Abstract
Background
Current therapy for allergic bronchopulmonary aspergillosis (ABPA) uses oral corticosteroids, exposing patients to the adverse effects of these agents. There are reports of the steroid-sparing effect of anti-IgE therapy with omalizumab for ABPA in patients with cystic fibrosis (CF), but there is little information on its efficacy against ABPA in patients with bronchial asthma without CF.
Objective
To examine the effects of omalizumab, measured by asthma control, blood eosinophilia, total serum immunoglobulin E (IgE), oral corticosteroid requirements, and forced expiratory volume spirometry in patients with ABPA and bronchial asthma.
Methods
A retrospective review of charts from 2004–2006 of patients treated with omalizumab at an academic allergy and immunology practice in the Bronx, New York were examined for systemic steroid and rescue inhaler usage, serum immunoglobulin E levels, blood eosinophil counts, and asthma symptoms, as measured by the Asthma Control Test (ACT).
Results
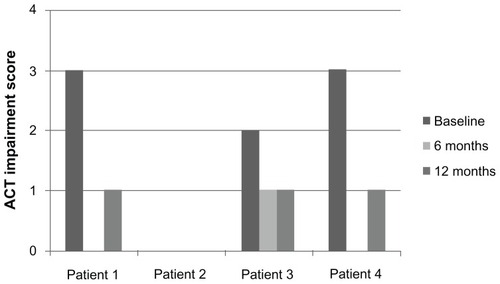
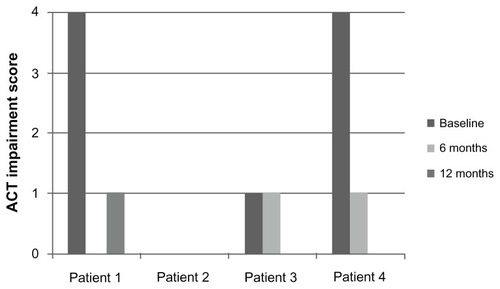
A total of 21 charts were screened for the diagnosis of ABPA and bronchial asthma. Four patients with ABPA were identified; two of these patients were male. The median monthly systemic corticosteroid use at 6 months and 12 months decreased from baseline usage. Total serum IgE decreased in all patients at 12 months of therapy. Pre-bronchodilator forced expiratory vital capacity at one second (FEV1) was variable at 1 year of treatment. There was an improvement in Asthma Control Test (ACT) symptom scores for both daytime and nighttime symptoms.
Conclusions
Treatment with omalizumab creates a steroid-sparing effect, reduces systemic inflammatory markers, and results in improvement in ACT scores in patients with ABPA.
Introduction
Allergic bronchopulmonary aspergillosis (ABPA), with variable prevalence in bronchial asthmatics, is a disease that is difficult to treat.Citation1,Citation2 Rosenberg-Patterson diagnostic criteria for ABPA include asthma, immediate skin reactivity to Aspergillus fumigatus or other Aspergillus species, a serum immunoglobulin (Ig) E level of >417 IU/mL, radiographic evidence of fleeting infiltrates or central bronchiectasis, and elevated serum levels of A. fumigatus specific IgE or IgG.Citation2 Traditional therapy involves systemic corticosteroids and adjunctive antifungal treatment in an attempt to prevent irreversible lung damage.Citation3–Citation5 However, these treatments are often insufficient to control symptoms or lead to intolerable side effects. Since 2001, omalizumab (Xolair, Genentech, Novartis, South San Francisco, CA) has been a useful treatment for moderate to severe allergic asthma, but limited literature exists on its use in ABPA with bronchial asthma in patients without cystic fibrosis (CF).Citation6–Citation8 In 2007, we presented preliminary data on three patients with treatment-resistant ABPA (ie, those with corticosteroid-dependent disease), who were treated with omalizumab with improvement regarding quality of life symptoms, medication usage and pulmonary function testing.Citation9 Recently, case reports of patients with cystic fibrosis and ABPA and adult asthmatics with ABPA have suggested that omalizumab, a recombinant anti-immunoglobulin E, might be effective for treating acute exacerbations and reducing systemic corticosteroid requirements.Citation7–Citation13 We sought to clarify whether omalizumab is a useful adjunctive therapy for adult asthmatics with ABPA.
Methods
Design
We conducted a retrospective chart review of all asthmatic patients treated with omalizumab from 2004–2006 at two allergy and immunology clinics in the Bronx, NY, looking for those that fulfilled the diagnostic criteria for ABPA. All patients were treated by the same board certified allergist and immunologist before and after omalizumab therapy was initiated. The chart review was performed by two physicians (GH and JC). Charts were not screened for patients with cystic fibrosis as the clinical features were not present.
Patients
Patients were considered to have ABPA if they fulfilled all of the following criteria: a history of asthma, positivity to Aspergillus fumigatus on skin prick test or intradermal skin testing, a total serum immunoglobulin E (IgE) of > 417 IU/mL, and radiographic findings of either fleeting chest x-ray infiltrates or evidence of bronchiectasis on chest computed topography (CT). Patients were evaluated for stage of disease using the categories proposed by Patterson et al: acute (stage I), remission (stage II), exacerbation (stage III), corticosteroid-dependent asthma (stage IV), and fibrotic (stage V).Citation3
Treatment
All patients received omalizumab dosage and dosing intervals based on package insert guidelines, except patients with IgE levels > 700 IU/L, who were given the maximum dosage of omalizumab, 375 mg every 2 weeks. No dosing adjustments were made throughout the treatment period.
Outcome measures
We collected data at three points: baseline (4 weeks prior to starting omalizumab), 6 months (months 1–6), and 12 months (months 7–12) after treatment with omalizumab. We collected data on: (1) medication usage, including systemic steroid and rescue medication use, (2) serum IgE at baseline and after 12 months of treatment, (3) eosinophil levels at baseline and after 12 months of treatment, and (4) quality of life symptoms at baseline, 6 months, and 12 months of treatment. Values after 6 and 12 months of treatment were compared to baseline values. Quality of life scores were taken from self-reported answers to the Asthma Control Test (ACT).Citation14
Symptom scores
Daytime symptoms and nighttime symptoms were rated as none of the time, some of the time, most of the time, and all of the time. These answers were converted into numerical scores of 0, 1, 2, or 3, respectively. Rescue inhaler usage was based on patient reporting at baseline, 6 months, and 12 months of treatment. Systemic steroid usage was based on patient reports collected from the medical records at baseline, 6 months, and 12 months of treatment. Average monthly steroid use was calculated based on an assumption of a 30-day month. For example, if a patient was taking a total of 30 mg of corticosteroids daily for 3 months and 10 mg daily for 3 months (30 mg × 30 days × 3 months) + (10 mg × 30 days × 3 months)/6 months = 600 mg, the monthly average would be 600 mg.
Statistical Analysis
ANOVA and paired t-tests were performed using Microsoft Excel.Citation15
Results
Patient demographics and clinical characteristics at baseline
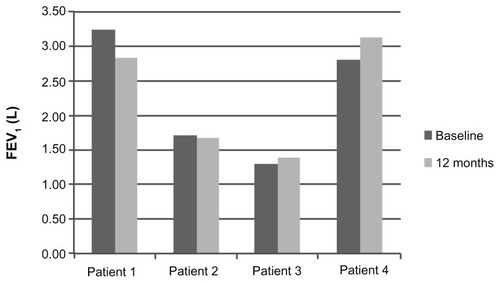
Twenty-one charts were reviewed and four patients satisfying the diagnostic criteria for ABPA were identified. Patient demographics and clinical characteristics are summarized in . Mean age was 50.5 years (range 44–58) and 50% were male. All patients were stage IV ABPA (corticosteroid-dependent). Patients, on average, had been followed for 16 months by the same allergist/immunologist and/or pulmonologist before starting omalizumab. Corticosteroid doses were stable prior to initiating omalizumab. All records from both physicians were reviewed. All four patients had treatment failure with systemic steroids; two had treatment failure with itraconazole. All patients had positive skin prick and/or intradermal testing to A. fumigatus or RAST testing. Baseline medications included inhaled corticosteroids and long acting beta-agonists combinations, leukotriene receptor antagonists, and leukotriene inhibitors. All baseline medications were continued throughout treatment. Patient 2 had ground glass opacities on CT scan; the other patients (1, 3, and 4) had evidence of fleeting infiltrates on chest radiography or chest CT. Six months prior to initiating omalizumab, the average serum IgE was 1730 IU/L (range = 565–4362 IU/L); the average pre-bronchodilator FEV1 at baseline was 2.28 L (range 1.3–3.28 L), and the average eosinophil level was 0.48/mm3 (0.4–0.6/mm3). All patients were treated with 375 mg every two weeks.
Table 1 Patient demographics prior to starting omalizumab
Changes in medication use
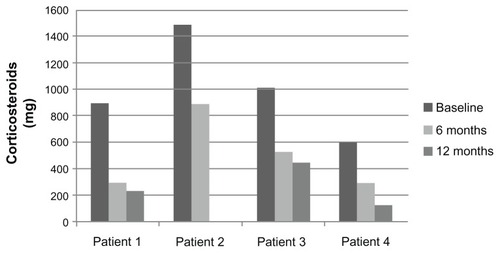
Average total monthly systemic steroid use after 6 months and 12 months of omalizumab decreased in all patients (). Average monthly steroid use at baseline, 6 months, and 12 months was 1002 mg, 506 mg, and 206 mg (P = 0.01). There was a 67%, 40%, 48%, and 50% reduction in total monthly corticosteroid use from baseline to 6 months, and 20%, 99%, 14%, and 58% reduction in total monthly steroid use from 6 months to 12 months in patients 1, 2, 3, and 4, respectively.
Figure 1 Average monthly systemic steroid use based on patient records 6 months prior to initiation of omalizumab, at 6 month point and at 12 months of therapy (P = 0.01).
Rescue inhaler use by the patients was variable. Patient 1 did not use any rescue inhaler, while patient 2 used it often. In contrast to decreased oral corticosteroid use, rescue inhaler use only changed significantly in the one high rescue inhaler-using patient (patient 2). In this patient, at 6 and 12 months after initiation of omalizumab, average weekly rescue inhaler use decreased from 49 times per month to 0; there was no significant change in usage by the other patients.
Changes in asthma symptoms and lung function
Along with the decrease in oral corticosteroid use, daytime and nighttime asthma symptoms, as measured by the ACT, improved after omalizumab. Patients 1, 3, and 4 reported significant improvement in both daytime and nighttime symptom ACT scores ( and ). There was no consistent effect on pre-bronchodilator FEV1 at 12 months ().
Changes in inflammatory markers
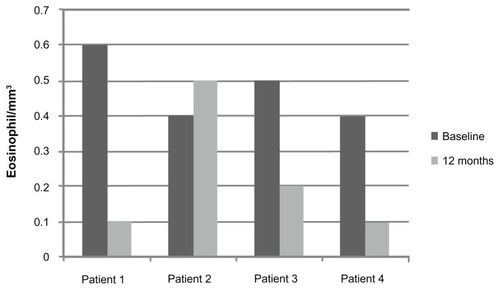
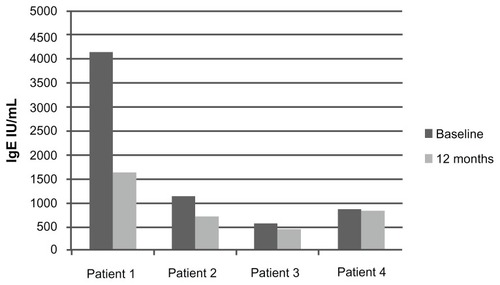
Total blood eosinophil counts and serum IgE were measured before and after approximately 1 year of treatment with omalizumab. Eosinophil counts decreased after 12 months of treatment in three of the patients (patients 1, 3, and 4). Mean eosinophil counts at 12 months decreased by 38% to 0.2/mm3 (range 0.1–0.5/mm3). These changes approached statistical significance (P = 0.06) ().
Total serum IgE at 12 months of treatment decreased in all four patients, going from a mean of 1730 IU/L at baseline (range 565–4362 IU/L) to a mean of 914 IU/L (range 466–1638 IU/L) after 12 months of treatment (). However, these changes were not significant (P = 0.40).
Discussion
These results indicate that omalizumab treatment for 12 months may offer a corticosteroid-sparing treatment option and improvement in asthma symptoms, as measured by the ACT. There was also a concomitant reduction in two inflammatory markers: blood eosinophilia and total serum IgE levels. In ABPA, symptoms and inflammatory marker levels are significantly improved by oral corticosteroid treatment,Citation16, Citation17 so these observed reductions in asthma symptoms and inflammatory markers are even more meaningful, since they were associated with a decrease in oral corticosteroid use by all four patients.
While no data exists on the incidence of adverse effects from oral corticosteroids in patients with ABPA, the side effects of increased diabetes, cataracts/glaucoma, hypertension, risk of fractures, immunosuppression, and gastrointestinal complaints are important concerns for patients on long term systemic corticosteroids. Therefore, the corticosteroid-sparing effect of omalizumab in these patients is a clinically relevant finding.
There are other reports of improved control of ABPA in adult asthmatics with the use of omalizumab. Perez-de-llano et al followed 18 patients with ABPA (16 adult asthmatics with ABPA and 2 with CF and ABPA) for a median of 16 weeks. They showed improvements in daily asthma symptoms, improved FEV1, and a reduction in oral corticosteroid usage.Citation7 Tillie-Leblond et al followed 16 adult asthmatics with ABPA for 12 months and showed a reduction in asthma exacerbations and reduced oral corticosteroid usage.Citation8
Unlike previously described patients with cystic fibrosis and ABPA,Citation10–Citation13 the improvement in FEV1 and quality of life symptom scores in our patients were not as immediate or dramatic. In reports on the use of omalizumab in patients with cystic fibrosis and ABPA, omalizumab was administered in most cases during an acute exacerbation and continued for up to 12 months. These patients may have had a better baseline FEV1 or there may have been a different pathophysiology of ABPA and omalizumab in patients with cystic fibrosis and ABPA as compared to ABPA and bronchial asthma.
The significance of the observed decrease in serum IgE is unclear. Serum IgE is typically used as a marker of disease exacerbation in ABPA, with a doubling indicating an exacerbation. Omalizumab specifically binds to the same portion of the Cɛ3 domain of IgE involved in binding to the high-affinity IgE receptor, thus blocking binding of specific IgE on mast cells and basophils, preventing degranulation. During treatment, free IgE levels in serum decline rapidly while bound anti-IgE-IgE complexes result in elevations of total IgE. Because of this phenomenon, serum IgE levels are generally not considered to be a reliable marker of a clinical response once therapy is initiated.Citation17,Citation18 However, in patients with severe asthma treated with omalizumab, Slavin et al report positive correlations between asthma symptoms and total serum IgE levels.Citation19 In another report, Lin et al also suggest that serum IgE in patients treated with omalizumab is still an important marker of disease activity.Citation20 It is unknown whether the reduction in total serum IgE and eosinophilia is part of the natural course of ABPA or a marker for successful treatment with omalizumab. The decrease in eosinophil levels is consistent with what has been previously reported as an effect of omalizumab.
Using a cutoff total IgE value of >417 kU/L, rather than the more stringent value of 1000 kU/L, as proposed by Agrawal, may have led to an over diagnosis of ABPA in our study population.Citation21 It is difficult to know what the true IgE level was, given that all patients were on oral corticosteroids at the time of measurement.
ABPA is a chronic disease with episodes of remission and exacerbation. Our treatment period of 1 year may have coincided with periods of disease quiescence. Patients 1, 3, and 4 are currently cared for by the same allergist and immunologist. Patient 3 continues omalizumab therapy. He required one burst of corticosteroids in December 2011. Patients 1 and 4 stopped omalizumab after 1 year, secondary to problems with insurance coverage. Both are currently on oral corticosteroids to manage their symptoms. Patient 1 now requires prednisone 5–20 mg daily to control symptoms. Patient 4 is maintained on 10 mg. Patient 2 was lost to follow up after 1 year; efforts to reach him were unsuccessful.
Questions remain about the effective dosage and the utility of omalizumab in asthmatics with ABPA. Patients 1, 2, and 4 had higher levels of baseline IgE (<700 IU/L) than is recommended for omalizumab therapy. Patients with such serum levels may require higher doses of omalizumab to achieve complete reduction of symptoms.
One use of systemic steroids in ABPA is to prevent the progression of disease to permanent fibrosis of the lungs. It is unclear if omalizumab has a similar effect. Our patients did not have repeat chest x-rays or CTs after the initiation of omalizumab to determine whether there was resolution or progression of the radiographic findings, but it would be interesting to determine whether there was improvement in this disease marker.
Omalizumab seems to represent a potential steroid-sparing, alternative treatment for patients with ABPA. However, we recognize that interpretation of these results is limited by the inherent problems associated with a retrospective chart review, by a lack of complete data collection, and by the small number of patients studied. Future studies including randomized double blind placebo trials are needed to clarify the true beneficial effects of omalizumab in the treatment of ABPA.
Acknowledgments
Dr Jennifer Collins, MD organized the data and was the primary author for this paper. Dr G deVos, MD, MS contributed to statistical analysis and revisions. Drs G Hudes, MD, PhD and D Rosenstreich, MD provided input into the organization of this paper, chart review, editorial advice, and information on patients with allergic bronchopulmonary aspergillosis. Dr Gopei Yu provided statistical advice. Drs Vivian Chou and Anita Gewurz provided editorial advice and information regarding one of their patients with ABPA who was treated with omalizumab.
Disclosure
The authors report no conflicts of interest in this work.
References
- AgarwalRAgarwalANGuptaDJindalSKAspergillus hypersensitivity and allergic bronchopulmonary aspergillosis in patients with bronchial asthma: systematic review and meta-analysisInt J Tuberc Lung Dis2009893694419723372
- GreenbergerPAPattersonRAllergic bronchopulmonary aspergillosis and the evaluation of the patient with asthmaJ Allergy Clin Immunol1988816466503356845
- PattersonRGreenbergerPARadinRCRobertsMAllergic bronchopulmonary aspergillosis: staging as an aid to managementAnn Intern Med19829632862917059089
- WarkPAHensleyMJSaltosNAnti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trialJ Allergy Clin Immunol2003111595295712743557
- JudsonMAStevensDACurrent pharmacotherapy of allergic bronchopulmonary aspergillosisExpert Opin Pharmacother2001271065107111583057
- Allergic Bronchopulmonary Aspergillosis [medical letter]South San Francisco, CAGenetech Novartis2011
- Pérez-de-LlanoLVenneraMParraAEffects of omalizumab in Aspergillus-associated airway diseaseThorax20116653954021398373
- Tillie-LeblondIGermaudPLeroyerCAllergic bronchopulmonary aspergillosis and omalizumabAllergy2011661254125621517902
- CollinsJHudesGRosenstreichDAllergic Bronchopulmonary Aspergillosis Treated Successfully with Omalizumab: Three Case Reports [oral presentation]Ann Allergy Asthma Immunol2008100Suppl 1A3A9718268819
- KanuAPatelKTreatment of allergic bronchopulmonary aspergillosis (ABPA) in CF with anti-IgE antibody (omalizumab)Pediatr Pulmonol200843121249125119009619
- LebecquePLeonardAPiletteCOmalizumab for treatment of ABPA exacerbations in cystic fibrosis patientsPediatr Pulmonol20094451619382216
- Van der EntCKHoekstraHRijkersGTSuccessful treatment of allergic bronchopulmonary aspergillosis with recombinant anti-IgE antibodyThorax200762327627717329558
- ZirbesJMMillaCESteroid-sparing effect of omalizumab for allergic bronchopulmonary aspergillosis and cystic fibrosisPediatr Pulmonol200843660761018433040
- NathanRASorknessCAKosinskiMDevelopment of the asthma control test: a survey for assessing asthma controlJ Allergy Clin Immuno200411315965
- Excel (Part of Microsoft Office) [computer program]Microsoft2007
- MarchantJLWarnerJOBushARise in total IgE as an indicator of allergic bronchopulmonary aspergillosis in cystic fibrosisThorax1994491010027974292
- RickettiAJGreenbergerPAPattersonRSerum IgE as an important aid in management of allergic bronchopulmonary aspergillosisJ Allergy Clin Immuno198474168
- BusseWCorrenJLanierBQOmalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthmaJ Allergy Clin Immunol2001108218419011496232
- SlavinRGFerioliCTannenbaumSJMartinCBloggMLowePJAsthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrationsJ Allergy Clin Immunol20091231107113e319130931
- LinRYSethiSBhargaveGAMeasured immunoglobulin E in allergic bronchopulmonary aspergillosis treated with omalizumabJ Asthma201047894294520831464
- AgrawalRAllergic bronchopulmonary aspergillosisChest2009135380582619265090