S A. 2015;112(17):5395–5400. doi:10.1073/pnas.1420508112
- Herbert CA, King CM, Ring PC, et al. Augmentation of permeability in the bronchial epithelium by the house dust mite allergen Der p1. Am J Respir Cell Mol Biol. 1995;12(4):369–378. doi:10.1165/ajrcmb.12.4.7695916
- Matsumura Y. Role of allergen source-derived proteases in sensitization via airway epithelial cells. J Allergy. 2012;2012:903659. doi:10.1155/2012/903659
- Wan H, Winton HL, Soeller C, et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J Clin Invest. 1999;104(1):123–133. doi:10.1172/JCI5844
- Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8(3):193–204. doi:10.1038/nri2275
- Looi K, Troy NM, Garratt LW, et al. Effect of human rhinovirus infection on airway epithelium tight junction protein disassembly and transepithelial permeability. Exp Lung Res. 2016;42(7):380–395. doi:10.1080/01902148.2016.1235237
- Kast JI, McFarlane AJ, Globinska A, et al. Respiratory syncytial virus infection influences tight junction integrity. Clin Exp Immunol. 2017;190(3):351–359. doi:10.1111/cei.13042
- Akdis CA. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat Rev Immunol. 2021;21(11):739–751. doi:10.1038/s41577-021-00538-7
- Heijink IH, Brandenburg SM, Postma DS, van Oosterhout AJ. Cigarette smoke impairs airway epithelial barrier function and cell-cell contact recovery. Eur Respir J. 2012;39(2):419–428. doi:10.1183/09031936.00193810
- Tamashiro E, Cohen NA, Palmer JN, Lima WT. Effects of cigarette smoking on the respiratory epithelium and its role in the pathogenesis of chronic rhinosinusitis. Braz J Otorhinolaryngol. 2009;75(6):903–907. doi:10.1016/s1808-8694(15)30557-7
- Schamberger AC, Staab-Weijnitz CA, Mise-Racek N, Eickelberg O. Cigarette smoke alters primary human bronchial epithelial cell differentiation at the air-liquid interface. Sci Rep. 2015;5:8163. doi:10.1038/srep08163
- Sokolowska M, Quesniaux VFJ, Akdis CA, Chung KF, Ryffel B, Togbe D. Acute respiratory barrier disruption by ozone exposure in mice. Front Immunol. 2019;10:2169. doi:10.3389/fimmu.2019.02169
- Bromberg PA, Ranga V, Stutts MJ. Effects of ozone on airway epithelial permeability and ion transport. Res Rep Health Eff Inst. 1991;1991(48):1–22;discussion 23–32.
- Schamberger AC, Mise N, Jia J, et al. Cigarette smoke-induced disruption of bronchial epithelial tight junctions is prevented by transforming growth factor-beta. Am J Respir Cell Mol Biol. 2014;50(6):1040–1052. doi:10.1165/rcmb.2013-0090OC
- Yamamoto N, Kan OK, Tatsuta M, et al. Incense smoke-induced oxidative stress disrupts tight junctions and bronchial epithelial barrier integrity and induces airway hyperresponsiveness in mouse lungs. Sci Rep. 2021;11(1):7222. doi:10.1038/s41598-021-86745-7
- Cao X, Lin H, Muskhelishvili L, Latendresse J, Richter P, Heflich RH. Tight junction disruption by cadmium in an in vitro human airway tissue model. Respir Res. 2015;16:30. doi:10.1186/s12931-015-0191-9
- Cullinan P, Harris JM, Newman Taylor AJ, et al. An outbreak of asthma in a modern detergent factory. Lancet. 2000;356(9245):1899–1900. doi:10.1016/S0140-6736(00)03264-5
- Hole AM, Draper A, Jolliffe G, Cullinan P, Jones M, Taylor AJ. Occupational asthma caused by bacillary amylase used in the detergent industry. Occup Environ Med. 2000;57(12):840–842. doi:10.1136/oem.57.12.840
- Xian M, Wawrzyniak P, Ruckert B, et al. Anionic surfactants and commercial detergents decrease tight junction barrier integrity in human keratinocytes. J Allergy Clin Immunol. 2016;138(3):890–893 e899. doi:10.1016/j.jaci.2016.07.003
- Wang M, Tan G, Eljaszewicz A, et al. Laundry detergents and detergent residue after rinsing directly disrupt tight junction barrier integrity in human bronchial epithelial cells. J Allergy Clin Immunol. 2019;143(5):1892–1903. doi:10.1016/j.jaci.2018.11.016
- Schleimer RP, Berdnikovs S. Etiology of epithelial barrier dysfunction in patients with type 2 inflammatory diseases. J Allergy Clin Immunol. 2017;139(6):1752–1761. doi:10.1016/j.jaci.2017.04.010
- Heijink IH, Kuchibhotla VNS, Roffel MP, et al. Epithelial cell dysfunction, a major driver of asthma development. Allergy. 2020;75(8):1902–1917. doi:10.1111/all.14421
- Hackett TL, Singhera GK, Shaheen F, et al. Intrinsic phenotypic differences of asthmatic epithelium and its inflammatory responses to respiratory syncytial virus and air pollution. Am J Respir Cell Mol Biol. 2011;45(5):1090–1100. doi:10.1165/rcmb.2011-0031OC
- Soyka MB, Wawrzyniak P, Eiwegger T, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012;130(5):1087–1096 e1010. doi:10.1016/j.jaci.2012.05.052
- Wawrzyniak P, Wawrzyniak M, Wanke K, et al. Regulation of bronchial epithelial barrier integrity by type 2 cytokines and histone deacetylases in asthmatic patients. J Allergy Clin Immunol. 2017;139(1):93–103. doi:10.1016/j.jaci.2016.03.050
- Xiao C, Puddicombe SM, Field S, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011;128(3):549–556 e541–512. doi:10.1016/j.jaci.2011.05.038
- Blume C, Swindle EJ, Dennison P, et al. Barrier responses of human bronchial epithelial cells to grass pollen exposure. Eur Respir J. 2013;42(1):87–97. doi:10.1183/09031936.00075612
- de Boer WI, Sharma HS, Baelemans SM, Hoogsteden HC, Lambrecht BN, Braunstahl GJ. Altered expression of epithelial junctional proteins in atopic asthma: possible role in inflammation. Can J Physiol Pharmacol. 2008;86(3):105–112. doi:10.1139/Y08-004
- Hackett TL, de Bruin HG, Shaheen F, et al. Caveolin-1 controls airway epithelial barrier function. Implications for asthma. Am J Respir Cell Mol Biol. 2013;49(4):662–671. doi:10.1165/rcmb.2013-0124OC
- Steelant B, Farre R, Wawrzyniak P, et al. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137(4):1043–1053 e1045. doi:10.1016/j.jaci.2015.10.050
- Sweerus K, Lachowicz-Scroggins M, Gordon E, et al. Claudin-18 deficiency is associated with airway epithelial barrier dysfunction and asthma. J Allergy Clin Immunol. 2017;139(1):72–81 e71. doi:10.1016/j.jaci.2016.02.035
- Hackett TL. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol. 2012;12(1):53–59. doi:10.1097/ACI.0b013e32834ec6eb
- Yang J, Antin P, Berx G, et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21(6):341–352. doi:10.1038/s41580-020-0237-9
- John M. Eisenberg Center for Clinical Decisions and Communications Science. Omega-3 fatty acids and cardiovascular disease: a review of the research for adults. In: Comparative Effectiveness Review Summary Guides for Consumers. Rockville (MD): Agency for Healthcare Research and Quality; 2005.
- Reuter S, Martin H, Beckert H, et al. The Wnt/beta-catenin pathway attenuates experimental allergic airway disease. J Immunol. 2014;193(2):485–495. doi:10.4049/jimmunol.1400013
- Yao L, Zhao H, Tang H, et al. Blockade of beta-catenin signaling attenuates toluene diisocyanate-induced experimental asthma. Allergy. 2017;72(4):579–589. doi:10.1111/all.13045
- Vroling AB, Fokkens WJ, van Drunen CM. How epithelial cells detect danger: aiding the immune response. Allergy. 2008;63(9):1110–1123. doi:10.1111/j.1398-9995.2008.01785.x
- Wills-Karp M. Allergen-specific pattern recognition receptor pathways. Curr Opin Immunol. 2010;22(6):777–782. doi:10.1016/j.coi.2010.10.011
- Laulajainen-Hongisto A, Toppila-Salmi SK, Luukkainen A, Kern R. Airway epithelial dynamics in allergy and related chronic inflammatory airway diseases. Front Cell Dev Biol. 2020;8:204. doi:10.3389/fcell.2020.00204
- Barnes PJ. The cytokine network in asthma and chronic obstructive pulmonary disease. J Clin Invest. 2008;118(11):3546–3556. doi:10.1172/JCI36130
- Kaur K, Bachus H, Lewis C, et al. GM-CSF production by non-classical monocytes controls antagonistic LPS-driven functions in allergic inflammation. Cell Rep. 2021;37(13):110178. doi:10.1016/j.celrep.2021.110178
- Hamilton LM, Torres-Lozano C, Puddicombe SM, et al. The role of the epidermal growth factor receptor in sustaining neutrophil inflammation in severe asthma. Clin Exp Allergy. 2003;33(2):233–240. doi:10.1046/j.1365-2222.2003.01593.x
- Bayarri MA, Milara J, Estornut C, Cortijo J. Nitric oxide system and bronchial epithelium: more than a barrier. Front Physiol. 2021;12:687381. doi:10.3389/fphys.2021.687381
- Hardiman KM, McNicholas-Bevensee CM, Fortenberry J, et al. Regulation of amiloride-sensitive Na(+) transport by basal nitric oxide. Am J Respir Cell Mol Biol. 2004;30(5):720–728. doi:10.1165/rcmb.2003-0325OC
- Olson N, Greul AK, Hristova M, Bove PF, Kasahara DI, van der Vliet A. Nitric oxide and airway epithelial barrier function: regulation of tight junction proteins and epithelial permeability. Arch Biochem Biophys. 2009;484(2):205–213. doi:10.1016/j.abb.2008.11.027
- Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J. 2017;50(1):1601805.
- Lee CG, Ma B, Takyar S, et al. Studies of vascular endothelial growth factor in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2011;8(6):512–515. doi:10.1513/pats.201102-018MW
- Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10(12):838–848. doi:10.1038/nri2870
- Wu CA, Peluso JJ, Zhu L, Lingenheld EG, Walker ST, Puddington L. Bronchial epithelial cells produce IL-5: implications for local immune responses in the airways. Cell Immunol. 2010;264(1):32–41. doi:10.1016/j.cellimm.2010.04.008
- Martinez FD, Vercelli D. Asthma. Lancet. 2013;382(9901):1360–1372. doi:10.1016/S0140-6736(13)61536-6
- Kau AL, Korenblat PE. Anti-interleukin 4 and 13 for asthma treatment in the era of endotypes. Curr Opin Allergy Clin Immunol. 2014;14(6):570–575. doi:10.1097/ACI.0000000000000108
- Sugita K, Steer CA, Martinez-Gonzalez I, et al. Type 2 innate lymphoid cells disrupt bronchial epithelial barrier integrity by targeting tight junctions through IL-13 in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):300–310 e311. doi:10.1016/j.jaci.2017.02.038
- Tan HT, Hagner S, Ruchti F, et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy. 2019;74(2):294–307. doi:10.1111/all.13619
- Major J, Crotta S, Llorian M, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369(6504):712–717. doi:10.1126/science.abc2061
- Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13(9):3218–3234. doi:10.1091/mbc.e02-03-0134
- Hardyman MA, Wilkinson E, Martin E, et al. TNF-alpha-mediated bronchial barrier disruption and regulation by src-family kinase activation. J Allergy Clin Immunol. 2013;132(3):665–675 e668. doi:10.1016/j.jaci.2013.03.005
- Zabner J, Winter MC, Shasby S, Ries D, Shasby DM. Histamine decreases E-cadherin-based adhesion to increase permeability of human airway epithelium. Chest. 2003;123(3 Suppl):385S. doi:10.1378/chest.123.3_suppl.385S
- Zabner J, Winter M, Excoffon KJ, et al. Histamine alters E-cadherin cell adhesion to increase human airway epithelial permeability. J Appl Physiol. 2003;95(1):394–401. doi:10.1152/japplphysiol.01134.2002
- Sanak M. Eicosanoid mediators in the airway inflammation of asthmatic patients: what is new? Allergy Asthma Immunol Res. 2016;8(6):481–490. doi:10.4168/aair.2016.8.6.481
- Luster AD, Tager AM. T-cell trafficking in asthma: lipid mediators grease the way. Nat Rev Immunol. 2004;4(9):711–724. doi:10.1038/nri1438
- Liu MC, Bleecker ER, Lichtenstein LM, et al. Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am Rev Respir Dis. 1990;142(1):126–132. doi:10.1164/ajrccm/142.1.126
- Liu MC, Proud D, Lichtenstein LM, et al. Effects of prednisone on the cellular responses and release of cytokines and mediators after segmental allergen challenge of asthmatic subjects. J Allergy Clin Immunol. 2001;108(1):29–38. doi:10.1067/mai.2001.116004
- Alving K, Matran R, Lundberg JM. The possible role of prostaglandin D2 in the long-lasting airways vasodilatation induced by allergen in the sensitized pig. Acta Physiol Scand. 1991;143(1):93–103. doi:10.1111/j.1748-1716.1991.tb09204.x
- Matsuoka T, Hirata M, Tanaka H, et al. Prostaglandin D2 as a mediator of allergic asthma. Science. 2000;287(5460):2013–2017. doi:10.1126/science.287.5460.2013
- Mondino C, Ciabattoni G, Koch P, et al. Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J Allergy Clin Immunol. 2004;114(4):761–767. doi:10.1016/j.jaci.2004.06.054
- Pavord ID, Ward R, Woltmann G, Wardlaw AJ, Sheller JR, Dworski R. Induced sputum eicosanoid concentrations in asthma. Am J Respir Crit Care Med. 1999;160(6):1905–1909. doi:10.1164/ajrccm.160.6.9903114
- Tager AM, Bromley SK, Medoff BD, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4(10):982–990. doi:10.1038/ni970
- Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol. 2003;4(10):965–973. doi:10.1038/ni972
- Snyder F. Platelet-activating factor: the biosynthetic and catabolic enzymes. Biochem J. 1995;305(Pt 3):689–705. doi:10.1042/bj3050689
- Schauberger E, Peinhaupt M, Cazares T, Lindsley AW. Lipid mediators of allergic disease: pathways, treatments, and emerging therapeutic targets. Curr Allergy Asthma Rep. 2016;16(7):48. doi:10.1007/s11882-016-0628-3
- Krishnamoorthy N, Abdulnour RE, Walker KH, Engstrom BD, Levy BD. Specialized proresolving mediators in innate and adaptive immune responses in airway diseases. Physiol Rev. 2018;98(3):1335–1370. doi:10.1152/physrev.00026.2017
- Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi:10.1038/ni1276
- Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40(3):315–327. doi:10.1016/j.immuni.2014.02.009
- Levy BD, Bonnans C, Silverman ES, et al. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172(7):824–830. doi:10.1164/rccm.200410-1413OC
- Vachier I, Bonnans C, Chavis C, et al. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115(1):55–60. doi:10.1016/j.jaci.2004.09.038
- Ono E, Dutile S, Kazani S, et al. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med. 2014;190(8):886–897. doi:10.1164/rccm.201403-0544OC
- Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145(6):1281–1284. doi:10.1164/ajrccm/145.6.1281
- Kong X, Wu SH, Zhang L, Chen XQ. Pilot application of lipoxin A4 analog and lipoxin A4 receptor agonist in asthmatic children with acute episodes. Exp Ther Med. 2017;14(3):2284–2290. doi:10.3892/etm.2017.4787
- Steinke JW, Liu L, Huyett P, Negri J, Payne SC, Borish L. Prominent role of IFN-gamma in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2013;132(4):856–865 e851–853. doi:10.1016/j.jaci.2013.05.008
- Soyombo O, Spur BW, Lee TH. Effects of lipoxin A4 on chemotaxis and degranulation of human eosinophils stimulated by platelet-activating factor and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Allergy. 1994;49(4):230–234. doi:10.1111/j.1398-9995.1994.tb02654.x
- Planaguma A, Kazani S, Marigowda G, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178(6):574–582. doi:10.1164/rccm.200801-061OC
- Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168(4):1064–1072. doi:10.2353/ajpath.2006.051056
- Barnig C, Cernadas M, Dutile S, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5(174):174ra126. doi:10.1126/scitranslmed.3004812
- Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9(8):873–879. doi:10.1038/ni.1627
- Aoki H, Hisada T, Ishizuka T, et al. Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun. 2008;367(2):509–515. doi:10.1016/j.bbrc.2008.01.012
- Rogerio AP, Haworth O, Croze R, et al. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J Immunol. 2012;189(4):1983–1991. doi:10.4049/jimmunol.1101665
- Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178(1):496–502. doi:10.4049/jimmunol.178.1.496
- Lin YJ, Flaczyk A, Wolfheimer S, et al. The fusion protein rFlaA:Betv1 modulates DC responses by a p38-MAPK and COX2-dependent secretion of PGE2 from epithelial cells. Cells. 2021;10:12. doi:10.3390/cells10123415
- Zhou Y, Wang W, Zhao C, et al. Prostaglandin E2 inhibits group 2 innate lymphoid cell activation and allergic airway inflammation through E-prostanoid 4-cyclic adenosine monophosphate signaling. Front Immunol. 2018;9:501. doi:10.3389/fimmu.2018.00501
- Aggarwal S, Moodley YP, Thompson PJ, Misso NL. Prostaglandin E2 and cysteinyl leukotriene concentrations in sputum: association with asthma severity and eosinophilic inflammation. Clin Exp Allergy. 2010;40(1):85–93. doi:10.1111/j.1365-2222.2009.03386.x
- Pavord ID, Wong CS, Williams J, Tattersfield AE. Effect of inhaled prostaglandin E2 on allergen-induced asthma. Am Rev Respir Dis. 1993;148(1):87–90. doi:10.1164/ajrccm/148.1.87
- Takahashi Y, Tokuoka S, Masuda T, et al. Augmentation of allergic inflammation in prostanoid IP receptor deficient mice. Br J Pharmacol. 2002;137(3):315–322. doi:10.1038/sj.bjp.0704872
- Nagao K, Tanaka H, Komai M, Masuda T, Narumiya S, Nagai H. Role of prostaglandin I2 in airway remodeling induced by repeated allergen challenge in mice. Am J Respir Cell Mol Biol. 2003;29(3pt 1):314–320. doi:10.1165/rcmb.2003-0035OC
- Yamabayashi C, Koya T, Kagamu H, et al. A novel prostacyclin agonist protects against airway hyperresponsiveness and remodeling in mice. Am J Respir Cell Mol Biol. 2012;47(2):170–177. doi:10.1165/rcmb.2011-0350OC
- Tripathi P, Awasthi S, Husain N, Prasad R, Mishra V. Increased expression of ADAM33 protein in asthmatic patients as compared to non-asthmatic controls. Indian J Med Res. 2013;137(3):507–514.
- Biswas S. Role of PCDH 1 gene in the development of childhood asthma and other related phenotypes: a literature review. Cureus. 2018;10(9):e3360. doi:10.7759/cureus.3360
- Koppelman GH, Meyers DA, Howard TD, et al. Identification of PCDH1 as a novel susceptibility gene for bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2009;180(10):929–935. doi:10.1164/rccm.200810-1621OC
- Bonnelykke K, Coleman AT, Evans MD, et al. Cadherin-related family member 3 genetics and rhinovirus C respiratory illnesses. Am J Respir Crit Care Med. 2018;197(5):589–594. doi:10.1164/rccm.201705-1021OC
- Everman JL, Sajuthi S, Saef B, et al. Functional genomics of CDHR3 confirms its role in HRV-C infection and childhood asthma exacerbations. J Allergy Clin Immunol. 2019;144(4):962–971. doi:10.1016/j.jaci.2019.01.052
- Chung LP, Baltic S, Ferreira M, Temple S, Waterer G, Thompson PJ. Beta2 adrenergic receptor (ADRbeta2) haplotype pair (2/4) is associated with severe asthma. PLoS One. 2014;9(4):e93695. doi:10.1371/journal.pone.0093695
- Biagini Myers JM, Martin LJ, Kovacic MB, et al. Epistasis between serine protease inhibitor Kazal-type 5 (SPINK5) and thymic stromal lymphopoietin (TSLP) genes contributes to childhood asthma. J Allergy Clin Immunol. 2014;134(4):891–899 e893. doi:10.1016/j.jaci.2014.03.037
- Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi:10.1056/NEJMoa0906312
- Saikumar Jayalatha AK, Hesse L, Ketelaar ME, Koppelman GH, Nawijn MC. The central role of IL-33/IL-1RL1 pathway in asthma: from pathogenesis to intervention. Pharmacol Ther. 2021;225:107847. doi:10.1016/j.pharmthera.2021.107847
- Gordon ED, Palandra J, Wesolowska-Andersen A, et al. IL1RL1 asthma risk variants regulate airway type 2 inflammation. JCI Insight. 2016;1(14):e87871. doi:10.1172/jci.insight.87871
- Yang R, Tan M, Xu J, Zhao X. Investigating the regulatory role of ORMDL3 in airway barrier dysfunction using in vivo and in vitro models. Int J Mol Med. 2019;44(2):535–548. doi:10.3892/ijmm.2019.4233
- James B, Milstien S, Spiegel S. ORMDL3 and allergic asthma: from physiology to pathology. J Allergy Clin Immunol. 2019;144(3):634–640. doi:10.1016/j.jaci.2019.07.023
- Laura G, Liu Y, Fernandes K, et al. ORMDL3 regulates poly I:C induced inflammatory responses in airway epithelial cells. BMC Pulm Med. 2021;21(1):167. doi:10.1186/s12890-021-01496-5
- Zhang YM. Orosomucoid-like protein 3, rhinovirus and asthma. World J Crit Care Med. 2021;10(5):170–182. doi:10.5492/wjccm.v10.i5.170
- Liu Y, Bochkov YA, Eickhoff JC, et al. Orosomucoid-like 3 supports rhinovirus replication in human epithelial cells. Am J Respir Cell Mol Biol. 2020;62(6):783–792. doi:10.1165/rcmb.2019-0237OC
- Yu F, Sun Y, Yu J, et al. ORMDL3 is associated with airway remodeling in asthma via the ERK/MMP-9 pathway. Mol Med Rep. 2017;15(5):2969–2976. doi:10.3892/mmr.2017.6413
- Anthoni M, Wang G, Leino MS, Lauerma AI, Alenius HT, Wolff HJ. Smad3 -signalling and Th2 cytokines in normal mouse airways and in a mouse model of asthma. Int J Biol Sci. 2007;3(7):477–485. doi:10.7150/ijbs.3.477
- Fan Q, Jian Y. MiR-203a-3p regulates TGF-beta1-induced epithelial-mesenchymal transition (EMT) in asthma by regulating Smad3 pathway through SIX1. Biosci Rep. 2020;40:2. doi:10.1042/BSR20192645
- Lund RJ, Osmala M, Malonzo M, et al. Atopic asthma after rhinovirus-induced wheezing is associated with DNA methylation change in the SMAD3 gene promoter. Allergy. 2018;73(8):1735–1740. doi:10.1111/all.13473
- Gibbs JD, Ornoff DM, Igo HA, Zeng JY, Imani F. Cell cycle arrest by transforming growth factor beta1 enhances replication of respiratory syncytial virus in lung epithelial cells. J Virol. 2009;83(23):12424–12431. doi:10.1128/JVI.00806-09
- Werder RB, Ullah MA, Rahman MM, et al. Targeting the P2Y13 receptor suppresses IL-33 and HMGB1 release and ameliorates experimental asthma. Am J Respir Crit Care Med. 2022;205(3):300–312. doi:10.1164/rccm.202009-3686OC
- Sordillo JE, Kelly R, Bunyavanich S, et al. Genome-wide expression profiles identify potential targets for gene-environment interactions in asthma severity. J Allergy Clin Immunol. 2015;136(4):885–892 e882. doi:10.1016/j.jaci.2015.02.035
- Alashkar Alhamwe B, Miethe S, Pogge von Strandmann E, Potaczek DP, Garn H. Epigenetic regulation of airway epithelium immune functions in asthma. Front Immunol. 2020;11:1747. doi:10.3389/fimmu.2020.01747
- Sugita K, Soyka MB, Wawrzyniak P, et al. Outside-in hypothesis revisited: the role of microbial, epithelial, and immune interactions. Ann Allergy Asthma Immunol. 2020;125(5):517–527. doi:10.1016/j.anai.2020.05.016
- Lee AY. The role of microRNAs in epidermal barrier. Int J Mol Sci. 2020;21:16.
- Potaczek DP, Harb H, Michel S, Alhamwe BA, Renz H, Tost J. Epigenetics and allergy: from basic mechanisms to clinical applications. Epigenomics. 2017;9(4):539–571. doi:10.2217/epi-2016-0162
- Kabesch M, Tost J. Recent findings in the genetics and epigenetics of asthma and allergy. Semin Immunopathol. 2020;42(1):43–60. doi:10.1007/s00281-019-00777-w
- Wang CM, Chang CB, Wu SF. Differential DNA methylation in allergen-specific immunotherapy of asthma. Cell Mol Immunol. 2020;17(9):1017–1018. doi:10.1038/s41423-020-0476-x
- Wawrzyniak P, Krawczyk K, Acharya S, et al. Inhibition of CpG methylation improves the barrier integrity of bronchial epithelial cells in asthma. Allergy. 2021;76(6):1864–1868. doi:10.1111/all.14667
- Vermeulen CJ, Xu CJ, Vonk JM, et al. Differential DNA methylation in bronchial biopsies between persistent asthma and asthma in remission. Eur Respir J. 2020;55:2. doi:10.1183/13993003.01280-2019
- Cardenas A, Fadadu RP, Van Der Laan L, et al. Controlled human exposures to diesel exhaust: a human epigenome-wide experiment of target bronchial epithelial cells. Environ Epigenet. 2021;7(1):dvab003. doi:10.1093/eep/dvab003
- Qin W, Brands X, Van’t Veer C, et al. Bronchial epithelial DNA methyltransferase 3b dampens pulmonary immune responses during Pseudomonas aeruginosa infection. PLoS Pathog. 2021;17(4):e1009491. doi:10.1371/journal.ppat.1009491
- Liberti DC, Zepp JA, Bartoni CA, et al. Dnmt1 is required for proximal-distal patterning of the lung endoderm and for restraining alveolar type 2 cell fate. Dev Biol. 2019;454(2):108–117. doi:10.1016/j.ydbio.2019.06.019
- Lawlor L, Yang XB. Harnessing the HDAC-histone deacetylase enzymes, inhibitors and how these can be utilised in tissue engineering. Int J Oral Sci. 2019;11(2):20. doi:10.1038/s41368-019-0053-2
- Steelant B, Wawrzyniak P, Martens K, et al. Blocking histone deacetylase activity as a novel target for epithelial barrier defects in patients with allergic rhinitis. J Allergy Clin Immunol. 2019;144(5):1242–1253 e1247. doi:10.1016/j.jaci.2019.04.027
- Ito K, Yamamura S, Essilfie-Quaye S, et al. Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med. 2006;203(1):7–13. doi:10.1084/jem.20050466
- Wei W, Chen W, He N. HDAC4 induces the development of asthma by increasing Slug-upregulated CXCL12 expression through KLF5 deacetylation. J Transl Med. 2021;19(1):258. doi:10.1186/s12967-021-02812-7
- Ren Y, Su X, Kong L, et al. Therapeutic effects of histone deacetylase inhibitors in a murine asthma model. Inflamm Res. 2016;65(12):995–1008. doi:10.1007/s00011-016-0984-4
- Wang J, Cui M, Sun F, et al. HDAC inhibitor sodium butyrate prevents allergic rhinitis and alters lncRNA and mRNA expression profiles in the nasal mucosa of mice. Int J Mol Med. 2020;45(4):1150–1162. doi:10.3892/ijmm.2020.4489
- Sekiyama A, Gon Y, Terakado M, et al. Glucocorticoids enhance airway epithelial barrier integrity. Int Immunopharmacol. 2012;12(2):350–357. doi:10.1016/j.intimp.2011.12.006
- Rimmer C, Hetelekides S, Eliseeva SI, Georas SN, Veazey JM, Deli MA. Budesonide promotes airway epithelial barrier integrity following double-stranded RNA challenge. PLoS One. 2021;16(12):e0260706. doi:10.1371/journal.pone.0260706
- Ma S, Xian M, Wang Y, Wang C, Zhang L. Budesonide repairs decreased barrier integrity of eosinophilic nasal polyp epithelial cells caused by PM2.5. Clin Transl Allergy. 2021;11(5):e12019. doi:10.1002/clt2.12029
- Doulaptsi M, Wils T, Hellings PW, et al. Mometasone furoate and fluticasone furoate are equally effective in restoring nasal epithelial barrier dysfunction in allergic rhinitis. World Allergy Organ J. 2021;14(9):100585. doi:10.1016/j.waojou.2021.100585
- Qian J, Xu Y, Yu Z. Budesonide and calcitriol synergistically inhibit airway remodeling in asthmatic mice. Can Respir J. 2018;2018:5259240. doi:10.1155/2018/5259240
- Southam DS, Ellis R, Wattie J, Young S, Inman MD. Budesonide prevents but does not reverse sustained airway hyperresponsiveness in mice. Eur Respir J. 2008;32(4):970–978. doi:10.1183/09031936.00125307
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi:10.1164/rccm.200401-033OC
- Kardas G, Kuna P, Panek M. Biological therapies of severe asthma and their possible effects on airway remodeling. Front Immunol. 2020;11:1134. doi:10.3389/fimmu.2020.01134
- Riccio AM, Dal Negro RW, Micheletto C, et al. Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients. Int J Immunopathol Pharmacol. 2012;25(2):475–484. doi:10.1177/039463201202500217
- Zastrzezynska W, Przybyszowski M, Bazan-Socha S, et al. Omalizumab may decrease the thickness of the reticular basement membrane and fibronectin deposit in the bronchial mucosa of severe allergic asthmatics. J Asthma. 2020;57(5):468–477. doi:10.1080/02770903.2019.1585872
- Hoshino M, Ohtawa J. Effects of adding omalizumab, an anti-immunoglobulin E antibody, on airway wall thickening in asthma. Respiration. 2012;83(6):520–528. doi:10.1159/000334701
- Laviolette M, Gossage DL, Gauvreau G, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–1096 e1085. doi:10.1016/j.jaci.2013.05.020
- Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol. 2016;170(2):122–131. doi:10.1159/000447692
- Turkeli A, Yilmaz O, Karaman M, et al. Anti-VEGF treatment suppresses remodeling factors and restores epithelial barrier function through the E-cadherin/beta-catenin signaling axis in experimental asthma models. Exp Ther Med. 2021;22(1):689. doi:10.3892/etm.2021.10121
- Globinska A, Boonpiyathad T, Satitsuksanoa P, et al. Mechanisms of allergen-specific immunotherapy: diverse mechanisms of immune tolerance to allergens. Ann Allergy Asthma Immunol. 2018;121(3):306–312. doi:10.1016/j.anai.2018.06.026
- Shamji MH, Layhadi JA, Sharif H, Penagos M, Durham SR. Immunological responses and biomarkers for allergen-specific immunotherapy against inhaled allergens. J Allergy Clin Immunol Pract. 2021;9(5):1769–1778. doi:10.1016/j.jaip.2021.03.029
- Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol. 2011;127(1):18–27; quiz 28–19. doi:10.1016/j.jaci.2010.11.030
- Yuan X, Wang J, Li Y, et al. Allergy immunotherapy restores airway epithelial barrier dysfunction through suppressing IL-25 -induced endoplasmic reticulum stress in asthma. Sci Rep. 2018;8(1):7950. doi:10.1038/s41598-018-26221-x
- Hesse L, Van ieperen N, Habraken C, et al. Subcutaneous immunotherapy with purified Der p1 and 2 suppresses type 2 immunity in a murine asthma model. Allergy. 2018;73(4):862–874. doi:10.1111/all.13382
- Hoshino M, Akitsu K, Kubota K. Effect of sublingual immunotherapy on airway inflammation and airway wall thickness in allergic asthma. J Allergy Clin Immunol Pract. 2019;7(8):2804–2811. doi:10.1016/j.jaip.2019.06.003
- Zissler UM, Jakwerth CA, Guerth F, et al. Allergen-specific immunotherapy induces the suppressive secretoglobin 1A1 in cells of the lower airways. Allergy. 2021;76(8):2461–2474. doi:10.1111/all.14756
- Wawrzyniak M, Groeger D, Frei R, et al. Spermidine and spermine exert protective effects within the lung. Pharmacol Res Perspect. 2021;9(4):e00837. doi:10.1002/prp2.837
- Sussan TE, Gajghate S, Chatterjee S, et al. Nrf2 reduces allergic asthma in mice through enhanced airway epithelial cytoprotective function. Am J Physiol Lung Cell Mol Physiol. 2015;309(1):L27–36. doi:10.1152/ajplung.00398.2014
- Angelina A, Martin-Fontecha M, Ruckert B, et al. The cannabinoid WIN55212-2 restores rhinovirus-induced epithelial barrier disruption. Allergy. 2021;76(6):1900–1902. doi:10.1111/all.14707
- Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27(5):401–415. doi:10.1080/019021401300317125
- Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol. 2021;21(6):347–362. doi:10.1038/s41577-020-00477-9
- Yang Y, Riccio P, Schotsaert M, et al. Spatial-temporal lineage restrictions of embryonic p63(+) progenitors establish distinct stem cell pools in adult airways. Dev Cell. 2018;44(6):752–761 e754. doi:10.1016/j.devcel.2018.03.001
- Morrisey EE. Basal cells in lung development and repair. Dev Cell. 2018;44(6):653–654. doi:10.1016/j.devcel.2018.03.004
- Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38(5):517–523. doi:10.1165/rcmb.2007-0050OC
- Bukowy-Bieryllo Z. Long-term differentiating primary human airway epithelial cell cultures: how far are we? Cell Commun Signal. 2021;19(1):63. doi:10.1186/s12964-021-00740-z
- Knoop KA, Newberry RD. Goblet cells: multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018;11(6):1551–1557. doi:10.1038/s41385-018-0039-y
- Rogers DF. The airway goblet cell. Int J Biochem Cell Biol. 2003;35(1):1–6. doi:10.1016/S1357-2725(02)00083-3
- Rogers DF. Airway goblet cell hyperplasia in asthma: hypersecretory and anti-inflammatory? Clin Exp Allergy. 2002;32(8):1124–1127. doi:10.1046/j.1365-2745.2002.01474.x
- Jackson AD. Airway goblet-cell mucus secretion. Trends Pharmacol Sci. 2001;22(1):39–45. doi:10.1016/S0165-6147(00)01600-X
- Yang S, Yu M. Role of goblet cells in intestinal barrier and mucosal immunity. J Inflamm Res. 2021;14:3171–3183. doi:10.2147/JIR.S318327
- Rokicki W, Rokicki M, Wojtacha J, Dzeljijli A. The role and importance of club cells (Clara cells) in the pathogenesis of some respiratory diseases. Kardiochir Torakochirurgia Pol. 2016;13(1):26–30. doi:10.5114/kitp.2016.58961
- Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30(4):469–475. doi:10.1046/j.1365-2222.2000.00760.x
- Wang SX, Liu P, Wei MT, et al. Roles of serum Clara cell protein 16 and surfactant protein-D in the early diagnosis and progression of silicosis. J Occup Environ Med. 2007;49(8):834–839. doi:10.1097/JOM.0b013e318124a927
- Pilon AL. Rationale for the development of recombinant human CC10 as a therapeutic for inflammatory and fibrotic disease. Ann N Y Acad Sci. 2000;923:280–299. doi:10.1111/j.1749-6632.2000.tb05536.x
- Tata PR, Mou H, Pardo-Saganta A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503(7475):218–223. doi:10.1038/nature12777
- Morimoto M, Nishinakamura R, Saga Y, Kopan R. Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development. 2012;139(23):4365–4373. doi:10.1242/dev.083840
- Guseh JS, Bores SA, Stanger BZ, et al. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136(10):1751–1759. doi:10.1242/dev.029249
- Thomas B, Rutman A, Hirst RA, et al. Ciliary dysfunction and ultrastructural abnormalities are features of severe asthma. J Allergy Clin Immunol. 2010;126(4):722–729 e722. doi:10.1016/j.jaci.2010.05.046
- Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379–406. doi:10.1146/annurev-physiol-021014-071931
- Van Lommel A. Pulmonary neuroendocrine cells (PNEC) and neuroepithelial bodies (NEB): chemoreceptors and regulators of lung development. Paediatr Respir Rev. 2001;2(2):171–176. doi:10.1053/prrv.2000.0126
- Noguchi M, Furukawa KT, Morimoto M. Pulmonary neuroendocrine cells: physiology, tissue homeostasis and disease. Dis Model Mech. 2020;13:12. doi:10.1242/dmm.046920
- Kobayashi Y, Tata PR. Pulmonary neuroendocrine cells: sensors and sentinels of the lung. Dev Cell. 2018;45(4):425–426. doi:10.1016/j.devcel.2018.05.009
- Johnson DE, Georgieff MK. Pulmonary neuroendocrine cells. Their secretory products and their potential roles in health and chronic lung disease in infancy. Am Rev Respir Dis. 1989;140(6):1807–1812. doi:10.1164/ajrccm/140.6.1807
- Sui P, Wiesner DL, Xu J, et al. Pulmonary neuroendocrine cells amplify allergic asthma responses. Science. 2018;360:6393. doi:10.1126/science.aan8546
- Goldfarbmuren KC, Jackson ND, Sajuthi SP, et al. Dissecting the cellular specificity of smoking effects and reconstructing lineages in the human airway epithelium. Nat Commun. 2020;11(1):2485. doi:10.1038/s41467-020-16239-z
- Montoro DT, Haber AL, Biton M, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560(7718):319–324. doi:10.1038/s41586-018-0393-7
- Plasschaert LW, Zilionis R, Choo-Wing R, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560(7718):377–381. doi:10.1038/s41586-018-0394-6
- Ruan YC, Wang Y, Da Silva N, et al. CFTR interacts with ZO-1 to regulate tight junction assembly and epithelial differentiation through the ZONAB pathway. J Cell Sci. 2014;127(Pt 20):4396–4408. doi:10.1242/jcs.148098
- Shah VS, Meyerholz DK, Tang XX, et al. Airway acidification initiates host defense abnormalities in cystic fibrosis mice. Science. 2016;351(6272):503–507. doi:10.1126/science.aad5589
- Vieira Braga FA, Kar G, Berg M, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med. 2019;25(7):1153–1163. doi:10.1038/s41591-019-0468-5
- Deprez M, Zaragosi LE, Truchi M, et al. A single-cell atlas of the human healthy airways. Am J Respir Crit Care Med. 2020;202(12):1636–1645. doi:10.1164/rccm.201911-2199OC
- Schneider C, O’Leary CE, Locksley RM. Regulation of immune responses by tuft cells. Nat Rev Immunol. 2019;19(9):584–593. doi:10.1038/s41577-019-0176-x
- Krasteva G, Canning BJ, Hartmann P, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci U S A. 2011;108(23):9478–9483. doi:10.1073/pnas.1019418108
- O’Leary CE, Schneider C, Locksley RM. Tuft cells-systemically dispersed sensory epithelia integrating immune and neural circuitry. Annu Rev Immunol. 2019;37:47–72. doi:10.1146/annurev-immunol-042718-041505
- Kohanski MA, Workman AD, Patel NN, et al. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018;142(2):460–469 e467. doi:10.1016/j.jaci.2018.03.019
- Patel NN, Kohanski MA, Maina IW, et al. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2018;8(8):900–906. doi:10.1002/alr.22142
