Abstract
Background
Cross-reacting carbohydrate determinants (CCD) exist in some pollen and food allergens, but they do not contribute to allergic symptoms. However, CCD can induce specific IgE (sIgE) production and may lead to incorrect allergen diagnosis and treatment. CCD inhibitor is a specific antibody adsorbent which can preclude CCD from binding to sIgE. Currently, the data of CCD inhibition in allergen sIgE test are limited.
Methods
The allergic patients with positive skin prick reactions to two or more pollen and/or food allergen extracts were included in our study. Their sera were obtained and sIgE was tested with an allergen panel that included 29 single and mixed allergens (MEDIWISS Analytic GmbH, China) before and after CCD inhibition. The changes of sIgE against these allergens and the correlations of sIgEs to clinical symptoms were analyzed.
Results
A total of 44 patients were included and 36 (81.82%) of those were multi-sensitized to house dust mites and pollen allergens based on skin prick tests. The sIgE levels and positive rates against most pollen and food allergens were significantly lower after CCD inhibition. The sIgE levels of pollen were positively correlated to those of food allergens before CCD inhibition. However, these correlations were weakened or no longer existed after CCD inhibition. The sIgE against pollen and food allergens showed significantly higher consistency with clinical symptoms after CCD inhibition.
Conclusion
Cross-sensitization caused by CCD is widespread in pollen and food. CCD inhibition test can improve the diagnostic accuracy of pollen and food allergy.
Introduction
Allergen provocation test (APT) has been regarded as “golden standard” for the etiological diagnosis of allergic diseases. However, APT is not always available in many countries including China. In this case, the diagnosis of allergic diseases highly relies on allergen skin test and/or specific IgE (sIgE).Citation1 Sometimes, patients present a negative history of allergy symptoms but positive allergen tests, which may have two explanations. One is that the patients have been “sensitized” by the allergens, but without symptoms of “allergy”.Citation2 Another explanation is that some components of allergen extracts have no clinical relevance, but can cause false-positive results of allergen sIgE tests, such as “cross-reacting carbohydrate determinants (CCD)”.Citation3
CCD is a carbohydrate residue with simple structure that exists widely in pollen, food, insect and latex allergen extracts.Citation4 Studies have revealed that CCD-sIgE can be found in 20% of allergic patientsCitation5 and up to 35% in young patients.Citation6 The false-positive results caused by CCD may lead physicians to provide erroneous avoidance measures and treatment plans to patients who are not really allergic to certain allergens.Citation7 Therefore, it is crucial to identify CCD-sIgE for the purpose of accurate diagnoses of allergic diseases.
Currently, there are two methods to identify the presence of CCD-sIgE. One is to screen CCD-sIgE directly through component-resolved diagnosis (CRD),Citation8 and the other is to use CCD inhibitor and then to identify the allergens sIgE subsequently.Citation9 However, the expense of CRD is relatively high; moreover, when the sIgE of allergen extracts and CCD are positive simultaneously, it is difficult to figure out whether the positive results are caused by CCD or by other allergen components. One solution proposed for the problem was to remove anti-CCD IgE with immobilized CCDs, but the idea was eventually abandoned because it was simply too laborious for routine application.Citation6,Citation10 Compared with these methods, CCD inhibition test provides a more economical and practical alternative. Studies have shown that CCD inhibition test is a simple pre-treatment method that can remove CCD-sIgE from serum and theoretically improve the accuracy of diagnostic tests in vitro.Citation10,Citation11 Some commercial tests, including CCD inhibitor such as ALEX, have been applied in clinical practice and showed the advantages in reducing false-positive IgE results without impact on diagnostic sensitivity towards relevant allergens.Citation12
Despite the advantages of CCD inhibition tests, the clinical relevance of the changes of sIgE levels against common airborne and food allergens before and after CCD inhibition remain unclear. In addition, the consistency between symptoms and sIgE levels after CCD inhibition still needs further research. In this study, we tried to investigate the clinical value of CCD inhibition test in the precise diagnosis of allergic diseases by focusing on the changes of sIgE levels against airborne and food allergens before and after CCD inhibition and their correlations to clinical symptoms.
Materials and Methods
Patients
This study was conducted from January 2021 to October 2021 in the allergy department of Tongji Hospital, Wuhan, China. All patients fulfilled the following inclusion criteria: 1) diagnosed with at least one of the following: allergic rhinitis (AR), allergic asthma (AA), atopic dermatitis (AD), urticaria, or IgE-mediated food allergy according to current guidelines, including Allergic Rhinitis and its Impact on Asthma (ARIA),Citation13 Global Initiative for Asthma (GINA) (http://ginasthma.org/), Diagnostic criteria for Atopic dermatitis in China,Citation14 Guideline for diagnosis and treatment of urticaria in China (2018),Citation15 and food allergy guidelines.Citation16 2) showed positive skin prick tests (SPT) to two or more pollen and/or food allergen extracts. 3) recurrent symptoms in spring and/or autumn, or after ingesting suspicious food. The exclusion criteria were: 1) had received allergen immunotherapy within 6 months or omalizumab injections within 1 month; 2) had a history of anaphylaxis within 4 weeks; 3) had a history of chronic diseases other than allergic diseases or immune deficiency diseases. The demographic data including gender, age, course of disease and SPT and sIgE results of allergen before and after CCD inhibition were recorded. On the day when the patients were recruited, all the patients completed a questionnaire including the following questions: What kinds of symptoms are you suffering from? Do the symptoms occur seasonally or perennially? When and in what situation will the symptoms become worsen? Do you have any symptoms within one hour after intake food? Specify all the suspected food. The study complied with the Declaration of Helsinki and had been approved by the independent Ethics Committee of Tongji Hospital (TJ-IRB20220202). Each included patient or his/her legal guardian had signed a written informed consent.
SIgE Before and After CCD Inhibition
Serum samples from patients were collected to detect sIgE levels of the following 29 allergen extracts (including single and mixed allergen): Dermatophagoidespteronyssinus (d1), Dermatophagoides farinae (d2), Blomia tropicalis (d201), cat dander (e1), dog dander (e5), cockroach (i6), silk (k74), ragweed (w1), Artemisia (w6), Humulus (w22), Chenopodium album/redroot amaranth (w10-w14), Juniperus formosana/birch (t6-t3), Platanus acerifolia/ash (t11-t15), alder/poplar/willow/beech/oak/walnut (txchn1), lipnica/ryegrass/timothy (gxchn), maple/mulberry/acacia/elm/cypress/broussonetia (txchn2), Aspergillus fumigatus (m3), Candida/Penicillium spot/mycospora/Alternaria/Aspergillus niger (mx4), egg yolk (f75), egg white (f1), milk (f2), peanut/soybean (f13-f14), sesame (f10), wheat/buckwheat (f4-f11), cashews/pistachios/hazelnuts/almonds/walnuts (fnutchn2), beef/lamb (f27-f88), fish (f3), shrimp/crab (f23-f24), peach/apple/mango/lychee/strawberry (ffruchn) (MEDIWISS Analytic GmbH, China). The allergen extracts panel covered airborne allergens such as house dust mites (HDMs), tree and weed pollen, pet dander and mold, as well as common food allergens. Positive sIgE were categorized into 6 classes: class 1 (≥0.35 to <0.70 IU/mL), class 2 (≥0.70 to <3.50 IU/mL), class 3 (≥3.50 to <17.50 IU/mL), class 4 (≥17.50 to <50 IU/mL), class 5 (≥50 to <100 IU/mL), and class 6 (≥100 IU/mL).
After the sIgE detection was completed, the CCD antibody adsorbent was taken out and restored to room temperature of 20–22°C, and 300ul serum was added to the centrifugal tube containing the lyophilized powder of 2mg CCD antibody adsorbent, which was mixed with three different glycoproteins containing bromelain (MMXF), horseradish peroxidase (MMUF) and ascorbate oxidase (ASOD). After the adsorbent and serum were fully mixed and dissolved, the mixed serums were re-tested with the same allergen panel. The CCD antibody adsorbent was provided by Shanghai Advanced Clinical Laboratory Science Co., Ltd.
Statistical Analysis
Continuous variables such as age and disease course which were non-normal distribution were represented as median and range interquartile. Quantitative data were compared before and after using paired t-test. The consistency of SPT (positive/negative) with allergic symptoms, and that of sIgE (positive/negative, cut-off value 0.35IU/mL) with allergic symptoms were expressed as percentages and evaluated using kappa statistics, with kappa value >0.4 being regarded as good consistency. Categorical variables were also expressed as percentages and were compared using the χ2 test or Fisher’s Exact test when suitable Spearman rank test was used for correlation evaluation of sIgE before and after CCD inhibition. Statistical analyses were performed using SPSS version 20.0 (IBM, Chicago, IL) and R package version 3.5.1. p<0.05 was considered as statistically significant.
Results
Patient Characteristics
A total of 44 patients with allergic diseases were included, 29 (65.91%) of whom were male, with the median age being 14. Among them, 17 (38.64%) were AR, 10 (22.73%) were AR combined with AA, 8 (18.18%) were AD, 3 (6.82%) were urticaria. The median course of diseases was 4 years and the most frequent allergens according to SPT results were Dermatophagoides pteronyssinus (81.82%), Dermatophagoides farinae (79.55%), Platanus acerifolia/Ash (79.55%), Humulus (79.55%) and tree pollen mixture (77.27%). 81.82% of the patients were multi-sensitized to HDMs and pollen allergens, 97.73% were sensitized to multiple pollen allergens ().
Table 1 Clinical Characteristics of the Included Patients
Changes of sIgE Levels Before and After CCD Inhibition
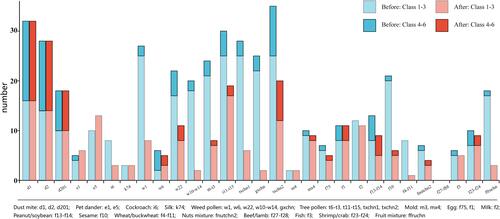
There were no significant differences in the sIgE levels against HDMs, pet dander and silk before and after CCD inhibition, but sIgE level of cockroach decreased significantly after CCD inhibition (p = 0.008). For autumn weed pollen, sIgE levels against ragweed, Humulus, Chenopodium album/redroot amaranth after CCD inhibition significantly decreased (all p < 0.01) except Artemisia. SIgE level against grass pollen mixture containing lipnica/ryegrass/timothy also decreased significantly after CCD inhibition (p < 0.001). For spring tree pollen, sIgE levels against formosana/birch, Platanus acerifolia/ash and tree pollen mixture after CCD inhibition significantly decreased (all p < 0.001). For mold allergens, the sIgE level of aspergillus fumigatus did not change significantly after CCD inhibition, but the level of mold mixture decreased significantly (p = 0.009). For food allergens, sIgEs against egg white, milk, beef/lamb, fish, shrimp/crab showed no differences after inhibition, but sIgE levels of peanut/soybean, sesame, wheat/buckwheat, nut mixture and fruit mixture decreased significantly (all p < 0.05) ().
Figure 1 sIgE level of the allergen extracts panel before and after CCD inhibition.
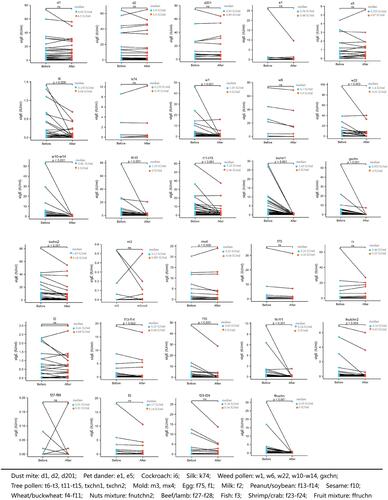
The positive rates of sIgE to autumn weed pollen including ragweed, Humulus, Chenopodium album/redroot amaranth and grass pollen mixture significantly decreased after CCD inhibition (all p < 0.05). For spring tree pollen, the positive rate of sIgE to Juniperus formosana/birch, Platanus acerifolia/ash and tree pollen mixture significantly decreased after CCD inhibition (all p < 0.05). For food allergens, the positive rates of sIgE to sesame, wheat/buckwheat and fruit mixture significantly decreased after CCD inhibition (all p < 0.05) (). The concentration of sIgE against weed pollen, tree pollen and food allergens decreased and most of them were in low concentration (class 1–3) after CCD inhibition ().
Table 2 Positive Rate of sIgE Before and After CCD Inhibition
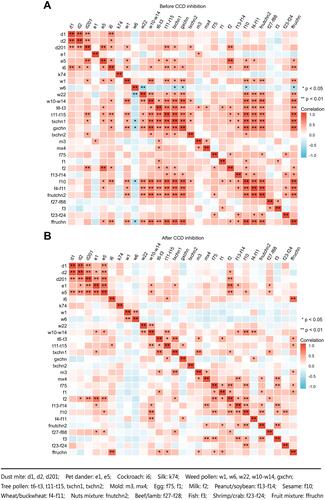
Correlations of sIgE Levels Before and After CCD Inhibition
Before CCD inhibition, there were widely positive correlations among the levels of sIgE against HDMs, Blomia tropicalis, pet dander, cockroaches, and positive correlation also existed extensively between the sIgE against weed pollen, tree pollen and food allergens. After CCD inhibition, these correlations were generally weakened; that is, only weed pollen such as Chenopodium album/redroot amaranth remained positively correlated with spring tree pollen such as Platanus acerifolia/ash and food allergens such as peanut/soybean, sesame, wheat/buckwheat. After CCD inhibition, the correlation among spring tree pollen such as formosana/birch and weed pollen no longer existed, and only positive correlation existed between formosana/birch and food allergens such as egg white and fruit mixture. Platanus acerifolia/ash was positively correlated with grass pollen mixture and fruit mixture, tree pollen mixture was positively correlated with food allergens such as milk, and grass pollen mixture was positively correlated with wheat/buckwheat ().
Consistency of SPT, sIgE Against Pollen and Food and Allergic Symptoms Before and After CCD Inhibition
In general, the SPT showed high consistency with sIgE before CCD inhibition. The consistent rates of weed pollen including ragweed, Artemisia, Humulus, spring tree pollen including Platanus acerifolia/ash, tree pollen mixture, and food allergens including egg white, milk, and fruit mixture decreased after CCD inhibition ().
Figure 4 Consistency of SPT, sIgE against pollen and food and allergic symptoms before and after CCD inhibition (A–D). (A) Consistency of SPT and sIgE against pollen and food before and after CCD inhibition. (B) Consistency of symptoms in spring and SPT, symptoms and sIgE against pollen and food before and after CCD inhibition. (C) Consistency of symptoms in autumn and SPT, symptoms and sIgE against pollen and food before and after CCD inhibition. (D) Consistency of symptoms after ingest food and SPT, symptoms and sIgE against pollen and food before and after CCD inhibition.
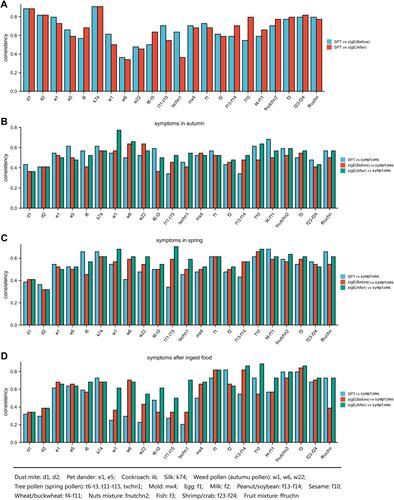
When we took recurrent autumn symptoms as the criterion of weed and/or grass pollen allergy, the allergic symptoms and sIgE against ragweed and Artemisia showed higher consistency after CCD inhibition, whereas sIgE against Humulus showed a lower consistency after inhibition ().
Similarly, when we took recurrent spring symptoms as the criterion of tree pollen allergy, the allergic symptoms showed higher consistencies with sIgEs against tree pollen after CCD inhibition ().
When we took immediate occurrence of allergic symptoms after food ingestion as the criterion of food allergy, the allergic symptoms and sIgE against most food showed higher consistencies after CCD inhibition, these food included egg white, fish and sesame, peanut/soybean, wheat/buckwheat, crab/shrimp, but except milk ().
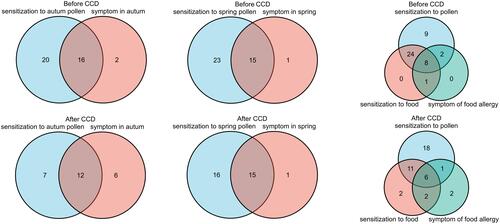
Symptoms and Sensitization to Pollen and Food Before and After CCD Inhibition
Before CCD inhibition, there were 36 patients sensitized to autumn pollen (positive sIgE), among whom 16 (44.44%) had symptoms in autumn; after CCD inhibition, 19 patients sensitized to autumn pollen and 12 (63.16%) had symptoms.
Before CCD inhibition, there were 38 patients sensitized to spring pollen, among whom 15 (39.47%) had symptoms in spring, after CCD, 31 patients sensitized to spring pollen and 15 (48.39%) had symptoms.
Before CCD inhibition, 33 patients were sensitized to food, among whom 9 (27.27%) patients showed symptoms after food ingestion, 32 patients were simultaneously sensitized to pollen and food, and 8 (25%) patients had symptoms after food ingestion. After CCD inhibition, there were 21 patients with food sensitization, 8 (38.10%) showed symptoms after food ingestion, 17 patients were simultaneously sensitized to pollen and food, 6 (35.29%) had symptoms after food ingestion ().
Discussion
Our study included patients with two or more pollen and/or food allergens according to positive SPT results. Interestingly, the most prevalent allergen in our study was HDMs, and the majority of our patients showed positive skin reactions to pollen and HDMs simultaneously, indicating that HDMs were still the major allergen in this area, which was consistent with our previous study.Citation17 The sensitization profile of SPT also showed that nearly 80% of the patients had positive reactions to pollen and food at the same time, which suggested that there might exist extensive cross-sensitization or co-sensitization between pollen and food allergens. Widespread cross-sensitization existing in pollen and food can lead to symptoms of food allergy, such as pollen-food allergy syndrome (PFAS), a symptom that generally occurs in the oropharynx when the individual with pollen allergy eats fresh fruits, nuts or vegetables.Citation18 However, this cross-sensitization may also be caused by CCD which can be widely found in pollen, food, insect venom and latex allergens, but it does not contribute to creating symptoms.Citation19 Therefore, screening or inhibition of CCD is important for the correct diagnosis and subsequent treatment of allergic diseases such as allergen avoidance and immunotherapy.
In our study, we found that the sIgE levels of most tree pollen, grass pollen, weed pollen and food allergens (including nut and fruit) were significantly lower after CCD inhibition, which, being basically consistent with the research of Holzweber, FCitation6 suggested that CCD exists in these allergen extracts. As a consequence, the positive rates of sIgEs to these pollen and food allergens also decreased significantly after CCD inhibition. Considering that CCD may not cause any symptoms, we believe that CCD inhibition has great advantage to figure out the true allergy patients in clinical practice. We also noticed that in most patients sensitized to multiple pollen or food in our region, the sIgE levels were low both before and after CCD inhibition, which was similar to the study launched by Luo et al in South China.Citation11 There were studies showing that sIgE level might not correlate with the severity of allergic diseases.Citation20 However, low sIgE level always suggested the individual might be asymptomatic.Citation21,Citation22 The relatively low sIgE level in our patients implied they might not really be allergic to the pollen or food, while CCD might lead to false-positive results in allergen IgE tests. Thus, it is essential to eliminate the potential impact of CCD on sIgE analysis, especially for those allergens that are already known to contain CCD components.
Apart from the fact that the CCD inhibition test decreased sIgE positive rates and levels, the correlation analysis also confirmed the prevalent existence of CCD among pollen and food. Before CCD inhibition, there were widely positive correlations of sIgE levels between pollen and food allergens. However, after CCD inhibition, these correlations were weakened or no longer existed, indicating that the correlations of sIgEs between pollen and food were mostly caused by CCD. Noticeably, we did not find the correlations of sIgE between birch and fruit and nuts, as well as Artemisia and peach after CCD inhibition, which had been reported to have cross-reactivity in patients with PFAS due to the similarity of allergen components other than pan-allergen such as CCD.Citation23–Citation26 A possible explanation might be that the nuts and fruit allergens we used in the study were mixed allergens instead of single allergen.
We found that sIgE of some weed pollen, tree pollen and fruit mixture before CCD inhibition had a better consistency with SPT compared to that after CCD inhibition, which did not conform to previous studies that suggested that CCD could lead to false-positive sIgE but not to false-positive SPT.Citation27 Our study indicated that SPT could not discriminate the false-positive sIgE caused by CCD. Therefore, for patients with sensitization to multiple-pollen or multiple-food, it is necessary to either test IgE against CCD component or adopt CCD inhibition. However, a few studies suggested that approximately one-third of CCD-sIgE was biologically active and can mediate up to 82% histamine release if stimulated with different glycoproteins,Citation28 and glycoproteins epitopes can induce responses at the cellular level, which implied that CCD IgE may play a role in vivo,Citation29 which might be the reason for the false-positive skin reaction induced by some CCD. Still, the exact mechanism needs further investigation.
Allergen tests coupled with clinical history are the cornerstone of etiological diagnosis for allergic diseases. In our study, we took the clinical history as a criterion to evaluate the diagnostic efficiency of IgE before and after CCD inhibition. We found that sIgE to tree pollen showed better consistency with symptoms after CCD inhibition in the patients with allergic symptoms in spring. In the patients with autumn symptoms, the sIgE to ragweed and Artemisia had better consistency with the symptoms, with the exception of Humulus. For patients with food allergy, we found that sIgE against most plant-derived food such as wheat, peanut and fruits showed better consistency with symptoms after CCD inhibition. These data support the application of CCD inhibition in the diagnosis of pollen and/or food allergic patients. Some kinds of allergen IgEs did not show better consistency with clinical history after CCD inhibition, which may be attributed to the absence of CCD in these allergens. Interestingly, for nut and fruit mixtures, SPT also showed good consistency with symptoms, suggesting that SPT or prick-to-prick test may be helpful in diagnosis of nut and fruit allergy.
Several studies suggested that CCD inhibition is a viable option to mitigate the influence of anti-CCD antibodies and to increase the reliability of allergen IgE tests, particularly in multiple sensitization.Citation30 In our study, we found that CCD inhibition increased the positive rates of IgE detection in truly pollen and/or food allergic patients (based on symptoms), which further demonstrated the importance of CCD inhibition in the diagnosis of seasonal allergic diseases and food allergy. We also found that most patients with food allergy were sensitized to pollen and food at the same time, after CCD inhibition, the number of patients sensitized simultaneously to pollen and food decreased by nearly 50%. However, 35% of the patients still had symptoms when ingesting food, which may be attributed to PFAS caused by cross-proteins other than CCD. Studies have shown that PR10 proteins, profilins, lipid transfer proteins, thaumatin-like proteins, isoflavone reductases, and β-1,3 glucanases are the most common cross-proteins that cause PFAS.Citation31 Therefore, CRD may be required for these patients.
There are some limitations in this study. Firstly, we included patients with multiple pollen or food sensitization, but most patients were sensitized to HDMs, a perennial allergen which may overshadow seasonal symptoms during pollen seasons and cause selection bias.Citation32 Secondly, CRD was not performed in our study, especially for sIgE of allergens with no difference before and after CCD inhibition. Further CRD can identify cross-sensitized component proteins, which is necessary for the diagnosis of PFAS; Thirdly, there were still some disadvantages of CCD inhibition. CCD inhibitor could not remove all the CCD-sIgE antibodies from the serum, according to the preliminary data, the CCD inhibitor used in our study could neutralize 92% but not 100% of the CCD IgEs. In addition, theoretically few patients would have no responses to current CCD inhibitors, but we did not test anti-CCD IgE directly in the study. Finally, the APT was not conducted in our study, and the identification of symptoms was mostly based on the history provided by patients, which may lead to certain diagnostic bias.
Conclusion
Cross-sensitization was widespread in pollen and food, and CCD might be the culprit for this cross-sensitization. CCD existed in pollen and food allergens can lead to the production of anti-CCD specific IgE and cause false-positive test results of the relevant allergens. CCD inhibition test can improve the diagnostic accuracy of seasonal allergic diseases and food allergy.
Acknowledgments
We would like to express our gratitude to Dr Xiubo Zhu for her invaluable assistance throughout the preparation of the original paper.
Disclosure
The authors report no conflicts of interest for this work.
Additional information
Funding
References
- Chen H, Li J, Cheng L, et al. China consensus document on allergy diagnostics. Allergy Asthma Immunol Res. 2021;13(2):177–205. doi:10.4168/aair.2021.13.2.177
- de Vos G. Skin testing versus serum-specific IgE testing: which is better for diagnosing aeroallergen sensitization and predicting clinical allergy? Curr Allergy Asthma Rep. 2014;14(5):430. doi:10.1007/s11882-014-0430-z
- Homann A, Schramm G, Jappe U. Glycans and glycan-specific IgE in clinical and molecular allergology: sensitization, diagnostics, and clinical symptoms. J Allergy Clin Immunol. 2017;140(2):356–368. doi:10.1016/j.jaci.2017.04.019
- Aalberse RC, Koshte V, Clemens JG. Immunoglobulin E antibodies that crossreact with vegetable foods, pollen, and Hymenoptera venom. J Allergy Clin Immunol. 1981;68(5):356–364. doi:10.1016/0091-6749(81)90133-0
- Altmann F. Coping with cross-reactive carbohydrate determinants in allergy diagnosis. Allergo J Int. 2016;25(4):98–105. doi:10.1007/s40629-016-0115-3
- Holzweber F, Svehla E, Fellner W, et al. Inhibition of IgE binding to cross-reactive carbohydrate determinants enhances diagnostic selectivity. Allergy. 2013;68(10):1269–1277. doi:10.1111/all.12229
- Sturm GJ, Jin C, Kranzelbinder B, et al. Inconsistent results of diagnostic tools hamper the differentiation between bee and vespid venom allergy. PLoS One. 2011;6(6):e20842. doi:10.1371/journal.pone.0020842
- Sinson E, Ocampo C, Liao C, et al. Cross-reactive carbohydrate determinant interference in cellulose-based IgE allergy tests utilizing recombinant allergen components. PLoS One. 2020;15(4):e231344. doi:10.1371/journal.pone.0231344
- Hemmer W, Altmann F, Holzweber F, Gruber C, Wantke F, Wohrl S. ImmunoCAP cellulose displays cross-reactive carbohydrate determinant (CCD) epitopes and can cause false-positive test results in patients with high anti-CCD IgE antibody levels. J Allergy Clin Immunol. 2018;141(1):372–381.
- Malandain H, Giroux F, Cano Y. The influence of carbohydrate structures present in common allergen sources on specific IgE results. Eur Ann Allergy Clin Immunol. 2007;39(7):216–220.
- Luo W, Huang H, Zheng P, Zheng J, Sun B. CCD inhibition test can improve the accuracy of the detection of pollen and seed food allergen-specific IgE in Southern China. J Asthma Allergy. 2021;14:439–447. doi:10.2147/JAA.S302920
- Akarsu A, Ocak M, Sahiner UM, Soyer O, Sekerel BE. Multiplex component-based allergen macroarray test is useful to predict clinical reactivity to tree nuts in children. Allergol Int. 2022;71(2):236–247. doi:10.1016/j.alit.2021.10.001
- Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
- Liu P, Zhao Y, Mu ZL, et al. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis. Chin Med J. 2016;129(7):757–762. doi:10.4103/0366-6999.178960
- Centre For Urticaria Research CSOD. Guideline for diagnosis and treatment of urticaria in China (2018). Chin J Dermatol. 2019;3(1):1–5.
- Lopes JP, Sicherer S. Food allergy: epidemiology, pathogenesis, diagnosis, prevention, and treatment. Curr Opin Immunol. 2020;66:57–64. doi:10.1016/j.coi.2020.03.014
- Wang J, Wu Y, Li J, Huang X, Zhu R. Eight aeroallergen skin extracts may be the optimal panel for allergic rhinitis patients in central China. Int Arch Allergy Immunol. 2017;173(4):193–198. doi:10.1159/000479429
- Scheurer S, Wangorsch A, Nerkamp J, et al. Cross-reactivity within the profilin panallergen family investigated by comparison of recombinant profilins from pear (Pyr c 4), cherry (Pru av 4) and celery (Api g 4) with birch pollen profilin Bet v 2. J Chromatogr B Biomed Sci Appl. 2001;756(1–2):315–325. doi:10.1016/S0378-4347(01)00090-1
- Mari A, Iacovacci P, Afferni C, et al. Specific IgE to cross-reactive carbohydrate determinants strongly affect the in vitro diagnosis of allergic diseases. J Allergy Clin Immunol. 1999;103(6):1005–1011. doi:10.1016/S0091-6749(99)70171-5
- Kulis M, Wright BL, Jones SM, Burks AW. Diagnosis, management, and investigational therapies for food allergies. Gastroenterology. 2015;148(6):1132–1142. doi:10.1053/j.gastro.2015.01.034
- Bao Y, Chen J, Cheng L, et al. Chinese guideline on allergen immunotherapy for allergic rhinitis. J Thorac Dis. 2017;9(11):4607–4650. doi:10.21037/jtd.2017.10.112
- Xu Q, Jiang Q, Yang L, et al. IgE and IgG4 Repertoire in asymptomatic HDM-Sensitized and HDM-Induced Allergic Rhinitis patients. Int Arch Allergy Immunol. 2021;182(12):1200–1211. doi:10.1159/000517824
- Ballmer-Weber BK, Hoffmann-Sommergruber K. Molecular diagnosis of fruit and vegetable allergy. Curr Opin Allergy Clin Immunol. 2011;11(3):229–235. doi:10.1097/ACI.0b013e3283464c74
- Hamada M, Kagawa M, Tanaka I. Evaluation of subcutaneous immunotherapy with birch pollen extract for pollen-food allergy syndrome. Asia Pac Allergy. 2021;11(4):e39. doi:10.5415/apallergy.2021.11.e39
- Uotila R, Kukkonen AK, Pelkonen AS, Mäkelä MJ. Cross‐sensitization profiles of edible nuts in a birch‐endemic area. Allergy. 2016;71(4):514–521. doi:10.1111/all.12826
- Ma S, Nie L, Li H, Wang R, Yin J. Component-resolved diagnosis of peanut allergy and its possible origins of sensitization in China. Int Arch Allergy Immunol. 2016;169(4):241–248. doi:10.1159/000446156
- van der Veen MJ, van Ree R, Aalberse RC, et al. Poor biologic activity of cross-reactive IgE directed to carbohydrate determinants of glycoproteins. J Allergy Clin Immunol. 1997;100(3):327–334. doi:10.1016/S0091-6749(97)70245-8
- Foetisch K, Westphal S, Lauer I, et al. Biological activity of IgE specific for cross-reactive carbohydrate determinants. J Allergy Clin Immunol. 2003;111(4):889–896. doi:10.1067/mai.2003.173
- Malandain H. IgE-reactive carbohydrate epitopes–classification, cross-reactivity, and clinical impact. Eur Ann Allergy Clin Immunol. 2005;37(4):122–128.
- Grzywnowicz M, Majsiak E, Gawel J, Miskiewicz K, Doniec Z, Kurzawa R. Inhibition of cross-reactive carbohydrate determinants in allergy diagnostics. Adv Exp Med Biol. 2018;1116:75–79.
- Poncet P, Senechal H, Charpin D. Update on pollen-food allergy syndrome. Expert Rev Clin Immunol. 2020;16(6):561–578. doi:10.1080/1744666X.2020.1774366
- Li J, Sun B, Huang Y, et al. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China. Allergy. 2009;64(7):1083–1092. doi:10.1111/j.1398-9995.2009.01967.x