Abstract
Introduction
Adherence with controller medication is a major challenge in asthma management. Thus, a reliable method of measurement is mandatory to assess adherence.
Aim
To examine the test-retest reliability on adherence with inhaled corticosteroids in adults with asthma using, a self-reported adherence score (Foster score).
Methods
Patients with asthma and >1 routine follow-up appointment at a university hospital outpatient clinic reported Foster scores. The objective Medication Possession Ratio (MPR) was calculated based on pharmacy redemption data and physician-prescribed doses of inhaled corticosteroids. The difference between Foster score and MPR at the first and second visit was assessed using a Bland–Altman plot, outcomes reported as limits of agreements and bias. Foster scores from both visits were used to calculate an intraclass correlation coefficient (ICC).
Results
Self-reported adherence with asthma controller medication measured by Foster score was significantly higher than the objective MPR (p < 0.0001). The Bland–Altman plot for MPR and Foster score at the first and second visit showed upper and lower limits of agreement of 83.5 – (−1.6) and 80.9 – (−6.9) and bias was 41.0 and 37.0, respectively. Of the included patients, 93.1% reported identical Foster scores between visits, resulting in an excellent ICC of 0.92. Absolute median difference between Foster scores and MPR at first and second visit was 8.7 percentage points (p = 0.049).
Conclusion
Foster score shows an excellent ICC; however, its poor agreement with objective measures of adherence suggests that clinicians should not rely on Foster scores alone to assess adherence with inhaled corticosteroids in patients with asthma.
Introduction
The goal of asthma management is to prevent asthma-related morbidity and mortality, such as exacerbations, persistent airflow limitation, asthma symptoms and reduce treatment side effects.Citation1 Adherence to controller medication for asthma range from 30% to 70% as reported by the WHO.Citation2 The Danish prevalence of asthma is 7500/100.000 citizens.Citation3 Studies indicate that 30–70% of asthma patients have low adherence.Citation4,Citation5 Previous studies have calculated that patients should take 80% or more of their prescribed medicine to be adherent,Citation6,Citation7 hence 80% is a widely used cut-point.Citation8–Citation14 Since low adherence is associated with steeper decline in lung function;Citation15 exacerbations;Citation4 increase in hospitalizations and emergency department visitsCitation16 and asthma-related death,Citation17 knowledge of patient adherence is important and a necessary point of intervention for clinicians.
There are several ways to investigate self-reported adherence. The Danish Society of Respiratory Medicine recommends using Foster score, a self-reported adherence measurement obtained by asking the patient a single question “How many days, out of seven, do you take your medicine as recommended?”.Citation18 However, it is stated that Foster score has a tendency towards overestimating adherence, hence the clinician should also assess adherence based on the number of purchased doses/prescribed doses.Citation18
Objective assessment of adherence can be calculated as Medication Possession Ratio (MPR), and is typically calculated as the total number of days supplied by medication, divided by the number of days in the period.Citation19 MPR has previously been shown to correlate with asthma control.Citation20 However, we have previously reported poor association between Foster score and MPR.Citation21
Adherence to medication may vary during the patients’ course of disease. The term persistency describes patients’ ability to withhold adherence to prescribed medication over time.Citation22 In chronic diseases, persistency is a challenge, albeit a key feature for disease control.Citation23 Although self-reported adherence has several potential pitfalls in persistency evaluation, it is often used given its advantages in clinical care, such as speed, efficiency, and low-cost.Citation23 Using self-reported adherence to investigate persistence is, however, poorly investigated.
In further analyses of data from the cohort included in the previously published study from our group,Citation21 we aim to investigate test-retest reliability of the Foster score over time, compared with MPR, in outpatient asthma patients.
Methods
This study is a cross-sectional, prospective study conducted at the respiratory outpatient clinics at the Department of Respiratory Diseases, Aalborg University Hospital (AaUH), Denmark (January 1-December 31, 2020) and Department of Respiratory Medicine, Copenhagen University Hospital-Hvidovre (CUHH), Copenhagen, Denmark (January 1-May 31, 2020).
Population
Patients diagnosed with asthma according to international guidelinesCitation1 were included by continuous enrolment at AaUH and CUHH, and patients with a routine follow-up appointment were screened for inclusion using electronic patient records. The inclusion criteria were i) more than one appointment at the outpatient clinic ii) age ≥18 years iii) diagnosed with asthma for at least 1 year prior to the index date. Exclusion criteria were i) prescribed ICS for less than one year prior to the index date ii) unclear dosage of medicine, that is prescriptions of multiple inhalers/changes in treatment regimen over a short period of time, where no reliable assessment of actual use of inhaled medicine could be obtained iii) receiving assisted medicine administration including living at a nursing home iv) inability to answer the questionnaire v) non-Danish residents.
Data Collection
Background characteristics were obtained on sex, age, Asthma Control Test (ACT)Citation24 or Asthma Control Questionnaire (ACQ).Citation25 Patients were considered highly symptomatic with an ACT ≤19Citation26 and an ACQ > 1.57.Citation27 Spirometry data consisting of Forced expiratory volume in the first second (FEV1), FEV1 in percent of predicted, Forced Expiratory Volume (FVC) and FVC in percent of predicted were collected through electronic patient records (Clinical Suite, CSC, Falls Church, Virginia, USA (AaUH) or Sundhedsplatformen, Epic Systems Inc., USA (CUHH)), and was obtained using Vyntus Spiro, Vyaire Medical, California, USA (AaUH) or Vitalograph Ltd., Buckinghamshire, UK (CUHH).
Foster scores were collected prior to or during physician consultation using either written questionnaires or verbally, as some appointments were conducted as telephone appointments due to the COVID-19 pandemic. Foster scores were obtained asking the question: “How many days, out of seven, do you take your medicine as recommended?”. The answer was subsequently divided by 7 and presented as percentages.
Pharmacy redemption data one year prior to the index date were obtained from the Common Medication Card (CMC) containing information on all of the redeemed medicine prescribed. Data included number of redeemed doses of SABA (short-acting β2-agonist), number of ICS doses redeemed, including combination therapy (ie, ICS/long-acting β2-agonist (LABA) or ICS/LABA/long-acting muscarinic antagonists (LAMA)). To account for inaccuracies in short prescription periods, any period shorter than 90 days was excluded. The MPR was calculated based on medication containing ICS, using the model described by Jensen et al.Citation21
Number of asthma exacerbations two years prior to the index date were assessed using number of redeemed prednisolone prescriptions from CMC, and number of hospitalizations due to asthma exacerbation, identified through ICD-10 discharge codes (J45.0-9) in the electronic patient records. Prescriptions of at least 37.5 mg P.O. prednisolone for at least 3 days, without concurrent hospitalizations equals a moderate asthma exacerbation, and hospital admission for over 24 hours and prescription of either P.O. or i.v. corticosteroids equals a severe asthma exacerbation.
Adherence and Persistency
Good adherence to prescribed inhalation medicine was defined as MPR > 0.8. Persistency was defined as the ability to maintain adherence >0.8 during the observation period.
Ethics
The study was approved by the Danish Patient Safety Authority (ref. 31–1521-118) and the Capital Region of Copenhagen’s Data Monitoring Board (ref. P-2020-648). The study was registered in the North Jutland Scientific Project Registry (ref 2020–101). All patients provided informed consent in accordance with the Declaration of Helsinki.
Statistics
Descriptive baseline characteristics were provided as means and standard deviations (SD) or medians and interquartile ranges (IQR), depending on distribution. For categorical data, numbers and percentages were used. Non-normally distributed data were compared by Wilcoxon signed rank test, and a paired t-test if data were normally distributed. A p-value of 0.05 was considered statistically significant.
A Bland-Altman test was performed to compare the potential differences between MPR and Foster score at both visits. Data were reported as limits of agreement (LOA) and bias.Citation28 A test retest, ICC (95% confidence intervals (CI)) was calculated for the Foster score and the Foster score/MPR-ratio, based on a mean-rating (k = 3), absolute-agreement, two-way mixed-effects model.Citation29 All statistical work was conducted using IBM SPSS Statistics 27.0.
Results
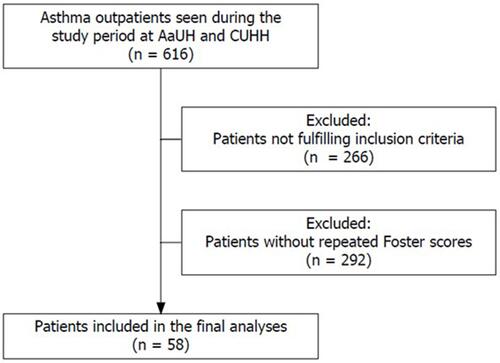
In total, 616 patients enrolled in this study, 58 had visited the out-patient clinic more than once and therefore met the inclusion criteria as shown in . The mean period between Foster score assessments was 148.2 days (74.1SD).
Figure 1 Flowchart for in- and exclusion of patients in the population.
Two-thirds of the population were female, and one-third had had a moderate- and one-fifth a severe exacerbation within the past two years prior to inclusion. shows further baseline characteristics of the population.
Table 1 Baseline Characteristics
provides an overview of the different treatment regimens prescribed to the patients as well as their MPRs and Foster scores.
Table 2 Treatment Regimens and Adherence Measurements
Persistence
There was no significant difference in Foster scores between first and second visit (p = 0.705), with 93.1% of patients reporting identical Foster scores and an ICC of 0.92 (0.86–0.95). A significant decline in MPR was observed from the first to the second out-patient visit (p = 0.046). Good adherence was recorded in 17.2% (10/58) of patients both at the first and the second visit, 10.3% (6/58) were persistently adherent, 7.0% (4/58) were adherent the first visit only, and 7.0% (4/58) at the second visit only.
Foster Score and MPR Agreement
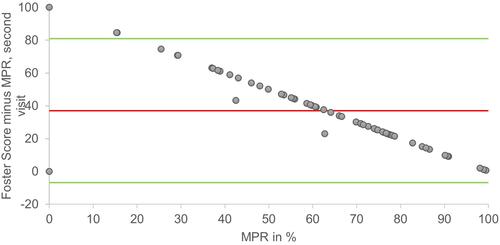
Bland-Altman plots for MPR and Foster score at the first and second visit is shown in and . At the first visit, LOA was −1.6–83.5 and bias was 41.0. At the second visit, LOA was −6.9–80.9 and bias was 37.0.
Figure 2 Bland Altman plot of MPR plotted against the differences of Foster score minus MPR from the first visit. Green lines = Upper and lower limits of agreement, (−1.6 and 83.5). Red line = mean (41.0).
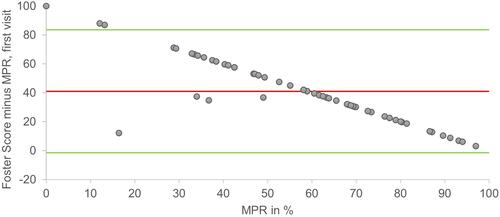
Figure 3 Bland Altman plot of MPR plotted against the differences of Foster score minus MPR from the second visit. Green lines = Upper and lower limits of agreement, (−6.9 and 80.9). Red line = mean (37.0).
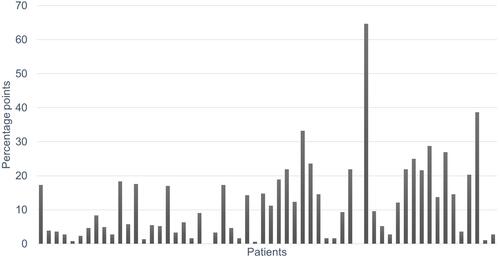
MPR was significantly lower than Foster score at both first and second visit (both p < 0.0001). ICC for the Foster score/MPR-ratio was 0.84 (95% CI 0.73–0.91). shows the difference between first and second Foster score/MPR-ratio in the individual patients and, thereby, the variety of the discrepancy over time in a group of asthma patients. The absolute median difference between Foster score/MPR-ratios from first to second visit was 8.7 [2.9;17.5] percentage points (range 0 to 64.7), and the absolute mean difference between those was significantly different (p = 0.049).
Discussion
When assessed by Foster score (that is self-reported adherence to ICS), we found a significant over-estimation of adherence to ICS, compared to objectively assessed adherence calculated by MPR. Overall MPR improved by 7.7% between the two visits, while Foster score remained unchanged. Less than one-fifth of the included patients were adherent and only 10% were persistently adherent during the observational period.
Persistence
This study demonstrated a slight increase in overall MPR. However, the increase is well below clinically relevant improvements, typically estimated to approx. 25%.Citation30 Similarly, due to the considerable variation in observation periods between subjects, any changes in adherence due to the increased clinical attention around the importance of adherence signaled by the questionnaire used are difficult to interpret, as interventions have mixed results in terms of lasting effects on adherence patternsCitation31 and follow-up time being an important variable.Citation32
Overall persistence was low, even compared to other studies, reporting persistency rates of 20–30%.Citation32,Citation33 These studies comprised larger populations, which may make direct comparison difficult. However, it is intriguing to see a low persistency in a group of patients followed in highly specialized units as the recruiting sites for this study. There are several plausible reasons for this, but use of more complex treatment regimens such as twice-daily or even twice-daily with multiple inhalers is known to influence adherence, and thus persistence, negatively.Citation32,Citation34 In the present study, patients were prescribed different inhalers. Second, the study period encompasses part of the COVID-19 pandemic, where the stance on ICS treatment and COVID-19 risk may possibly affect adherence, although trends to increase in adherence during the pandemic have been shown.Citation35 It would be of interest to repeat the study at a larger scale. Nonetheless, the results underline the importance of focusing on persistency in patients followed in tertiary clinics.
Foster Score and MPR Agreement
In concordance with previous findings, this study demonstrated over-estimation of adherence when based on self-reporting.Citation36–Citation38 However, self-reported adherence is seemingly significantly higher in the present study, with approx. 94% of patients claiming to be adherent to approx. 70% in previously referred studies.Citation29,Citation30 There may be several explanations for this. For one, different self-reporting tools are used in these studies. Some enquire into greater detail and present different possible answers, which may be favorable compared to the simple, one-question Foster score, especially considering the multifaceted nature of controller adherence.Citation34,Citation39 Second, the methods for judging objective adherence differ between studies. The present study uses a nationwide register comprising all pharmacies in Denmark, allowing for complete follow-up in terms of MPR, making it a valid estimation for actual ICS purchase.Citation40 Third, the statistical methods for evaluating the correlation between self-reported and objective adherence differ between studies.
A previous study on a larger patient population from the same cohort showed a weak, yet statistically significant association between Foster score and MPR.Citation21 It may be argued that correlation and regression analyses are less suited when neither modality of measurement are deemed as gold standard.Citation41 Based on these two studies, the use of Foster score only is not recommendable as there is a large discrepancy between patient assessed- and objective adherence.Citation36–Citation38
In the present study, Bland-Altman testing revealed wide LOAs between Foster score and MPR with a skew towards increasing differences with lower MPR due to the remarkably large proportion of patients claiming perfect adherence. The imprecision of Foster scores, the resulting LOAs and large bias measurements suggest a significant risk of erroneous adherence assessment in a clinical setting when relying on Foster scores alone. The overall trend of imprecise self-reported adherence scores has resulted in multidimensional questionnaires regarding adherence, such as the Adherence Starts with Knowledge test, Test of Adherence to Inhalers, Medication Intake Survey-Asthma and the Medication Adherence Report Scale for AsthmaCitation36,Citation42–Citation44 that either directly incorporate objective adherence assessment or general recommendations to use both self-reported and objective measurements.Citation18
In conclusion, Foster score shows an excellent intraclass correlation coefficient, but poor agreement with objective measures of adherence clearly suggests that clinicians should not rely entirely on Foster scores alone to assess adherence. Furthermore, we find very poor persistency in adherence over time. This paper therefore underlines the importance of focusing on persistency when meeting the patient in a clinical setting.
Limitations
The use of MPR as a measurement of objective adherence is established as a valid form of assessing adherence but fails to reflect some forms of non-adherence such as redeemed but unused doses, and can be skewed by redemption in bulk or repeated redemptions in close intervals.Citation45
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
BOH, FFJ, and UMW have no conflicts of interest to disclose related to the present article. KEJH reports personal fees and/or travel grants from AstraZeneca, Chiesi and TEVA unrelated to the present article. CSU has received personal fees for oral presentations, advisory board meeting from AstraZeneca, Novartis, GSK, Boehringer-Ingelheim, ALK-Abello, Chiesi, Sanofi, MundiPharma, Actelion, Orion Pharma and TEVA outside the present paper. The authors report no other conflicts of interest in this work.
References
- Global Initiative for Asthma. Global strategy for asthma management and prevention, 2020; 2020. Available from: www.ginasthma.org. Accessed December 29, 2021.
- Sabaté E. Adherence to Long-Term Therapies Evidence for Action. WHO; 2003.
- Statista. Number of individuals with asthma in Denmark from 2008 to 2018. Available from: https:// www.statista.com/ statistics/950546/ number- ofindividuals-with-asthma-in-denmark/. Accessed December 29, 2021.
- Engelkes M, Janssens HM, De Jongste JC, Sturkenboom MCJM, Verhamme KMC. Medication adherence and the risk of severe asthma exacerbations: a systematic review. Eur Respir J. 2015;45(2):396–407. doi:10.1183/09031936.00075614
- McDonald VM, Yorke J. Adherence in severe asthma: time to get it right. Eur Respir J. 2017;50(6):1702191. doi:10.1183/13993003.02191-2017
- Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin. 2009;25(9):2303–2310. doi:10.1185/03007990903126833
- Lasmar L, Camargos P, Champs N, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy. 2009;64(5):784–789. doi:10.1111/J.1398-9995.2008.01877.X
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. doi:10.1016/j.rmed.2013.04.005
- Bae YJ, Kim TB, Jee YK, et al. Severe asthma patients in Korea overestimate their adherence to inhaled corticosteroids. J Asthma. 2009;46(6):591–595. doi:10.1080/02770900902980908
- Hesso I, Nabhani Gebara S, Greene G, Co Stello RW, Kayyali R. A quantitative evaluation of adherence and inhalation technique among respiratory patients: an observational study using an electronic inhaler assessment device. Int J Clin Pract. 2020;74(2):e13437. doi:10.1111/ijcp.13437
- Erickson SR, Coombs JH, Kirking DM, Azimi AR. Compliance from self-reported versus pharmacy claims data with metered-dose inhalers. Ann Pharmacother. 2001;35(9):997–1003. doi:10.1345/aph.10379
- Van Steenis MNA, Driesenaar JA, Bensing JM, et al. Relationship between medication beliefs, self-reported and refill adherence, and symptoms in patients with asthma using inhaled corticosteroids. Patient Prefer Adherence. 2014;8:83–91. doi:10.2147/PPA.S44185
- Moran C, Doyle F, Sulaiman I, et al. The INCATM (Inhaler Compliance AssessmentTM): a comparison with established measures of adherence. Psychol Heal. 2017;32(10):1266–1287. doi:10.1080/08870446.2017.1290243
- von Bülow A, Backer V, Bodtger U, et al. Differentiation of adult severe asthma from difficult-to-treat asthma – outcomes of a systematic assessment protocol. Respir Med. 2018;145:41–47. doi:10.1016/j.rmed.2018.10.020
- Vähätalo I, Ilmarinen P, Tuomisto LE, et al. 12-year adherence to inhaled corticosteroids in adult-onset asthma. ERJ Open Res. 2020;6(1):00324–02019. doi:10.1183/23120541.00324-2019
- Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–1293. doi:10.1016/j.jaci.2004.09.028
- D’Amato G, Vitale C, Molino A, et al. Asthma-related deaths. Multidiscip Respir Med. 2016;11(1):1–5. doi:10.1186/s40248-016-0073-0
- von Bülow A, Urik CS, Sidenius K, et al. Astma - Svær - Udredning og behandling af patienter med mulig svær astma. [Asthma - Severe - Investigation and treatment of patients with possible severe asthma]. Available from: https://https://lungemedicin.dk/astma-svaer-udredning-og-behandling-af-patienter-med-mulig-svaer-astma/. Accessed December 29, 2021.
- Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother. 2009;43(1):36–44. doi:10.1345/aph.1K671
- Kang HR, Song HJ, Nam JH, et al. Risk factors of asthma exacerbation based on asthma severity: a nationwide population-based observational study in South Korea. BMJ Open. 2018;8(3):e020825. doi:10.1136/bmjopen-2017-020825
- Jensen FF, Håkansson KEJ, Nielsen BO, Weinreich UM, Ulrik CS. Self-reported vs. objectively assessed adherence to inhaled corticosteroids in asthma. Asthma Res Pract. 2021;7(1):1–9. doi:10.1186/s40733-021-00072-2
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi:10.1111/j.1524-4733.2007.00213.x
- Stirratt MJ, Dunbar-Jacob J, Crane HM, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med. 2015;5(4):470–482. doi:10.1007/s13142-015-0315-2
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
- Schatz M, Sorkness CA, Li JT, et al. Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117(3):549–556. doi:10.1016/j.jaci.2006.01.011
- Athanazio R, Carvalho-Pinto R, Fernandes FLA, et al. Can severe asthmatic patients achieve asthma control? A systematic approach in patients with difficult to control asthma followed in a specialized clinic. BMC Pulm Med. 2016;16(1):1–8. doi:10.1186/s12890-016-0314-1
- Dewitte K, Fierens C, Stöckl D, Thienpont LM. Application of the bland–Altman plot for interpretation of method-comparison studies: a critical investigation of its practice. Clin Chem. 2002;48(5):799–801. doi:10.1093/clinchem/48.5.799
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi:10.1016/j.jcm.2016.02.012
- Williams LK, Peterson EL, Wells K, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011;128(6):1185. doi:10.1016/j.jaci.2011.09.011
- Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2). doi:10.1002/14651858.CD000011.pub3
- Laforest L, Belhassen M, Devouassoux G, Didier A, Ginoux M, Van Ganse E. Long-term inhaled corticosteroid adherence in asthma patients with short-term adherence. J Allergy Clin Immunol Pract. 2016;4(5):890–899.e2. doi:10.1016/j.jaip.2016.07.008
- Covvey JR, Mullen AB, Ryan M, et al. A comparison of medication adherence/persistence for asthma and chronic obstructive pulmonary disease in the United Kingdom. Int J Clin Pract. 2014;68(10):1200–1208. doi:10.1111/ijcp.12451
- Bårnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respir Care. 2015;60(3):455–468. doi:10.4187/respcare.03200
- Jordan A, Sivapalan P, Jensen J-U. Does inhaled corticosteroid use affect the risk of COVID-19-related death? Breathe. 2021;17(1):200275. doi:10.1183/20734735.0275-2020
- Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: the medication adherence report scale for asthma. Ann Allergy Asthma Immunol. 2009;103(4):325–331. doi:10.1016/S1081-1206(10)60532-7
- Skurtveit S, Selmer R, Tverdal A, Furu K. The validity of self-reported prescription medication use among adolescents varied by therapeutic class. J Clin Epidemiol. 2008;61(7):714–717. doi:10.1016/j.jclinepi.2007.11.013
- Laforest L, El Hasnaoui A, Pribil C, et al. Asthma patients’ self-reported behaviours toward inhaled corticosteroids. Respir Med. 2009;103(9):1366–1375. doi:10.1016/j.rmed.2009.03.010
- Foster JM, Smith L, Bosnic-Anticevich SZ, et al. Identifying patient-specific beliefs and behaviours for conversations about adherence in asthma. Intern Med J. 2012;42(6):e136–e144. doi:10.1111/j.1445-5994.2011.02541.x
- Kildemoes HW, Sørensen HT, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi:10.1177/1403494810394717
- Giavarina D. Understanding Bland Altman analysis. Biochem Med. 2015;25(2):141–151.
- Plaza V, Fernández-Rodríguez C, Melero C, et al. Validation of the “Test of the Adherence to Inhalers” (TAI) for asthma and COPD patients. J Aerosol Med Pulm Drug Deliv. 2016;29(2):142–152. doi:10.1089/jamp.2015.1212
- Dima AL, van Ganse E, Laforest L, Texier N, de Bruin M. Measuring medication adherence in asthma: development of a novel self-report tool. Psychol Health. 2017;32(10):1288–1307. doi:10.1080/08870446.2017.1290248
- Matza LS, Park J, Coyne KS, Skinner EP, Malley KG, Wolever RQ. Derivation and validation of the ASK-12 adherence barrier survey. Ann Pharmacother. 2009;43(10):1621–1630. doi:10.1345/aph.1M174
- Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. doi:10.1002/pds.1230