Abstract
Patients with asthma frequently have comorbid chronic rhinosinusitis (CRS) with or without nasal polyps, increasing disease burden and complicating treatment. These post hoc analyses investigated disease-specific health-related quality of life (HRQoL) and general health status in the randomized, placebo-controlled QUEST study (NCT02414854) in patients treated with dupilumab for moderate-to-severe asthma with comorbid CRS. Patients received 300 mg of dupilumab or placebo every 2 weeks for 52 weeks. CRS HRQoL was assessed by the 22-item Sino-Nasal Outcome Test (SNOT-22; items scored 0–5). The 22 items are categorized into 5 domains (nasal, ear/facial, sleep, function, and emotion), and patients report the top 5 most important items affecting their health. General health status was assessed by Euro-QoL visual analog scale (EQ-VAS). Of 1902 patients, 382 (20.1%) self-reported comorbid CRS; 193 patients receiving dupilumab 300 mg q2w or matched placebo were included in this analysis. At baseline, the most impacted SNOT-22 domain was nasal, and general health status was below population norms. Patients rated “decreased sense of taste/smell,” “nasal blockage,” “cough,” “reduced productivity,” and “wake up tired” as the 5 most important SNOT-22 items affecting their health. Percentage change from baseline in SNOT-22 total score was significantly greater for dupilumab vs placebo at Weeks 24, 36, and 52 (all p < 0.05). Improvements from baseline were significantly greater for dupilumab vs placebo at Week 52 for all SNOT-22 domains (p < 0.05), except emotion. At Week 52, significant changes from baseline with dupilumab vs placebo were observed for all 5 most important SNOT-22 items affecting their health (all p < 0.05). EQ-VAS was significantly improved with dupilumab vs placebo by Week 12, with improvements sustained to Week 52 (all p < 0.01). In patients with moderate-to-severe asthma who self-reported comorbid CRS, dupilumab treatment vs placebo improved CRS-specific HRQoL and general health status.
Introduction
Approximately 20% of patients with asthma have uncontrolled, moderate-to-severe disease despite standard-of-care. These patients are at increased risk for illness, utilize considerable health-care resources,Citation1 and have coexisting type 2 inflammatory conditions. In an international cohort of patients with moderate-to-severe asthma (n = 899), 17% were found to have comorbid chronic rhinosinusitis with nasal polyps (CRSwNP).Citation2 Patients with asthma with comorbid chronic rhinosinusitis (CRS) have a high disease burden, with poorer lung function,Citation3 greater eosinophilic inflammation,Citation3,Citation4 and reduced asthma control,Citation4,Citation5 leading to a phenotype of difficult-to-treat asthma.Citation6 Consequently, these patients also have more severe impairment of disease-specific (asthma control and sinonasal symptoms) and general health-related quality of life (HRQoL).Citation7,Citation8
Dupilumab is a fully human VelocImmune®-derived monoclonal antibody that blocks interleukin (IL)-4Rα, the shared receptor component for IL-4 and IL-13, key cytokines in the type 2 inflammatory response.Citation9 In the Phase 3 LIBERTY ASTHMA QUEST study (NCT02414854) in patients with uncontrolled, moderate-to-severe asthma, dupilumab significantly reduced severe asthma exacerbations, and improved lung function, asthma control, and asthma-related HRQoL vs placebo, with incidence of treatment-emergent adverse events similar across treatment groups.Citation10 These effects of dupilumab were also demonstrated in the subgroup of patients in QUEST with comorbid CRS, who have a higher asthma symptom burden and are harder to treat.Citation11 There are multiple links between the upper and lower airwaysCitation12 and research has shown that improving the upper airway leads to an improvement in the lower airway.Citation13 Reducing lower airway burden in patients with asthma and CRS (who have a higher symptom load) may have the potential to improve quality of life. We wanted to assess whether in this patient group, receiving treatment for asthma (thus improving the lower airway) would demonstrate an improvement in CRS-specific HRQoL and general health status. We therefore undertook a post hoc analysis to assess whether improvement in asthma with dupilumab had an impact on patient-reported CRS-specific HRQoL and general health status in patients with uncontrolled, moderate-to-severe asthma and a history of comorbid CRS with or without nasal polyps from the QUEST study.
Materials and Methods
Study Design and Patients
Full details of the QUEST study (NCT02414854) have been published previously.Citation10 In brief, eligible patients were adults/adolescents with uncontrolled, moderate-to-severe asthma, and were receiving continuous treatment with medium-to-high doses of inhaled corticosteroids plus 1 or 2 additional asthma controller medications. Patients were randomized (2:2:1:1) to receive dupilumab 200 mg subcutaneously (SC), dupilumab 300 mg SC, or matched placebo every 2 weeks (q2w) for 52 weeks. All patients provided written informed consent. The local institutional review board or ethics committee at each study center oversaw trial conduct and documentation. This analysis included patients who self-reported having either CRS or nasal polyps (via an eDiary, no formal diagnosis of CRS was made). Objective measures of nasal polyps were not available in these patients, therefore the term CRS is used throughout and includes those with and without nasal polyps. The analysis focused on patients who received dupilumab 300 mg q2w (the approved dose for CRSwNP) or matched placebo. The 200 mg q2w and matched placebo group were not included in this analysis as this dose is not approved for CRSwNP. Access to data and related study documents from the QUEST study may be requested by qualified researchers. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.vivli.org/.
Outcomes
CRS-specific HRQoL was assessed using the 22-item Sino-Nasal Outcome Test (SNOT-22). The items are scored on a Likert-like scale of 0 (“no problem”) to 5 (“problem as bad as it can be”) and allows patients to report up to 5 most important items affecting their health. The 22 items are categorized into the following 5 validated domains for CRSwNP: nasal (8 items), ear/facial (4 items), sleep (4 items), function (3 items), and emotion (3 items).Citation14 The total score ranges from 0 to 110, and the domain scores are presented as the average item score per domain (0–5). In each case, higher scores represent worse CRS-specific HRQoL. Percentage change in total score from baseline, change from baseline in domain scores, and change from baseline scores of the top 5 items were assessed at Weeks 12, 24, 36, and 52. Individual response thresholds were defined by SNOT-22 improvement from baseline ≥8.9 (minimal clinically important difference). General health status was self-reported by the patient and measured with the Euro-QoL visual analog scale (EQ-VAS; collected within the EQ-5D-5L instrument), which scores from 0 “worst imaginable health state” to 100 “best imaginable health state” and whose change from baseline was assessed at Weeks 12, 24, 36, and 52.
Statistical Analyses
Least squares (LS) mean changes from baseline vs placebo were derived from a mixed model with repeated measures with percent change from baseline in SNOT-22 total score or change from baseline in SNOT-22 domain or item score values up to Week 52 as the response variable, and treatment, age, region (pooled country), baseline eosinophil strata, baseline inhaled corticosteroid dose level, visit, treatment-by-visit interaction, baseline SNOT-22 total or domain or item score value, and baseline-by-visit interaction as covariates. The proportion of patients achieving response thresholds for SNOT-22 was compared between dupilumab and placebo at a given visit using a logistic regression, with treatment, age, region (pooled country), baseline eosinophil strata, baseline inhaled corticosteroid dose level, and baseline SNOT-22 total score as covariates; the odds ratio vs placebo was computed with its 95% confidence interval. There was no imputation for missing values; all reported p-values are nominal.
Results
Baseline Characteristics
The intention-to-treat population comprised 1902 patients, of whom 382 (20.1%) reported a history of CRS at baseline. A total of 193 of these patients received dupilumab 300 mg q2w or matched placebo and were included in the current analyses. Baseline characteristics were generally balanced between the dupilumab 300 mg q2w and placebo groups (Supplementary Table S1).
Most Affected Domains and Most Important SNOT-22 Items
The most affected SNOT-22 domain at baseline was nasal, followed by function, sleep, emotion, and ear/facial (Supplementary Table S1). The SNOT-22 items rated as 1 of the top 5 most important by patients at baseline were “decreased sense of smell/taste” (rated most important by 53.8% of patients), “nasal blockage” (51.5%), “cough” (42.0%), “reduced productivity” (37.3%), and “wake up tired” (24.3%) (Supplementary Table S2). Items were rated “severe problem or problem as bad as it can be” by 43% of patients for “decreased sense of smell/taste,” 28% for “nasal blockage,” 17% for “cough,” 17% for “reduced productivity,” and 22% for “wake up tired.”
SNOT-22 Scores
Mean (standard deviation [SD]) baseline SNOT-22 score for placebo was 43.8 (19.3) and for dupilumab was 42.8 (18.0; range 0–110; Supplementary Table S1). LS mean percentage change from baseline in SNOT-22 total score was significantly greater for dupilumab vs placebo at Week 24 (LS mean difference vs placebo [95% confidence interval] −18.67% [−34.37, −2.98] p = 0.02), Week 36 (−31.44% [−48.73, −14.14] p = 0.0004), and Week 52 (−33.70 [−50.06, −17.34] p < 0.0001; ). At Week 52, 47.8% of patients in the dupilumab group achieved a clinically meaningful improvement of ≥8.9 pointsCitation15 vs 40.9% of patients in the placebo group (odds ratio vs placebo: 1.60, 95% confidence interval (0.79, 3.26); p = 0.20).
Figure 1 LS mean percentage change from baseline in SNOT-22 total score (A), LS mean change from baseline in top 5 important SNOT-22 items: (B) decreased sense of smell/taste, (C) nasal blockage, (D) cough, (E) reduced productivity, and (F) wake up tired, in patients with asthma with comorbid CRS.
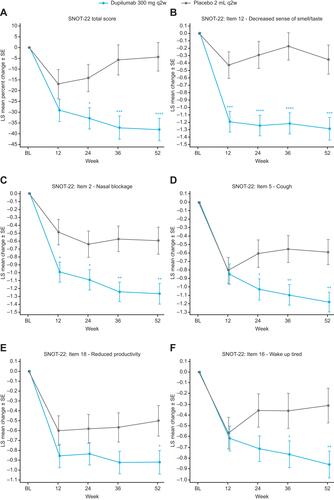
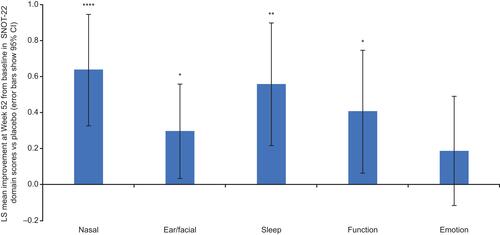
At Week 52, significant changes from baseline with dupilumab vs placebo were observed for all 5 SNOT-22 items rated most important by patients (; all p < 0.05). Changes from baseline for “decreased sense of smell/taste” and “nasal blockage” were significantly greater for dupilumab vs placebo at all timepoints (Weeks 12, 24, 36, 52; all p < 0.05). Improvements from baseline were significantly greater for dupilumab vs placebo at Week 52 across all SNOT-22 domains (all p < 0.05), except emotion (). Changes from baseline for nasal and ear/facial domains were even significantly greater for dupilumab vs placebo at all timepoints (Weeks 12, 24, 36, 52; all p < 0.05; data not shown).
Figure 2 LS mean change from baseline in SNOT-22 domain (nasal, ear/facial, sleep, function, emotion) scores dupilumab vs placebo at Week 52 in patients with asthma with comorbid CRS.
EQ-VAS
Mean (SD) baseline EQ-VAS scores (measuring general health status) were 63.6 (17.4) for placebo and 62.8 (19.3) for dupilumab (range 0–100; Supplementary Figure S1) and were below population norms, which range from 70.4 to 83.3 depending upon country.Citation16 EQ-VAS was significantly improved with dupilumab vs placebo by Week 12 (LS mean difference vs placebo [95% confidence interval] 7.04 [2.01, 12.08], p < 0.01), with improvements sustained to Week 52 (7.09 [2.27, 11.90], p < 0.01; Figure S1). At Week 52, mean (SD) EQ-VAS scores for placebo (74.3 [16.6]) and dupilumab (79.6 [15.3]) were both within population norms.
Discussion
Patients with uncontrolled moderate-to-severe asthma with comorbid CRS have greater disease burden, more severely impaired HRQoL, and are often more difficult to treat than patients with either condition alone.Citation3,Citation5–Citation7 Here we report on the effect of dupilumab on CRS-specific HRQoL and general health status in asthma patients with a patient-reported history of CRS. SNOT-22 has been identified as the most suitable patient reported outcome for assessing HRQoL in patients with CRS, while EQ-VAS has shown sensitivity to clinical change in rhinosinusitis that supports its use for monitoring patient outcomes in this condition.Citation17–Citation20 Previously it has been suggested that greater efficacy with dupilumab in those with comorbid asthma and CRSwNP than either alone may be because of simultaneous control of upper and lower airway type 2 inflammation,Citation21 which may translate into improved quality of life for the patient.
The current post hoc analyses were conducted as the impact of dupilumab on the most important aspects affecting patients’ health, different aspects of CRS-specific HRQoL, and overall general health status in patients with moderate-to-severe asthma and CRS with/without nasal polyps had not been previously reported. Dupilumab-treated patients demonstrated improvements in the top 5 items on SNOT-22 and across SNOT-22 domains, along with significant percent change improvement in SNOT-22 total score at 24 weeks, which were sustained over 52 weeks. Percent change gives a precise description of change over time as it accounts for baseline measurements. Improvements vs placebo were statistically significant at Week 52 for all domains of SNOT-22 (except emotional). The lack of statistical significance for the improvement in emotion domain score could be due to the low number of patients reporting the 3 emotion domain items as most important to them (frustrated/restless/irritable [18% of patients]; sad [8%]; embarrassed [7%]). It may also show that in patients who have asthma as the primary disease, the impact on CRS-related emotional health may be less prominent, as previous analyses have demonstrated significant improvement with dupilumab in all domains in patients with moderate-to-severe CRSwNP.Citation22 Multiple symptoms of CRSwNP have a negative impact on patients’ physical and mental wellbeing, with loss of smell being one of the most troublesome symptoms.Citation23 In the current study, improvements were seen in the top 2 SNOT-22 items (“sense of smell/taste” and “nasal blockage”) from first assessment at Week 12 and sustained to Week 52. In this population that had general health status (as per EQ-VAS scores) below population norms at baseline, dupilumab treatment rapidly improved general health status vs placebo at Week 12, with improvements maintained to Week 52.
Similarly, dupilumab added to standard of care has previously been shown to improve symptoms, lung function, and asthma-related HRQoL in the SINUS-24/SINUS-52 Phase 3 study of patients with severe CRSwNP with asthma.Citation24 Dupilumab also improved asthma-related outcomes: annualized severe exacerbation rates, lung function, patient-reported asthma control, and asthma-related HRQoL in patients with moderate-to-severe asthma with CRS.Citation11 This report demonstrates dupilumab efficacy for improving CRS-specific HRQoL and general health status in moderate-to-severe asthma with CRS.
Limitations of this analysis include its post hoc nature and the relatively small number of patients with coexisting CRS, and that comorbidity was self-reported. In addition, the severity of nasal polyposis was not recorded as part of the QUEST study, so the effect of dupilumab at different nasal polyp severity levels could not be determined.
Given the post hoc nature of these data, randomized controlled trials in patients with moderate-to-severe asthma and CRS (with or without nasal polyps) are needed. Currently, dupilumab is being studied in a Phase 3, randomized trial in patients with CRS without nasal polyps (Liberty CRSsNP ORION; NCT04678856), which includes a subgroup of patients with comorbid asthma, and a Phase 4, randomized, head-to-head trial (vs omalizumab) in patients with CRSwNP and comorbid asthma (EVEREST; NCT04998604), which might provide further evidence on the efficacy of dupilumab in these populations.
Conclusion
In patients with moderate-to-severe asthma who self-reported a history of comorbid CRS, dupilumab treatment improved CRS-specific HRQoL and general health status. These data add to our understanding of the effects of targeting type 2 inflammation in patients with coexisting upper and lower airways disease.
Abbreviations
BL, baseline; CRS, chronic rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyps; EQ-VAS, Euro-QoL visual analog scale; HRQoL, health-related quality of life; IL, interleukin; LS, least squares; q2w, every 2 weeks; SC, subcutaneous; SD, standard deviation; SE, standard error; SNOT-22, 22-item Sino-Nasal Outcome Test.
Acknowledgments
Medical writing/editorial assistance provided by Stephen Whiting, PhD, of Adelphi Group, funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Disclosure
C.H. reports advisory board fees from AstraZeneca, Dianosic, GlaxoSmithKline, and Sanofi. K.M.B. reports advisory board fees from AstraZeneca, GlaxoSmithKline, Regeneron, and Sanofi. E.H. reports research grants from AstraZeneca, Boehringer Ingelheim, Circassia, GlaxoSmithKline, Nestlé Purina, Novartis, Sanofi, Stallergenes-Greer, Regeneron, Teva, and Valeas. N.A.C. reports consultancy from Novartis, Oysterpoint Pharmaceuticals, and Sanofi/Regeneron, as well as licensing agreement with GeneOne Life Sciences. H.O. reports research funds from AstraZeneca GmbH, GlaxoSmithKline GmbH & Co. KG, and F. Hoffmann-La Roche Ltd, as well as advisory board fees and lecture fees from Novartis Pharma GmbH and Sanofi-Aventis Deutschland GmbH. A.H.K., J.M, J.A.J.-N., P.J.R. are employees and may hold stock and/or stock options in Sanofi. S.S, S.N., Y.D. are employees and may hold stock and/or stock options in Regeneron Pharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Kerkhof M, Tran TN, Soriano JB, et al. Healthcare resource use and costs of severe, uncontrolled eosinophilic asthma in the UK general population. Thorax. 2018;73(2):116–124. doi:10.1136/thoraxjnl-2017-210531
- Khan A, Gouia I, Kamat S, et al. Type 2 inflammation-related comorbidities among patients with asthma, chronic rhinosinusitis with nasal polyps, and atopic dermatitis. Eur Respir J. 2020;56:232. doi:10.1183/13993003.congress-2020.232
- Novelli F, Bacci E, Latorre M, et al. Comorbidities are associated with different features of severe asthma. Clin Mol Allergy. 2018;16:25. doi:10.1186/s12948-018-0103-x
- Bilodeau L, Boulay ME, Prince P, Boisvert P, Boulet LP. Comparative clinical and airway inflammatory features of asthmatics with or without polyps. Rhinology. 2010;48(4):420–425. doi:10.4193/Rhino09.095
- Tay TR, Radhakrishna N, Hore-Lacy F, et al. Comorbidities in difficult asthma are independent risk factors for frequent exacerbations, poor control and diminished quality of life. Respirology. 2016;21(8):1384–1390. doi:10.1111/resp.12838
- Tay TR, Hew M. Comorbid “treatable traits” in difficult asthma: current evidence and clinical evaluation. Allergy. 2018;73(7):1369–1382. doi:10.1111/all.13370
- Alobid I, Benítez P, Bernal-Sprekelsen M, et al. Nasal polyposis and its impact on quality of life: comparison between the effects of medical and surgical treatments. Allergy. 2005;60(4):452–458. doi:10.1111/j.1398-9995.2005.00725.x
- Huang CC, Chang PH, Wu PW, et al. Impact of nasal symptoms on the evaluation of asthma control. Medicine. 2017;96(8):e6147. doi:10.1097/MD.0000000000006147
- Murphy AJ, Macdonald LE, Stevens S, et al. Mice with megabase humanization of their immunoglobulin genes generate antibodies as efficiently as normal mice. Proc Natl Acad Sci U S A. 2014;111(14):5153–5158. doi:10.1073/pnas.1324022111
- Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092
- Maspero JF, Katelaris CH, Busse WW, et al. Dupilumab efficacy in uncontrolled, moderate-to-severe asthma with self-reported chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(2):527–539.e9. doi:10.1016/j.jaip.2019.07.016
- Licari A, Castagnoli R, Denicolò CF, Rossini L, Marseglia A, Marseglia GL. The nose and the lung: united airway disease? Front Pediatr. 2017;5:44. doi:10.3389/fped.2017.00044
- Hamada K, Oishi K, Chikumoto A, et al. Impact of sinus surgery on type 2 airway and systemic inflammation in asthma. J Asthma. 2021;58(6):750–758. doi:10.1080/02770903.2020.1729380
- Khan A, Reaney M, Guillemin I, et al. Development of Sinonasal Outcome Test (SNOT-22) domains in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2021. doi:10.1002/lary.29766
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item sinonasal outcome test. Clin Otolaryngol. 2009;34(5):447–454. doi:10.1111/j.1749-4486.2009.01995.x
- Janssen MF, Szende A, Cabases J, Ramos-Goñi JM, Vilagut G, König HH. Population norms for the EQ-5D-3L: a cross-country analysis of population surveys for 20 countries. Eur J Health Econ. 2019;20(2):205–216. doi:10.1007/s10198-018-0955-5
- Phillips KM, Talat R, Caradonna DS, Gray ST, Sedaghat AR. Quality of life impairment due to chronic rhinosinusitis in asthmatics is mediated by asthma control. Rhinology. 2019;57(6):430–435. doi:10.4193/Rhin19.207
- Remenschneider AK, D’Amico L, Gray ST, Holbrook EH, Gliklich RE, Metson R. The EQ-5D: a new tool for studying clinical outcomes in chronic rhinosinusitis. Laryngoscope. 2015;125(1):7–15. doi:10.1002/lary.24715
- Kim Do H, Han K, Kim SW. Effect of chronic rhinosinusitis with or without nasal polyp on quality of life in South Korea: 5th Korea National Health and nutrition examination survey Korean. Clin Exp Otorhinolaryngol. 2016;9(2):150–156. doi:10.21053/ceo.2015.01053
- Bachert C, Hellings PW, Mullol J, et al. Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy. 2020;75(1):148–157. doi:10.1111/all.13984
- Laidlaw TM, Mullol J, Woessner KM, Amin N, Mannent LP. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021;9(3):1133–1141. doi:10.1016/j.jaip.2020.09.063
- Lee SE, Hopkins C, Mullol J, et al. Dupilumab improves health related quality of life: results from the phase 3 SINUS studies. Allergy. 2022. doi:10.1111/all.15222
- Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2021;14:127–134. doi:10.2147/JAA.S290424
- Laidlaw TM, Bachert C, Amin N, et al. Dupilumab improves upper and lower airway disease control in chronic rhinosinusitis with nasal polyps and asthma. Ann Allergy Asthma Immunol. 2021;126(5):584–592.e1. doi:10.1016/j.anai.2021.01.012
