Abstract
The immune responses of T-helper (Th) and T-regulatory cells are thought to play a crucial role in the pathogenesis of allergic airway inflammation observed in asthma. The correction of immune response by these cells should be considered in the prevention and treatment of asthma. Native antigen 85B (Ag85B) of mycobacteria, which cross-reacts among mycobacteria species, may play an important biological role in host–pathogen interaction since it elicits various immune responses by activation of Th cells. The current study investigated the antiallergic inflammatory effects of DNA administration of Ag85B from Mycobacterium kansasii in a mouse model of asthma. Immunization of BALB/c mice with alum-adsorbed ovalbumin followed by aspiration with aerosolized ovalbumin resulted in the development of allergic airway inflammation. Administration of Ag85B DNA before the aerosolized ovalbumin challenge protected the mice from subsequent induction of allergic airway inflammation. Serum and bronchoalveolar lavage immunoglobulin E levels, extent of eosinophil infiltration, and levels of Th2-type cytokines in Ag85B DNA-administered mice were significantly lower than those in control plasmid-immunized mice, and levels of Th1-and T-regulatory-type cytokines were enhanced by Ag85B administration. The results of this study provide evidence for the potential utility of Ag85B DNA inoculation as a novel approach for the treatment of asthma.
Introduction
Asthma is characterized by airway hyperresponsiveness to a variety of specific and nonspecific stimuli, chronic pulmonary inflammation with eosinophilia, excessive mucus production, and high serum immunoglobulin E (IgE) levels. T-helper-2 (Th2) cells are thought to play a crucial role in the initiation, progression, and persistence of asthma in association with the production of interleukin-4 (IL-4), IL-5, and IL-13.Citation1–Citation3 Bronchoalveolar lavage (BAL) T-cells from human asthmatics have been reported to express elevated levels of IL-4 and IL-5 messenger ribonucleic acid (mRNA).Citation4,Citation5 Although the correction of this deviation to Th2-type immune responses is considered to be necessary to achieve therapeutic and preventive effects on asthma, it is not sufficient to obtain therapeutic effects in many cases. Another subset of T-cells, T-regulatory (Treg) cells, has been reported to be important in the development of allergic diseases such as asthma.Citation6 Many studies have suggested that effective immunotherapy for allergic diseases is associated with immune deviation from a disease-promoting Th2 response towards a Th1 response, with Treg cells having appropriate functions.Citation7 However, the induction of both subsets of cells – Th1 and Treg cells – for the treatment of asthma using immunological strategic tools is very difficult.
Administration of mycobacteria, including the bacillus Calmette–Guerin, has been thought to be effective for preventing the development of asthma by induction of Th1-type immune responses and inhibition of IgE by the production of IL-21 from natural killer T-cells.Citation8–Citation10 However, the relationship between bacillus Calmette–Guerin infection or mycobacteria immunization and asthma in humans is controversial because of the many causative factors affecting the induction of immune responses by mycobacteria, eg, human genetic background, mycobacteria strains, and environmental factors (reviewed in Arnoldussen et al).Citation11 From these findings, bacterial products from mycobacteria for immunotherapy against allergic disease should eliminate the harmful effects of host genetic factors, environmental factors, and strain specificity of mycobacteria.
Antigen 85B (Ag85B) is one of the most dominant protein antigens secreted from all mycobacterial species and has been shown to induce substantial Th cell proliferation and vigorous Th1 cytokine production.Citation12 Moreover, the induction of Th1-type immune responses by immunization of Ag85B was enhanced by presensitization with bacillus Calmette– Guerin.Citation13,Citation14 From these findings, the effectiveness of Ag85B DNA as immunotherapy for tumor disease and as a vaccine adjuvant for infectious disease, by its ability to induce Th1- type immune responses, was also reported.Citation13,Citation14 The current study investigated whether Ag85B DNA from Mycobacterium kansasii can inhibit the development of allergic airway inflammation as a novel immunotherapy.
Material and methods
Induction of allergic inflammation in mice
BALB/c female mice used in this study were handled according to ethical guidelines approved by the Institutional Animal Care and Use Committee of National Institute of Biomedical Innovation, Japan. The mice were sensitized to ovalbumin (OVA; Sigma-Aldrich, St Louis, MO) and challenged with aerosolized OVA according to a modification of the method of Nishikubo et al.Citation15 Briefly, mice were subcutaneously immunized with 10 μg OVA complexed with alum on days zero and 14. On days 21–25 after the first immunization, mice were challenged with an aerosol of 5% OVA in phosphate-buffered saline in a chamber for 20 minutes.
Administration of DNA
Mice were intraperitoneally administered 50 μg plasmid DNA encoding Ag85B DNA once on day –7, zero, 14, or 21. An empty plasmid vector (pcDNA™ 3.1; Life Technologies, Carlsbad, CA) was used as a control ().
Figure 1 Inhibition of the development of allergic inflammation in lungs by administration of Ag85B DNA vaccine. (A) Experimental design used to investigate the effects of Ag85B DNA vaccine on OVA-induced asthma. Mice were subjected to an OVA sensitization scheme,Citation15 and 50 μg of Ag85B DNA vaccine was intraperitoneally injected once on days −7, 0, 14, or 21. A control plasmid was also administered on the same day. (B) Results of histopathological examination of lungs of mice that had been administered Ag85B DNA or control DNA. All tissues were obtained 25 days after the first OVA immunization. The tissues were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
BAL fluid collection
BAL fluid was obtained by injecting and recovering two 0.5 mL aliquots of phosphate-buffered saline via a tracheal cannula. BAL fluid and sera were collected 25 days after the first OVA immunization. Cells in the BAL fluid were counted using a hematocytometer, and the differentials were determined by utilizing light microscopy to count 300 cells on Cytospin® preparations (Thermo Fisher Scientific, Waltham, MA). The concentration of inflammatory protein was measured by Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA).
Quantitation of IgE
IgE levels in sera were measured using enzyme-linked immunosorbent assay (ELISA) kits according to the procedure recommended by the manufacturer (Shibayagi Co, Ltd, Shibukawa, Japan).
Determination of cytokine production
Lymphocytes obtained from thoracic lymph nodes of immunized mice (5 × 106) were cultured with 10 μg/mL OVA in 24-well culture plates at a volume of 2 mL. After incubation at 37°C in a humidified incubator (5% carbon dioxide) for 48 hours, culture supernatants were collected and analyzed for production of interferon-γ (IFN-γ; Life Technologies) or IL-4 (Quantikine®; R&D Systems, Minneapolis, MN) by an ELISA assay according to the manufacturer’s protocol (Life Technologies). The amounts of IL-5 and IL-13 in BAL fluid were also measured by an ELISA kit (R&D Systems) 25 days after the first OVA immunization.
Detection of cytokine mRNA from lymphocytes using real-time polymerase chain reaction
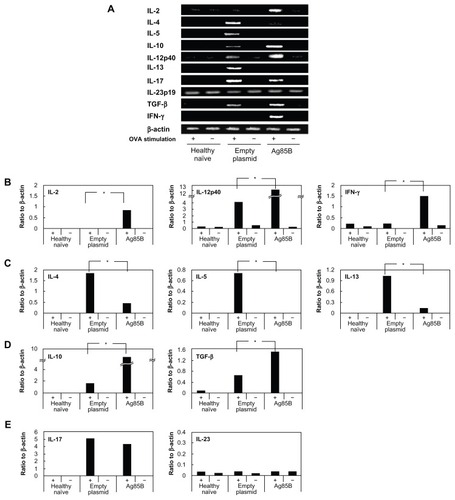
Total RNA was purified from OVA-stimulated or fetal calf serum (control)-stimulated spleen cells using Isogen (Nippon Gene Co, Ltd, Tokyo, Japan) following the manufacturer’s instructions. For the real-time reaction, a reverse transcription system (Promega Corporation, Fitchburg, WI) was used. Polymerase chain reaction was performed in a total volume of 50 μL of 1 × polymerase chain reaction buffer (Takara Shuzo, Kyoto, Japan) containing 0.5–1.0 μg of complementary DNA, 0.25 mM of each deoxyribonucleotide triphosphate, 2 μM of each primer, and 2.5 U of Taq DNA polymerase (Takara Shuzo). The specific primer pairs used were described previously.Citation15 The samples were amplified for 30–35 cycles under the following conditions: annealing for 30 seconds at 56°C, extension for 1 minute at 73°C, and denaturation for 30 seconds at 93°C. The reaction products were analyzed on 2% agarose, Tris-buffered ethylenediaminetetraacetic acid gel. Photographs of the gels were scanned, and band intensities were measured using a densitometer (CS Analyzer 3.0; ATTO Corporation, Tokyo, Japan). The quantity of cytokine mRNA was determined by the ratio of cytokine and beta actin band intensities. The profiles shown are representative of three independent experiments.
Histopathological examinations
Histopathological examinations of the lungs of the mice that had been administered Ag85B DNA or control DNA were performed. All tissues were obtained 25 days after the first OVA immunization. The tissues were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Results for healthy naïve mice and control plasmid DNA-immunized mice are also shown.
Statistical analysis
Statistical analyses were performed using the Mann–Whitney U test and the Kruskal–Wallis test. Values are expressed as mean ± standard deviation. A 95% confidence limit was considered to be significant (P < 0.05).
Results
Inhibition of the development of allergic inflammation in the lung by administration of Ag85B DNA
Mice were sensitized to OVA and challenged with aerosolized OVA as described previously.Citation11 These mice were intraperitoneally administered 50 μg plasmid DNA encoding Ag85B once on day –7, zero, 14, or 21. An empty plasmid vector (pcDNA 3.1) was used as a control (). Histopathological examinations of the lungs of mice injected with Ag85B DNA or control DNA and the lungs of healthy naïve mice were performed 25 days after the first inoculation of the plasmid. The lungs of mice that were administered Ag85B DNA on days –7, zero, and 14 did not show any pathological abnormalities compared with those of healthy naïve mice, but the lungs of mice that were administered Ag85B DNA on day 21 showed mild inflammation due to infiltration of eosinophils (). Mice administered the control plasmid did not show any inhibitory effects on the development of allergic inflammations. These results indicated that Ag85B DNA administration was effective for inhibiting the development of allergic inflammation, especially in the early phase of antigen sensitization.
Marked inhibition of allergic immune responses by administration of Ag85B DNA
The levels of protein, total cells, eosinophils, lymphocytes, and neutrophils in BAL fluid from mice immunized with Ag85B DNA vaccine were significantly lower than those in BAL fluid from mice vaccinated with control DNA (). Administration of Ag85B DNA also resulted in a significant reduction in the level of OVA-specific IgE (). The concentrations of Th2-type cytokines (IL-5 and IL-13) in BAL fluid from mice immunized with Ag85B DNA vaccine were significantly lower than those in BAL fluid from control mice (). These inhibitory effects on the development of allergic inflammation were correlated with day of Ag85B DNA injection. Injection on an early day was more effective for inhibiting the development of allergic inflammation. These results were also confirmed by histopathological observation.
Figure 2 Marked inhibition of the development of allergic inflammation by administration of Ag85B DNA. BAL fluid was obtained by injecting and recovering two 0.5 mL aliquots of phosphate-buffered saline via a tracheal cannula. Cells in the lavage fluid were counted using a hematocytometer, and the differentials were determined by utilizing light microscopy to count 300 cells on Cytospin® preparations (Thermo Fisher Scientific, Waltham, MA). The concentration of inflammatory protein was measured by Protein Assay Reagent (Bio-Rad Laboratories, Hercules, CA). Results for healthy naïve mice and control plasmid DNA-immunized mice are also shown. (A) Total protein, (B) number of cells, (C) eosinophils, (D) lymphocytes, (E) neutrophils, and (F) monocytes in BAL fluid from experimental animals were investigated. (G) The degrees of ovalbumin-specific immunoglobulin E responses in sera collected from experimental mice were also analyzed. Immunoglobulin E levels in sera were measured using enzyme-linked immunosorbent assay kits according to the procedure recommended by the manufacturer (Shibayagi Co, Ltd, Shibukawa, Japan). BAL fluid and sera were collected 25 days after the first ovalbumin immunization.
Abbreviations: Ag85B, antigen 85B; BAL, bronchoalveolar lavage.
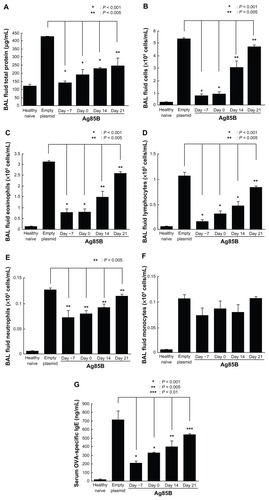
Figure 3 IL-5 and IL-13 production in BAL fluid. Amounts of (A) IL-5 and (B) IL-13 in BAL fluid were measured using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) 25 days after the first ovalbumin immunization.
Abbreviations: Ag85B, antigen 85B; BAL, bronchoalveolar lavage; IL, interleukin.
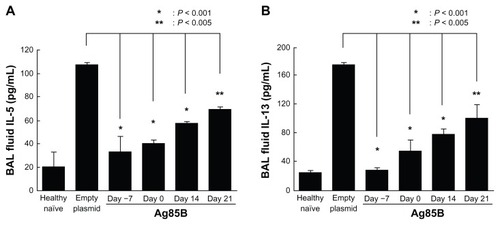
Effects of Ag85B DNA administration on the production of IL-4 and IFN-γ in response to OVA
The production of OVA-specific cytokines in lymph node cells after in vitro stimulation with OVA were assessed. The lymphocytes obtained from thoracic lymph nodes were stimulated in vitro with OVA for 48 hours. IL-4 and IFN-γ levels were measured in culture supernatants by ELISA. The level of IL-4 in culture supernatants from cells of Ag85B DNA-immunized mice was much lower than in culture supernatants from cells of control mice (). On the other hand, the production level of IFN-γ in Ag85B DNA-immunized mice was significantly higher than in control DNA-immunized mice ().
Figure 4 IFN-γ and IL-4 production in culture supernatant. Amounts of (A) IFN-γ and (B) IL-4 in culture supernatant were measured by enzyme-linked immunosorbent assay 25 days after the first OVA immunization. Spleen cells from immunized mice (5 × 106) were cultured with 10 μg/mL OVA in 24-well culture plates at a volume of 2 mL. After incubation at 37°C in a humidified incubator (5% carbon dioxide) for 96 hours, culture supernatants were quantified by using a standard enzyme-linked immunosorbent assay kit (Life Technologies, Carlsbad, CA).
Abbreviations: Ag85B, antigen 85B; IFN-γ, interferon-γ; IL-4, interleukin-4; OVA, ovalbumin.
Expression of cytokine mRNA in pulmonary lymph node cells after stimulation with OVA
The production of OVA-specific cytokines was also confirmed by mRNA levels of Th1-type cytokines (IFN-γ, IL-2, and IL-12) and Th2-type cytokines (IL-4, IL-5, and IL-13) (). Lymph node cells from Ag85B DNA vaccine-immunized mice showed strong IFN-γ, IL-2, and IL-12 expression and weak IL-4, IL-5, and IL-13 expression of mRNA, whereas control DNA-immunized mice showed the completely opposite results. The cells from control mice showed strong mRNA expression of Th2-type cytokines and weak mRNA expression of Th1-type cytokines (). It has been reported that therapeutic effects against asthma by administration of the culture supernatant of M. vaccae were derived from Treg cells by the induction of IL-10 and transforming growth factor-β.Citation16 In the current study, mRNA expression levels of IL-10 and transforming growth factor-β in lymph node cells obtained from mice immunized with Ag85B DNA were much higher than those in lymph node cells obtained from control mice after in vitro stimulation with OVA (). Another Th17 cell lineage, which is associated with allergen-induced airway allergic inflammation, was also assessed by the mRNA expression of cytokines. In the current experiment, mRNA expression of IL-17 was seen in both control DNA-immunized and Ag85B-DNA immunized mice after stimulation with OVA, with no difference in the mRNA expression levels of IL-17 between these groups (). The mRNA expression of IL-23 was also assessed since IL-23 is associated with the maturation of Th17 cells.Citation17 Expression of IL-23 mRNA was observed at the same level in all samples (). Inhibitory effects on the development of allergic inflammation are readily obtained in a mouse model of asthma through the administration of Ag85B DNA. These effects of immunotherapy by Ag85B DNA are due to activation of the immune responses of Th1 and Treg cells and inhibition of the responses of Th2 cells as a result of the enhancement of responses of Th1 and Treg cells.
Figure 5 Detection of cytokine messenger ribonucleic acid from lymphocytes using real-time polymerase chain reaction. Spleen cells were stimulated in vitro with OVA for 1 day in culture. Spleen cells stimulated with fetal calf serum were used as controls. Total ribonucleic acid was purified from the OVA-stimulated or fetal calf serum (control)-stimulated spleen cells using Isogen (Nippon Gene Co, Ltd, Tokyo, Japan) following the manufacturer’s instructions. For the real-time reaction, a reverse transcription system (Promega Corporation, Fitchburg, WI) was used. Polymerase chain reaction was performed in a total volume of 50 μL of 1 × polymerase chain reaction buffer (Takara Shuzo, Kyoto, Japan) containing 0.5–1.0 μg of complementary DNA, 0.25 mM of each deoxyribonucleotide triphosphate, 2 μM of each primer, and 2.5 U of Taq DNA polymerase (Takara Shuzo). The specific primer pairs used were previously described.Citation15 The samples were amplified for 30–35 cycles under the following conditions: annealing for 30 seconds at 56°C, extension for 1 minute at 73°C, and denaturation for 30 seconds at 93°C. (A) The reaction products were analyzed on 2% agarose, Tris-buffered ethylenediaminetetraacetic acid gels. (B–E) Photographs of the gels were scanned, and band intensities were measured using a densitometer (CS Analyzer 3.0; ATTO Corporation, Tokyo, Japan). The quantity of cytokine messenger ribonucleic acid was determined by the ratio of cytokine and beta actin band intensities.
Abbreviations: Ag85B, antigen 85B; IFN-γ, interferon-γ; IL, interleukin; OVA, ovalbumin; TGF-β, transforming growth factor-β.
Discussion
Current treatments of nonspecific immunosuppressive therapy for asthma, such as administration of glucocorticoids, are not satisfactory. Although these treatments are highly effective for controlling disease, most patients must continue to take these drugs throughout their lives. Moreover, these drugs have side effects, and asthma cannot be controlled by these drugs in up to 30% of patients. Given the high prevalence of this disease, improved and more effective therapeutic strategies are needed. The results of many studies have suggested that effective immunotherapy for allergic disease is associated with immune deviation from a disease-promoting Th2 response towards a Th1 response, with Treg cells having appropriate functions (reviewed in Takeda et al).Citation18 In the current study, the applicability of plasmid encoding complementary DNA of Ag85B from mycobacteria DNA to gene therapy of asthma was assessed. Although the introduced DNA is expressed predominantly by somatic cells, it is known that a relatively small but biologically significant number of dendritic cells are transfected with the inoculated DNA.Citation19–Citation21 Moreover, it was recently reported that systemic inoculation of a plasmid DNA may cause dendritic cell activation through direct transfection into dendritic cells.Citation22 It was demonstrated that inhibitory effects on the development of allergic inflammation are readily obtained in a mouse model of asthma through the administration of Ag85B DNA, even with only a single administration before or after antigen sensitization.
The mechanism of immune responses induced by Ag85B remains unclear. Various products having adjuvant activities, eg, lipopolysaccharide, cytosine-phosphodiester-guanine motif, and polyinosinic:polycytidylic acid, involve toll-like receptors (TLRs) and show augmentation of Th1-type immune responses.Citation18 It was previously reported that plasmid DNA encoding Ag85B stimulated the expression of TLR2, TLR3, and TLR4 mRNA. One possibility is that the induction of Th1-type immune responses by Ag85B is involved in innate immune responses. From this result, the activation of Th1 and Treg cells by Ag85B administration was thought to be involved in responses through stimulation of TLR2, TLR3, and TLR4, but not TLR9.Citation14 Various proteins derived from pathogens promote Th1 responses through stimulation of TLRs and subsequently through secretion of cytokines.Citation18 It has also been reported that TLR signaling induces not only Th1-type immune responses but also secretion of various cytokines from Treg cells.Citation23–Citation27 Moreover, recent studies have indicated that Th1 cells produce IL-10 as well as Th1-type cytokines by Notch regulation-dependent signal transducer and activator of transcription-4 signaling.Citation28 From these findings, effective immunotherapy by induction of both Th1 cell and Treg cell responses is thought to be possible by using appropriate materials. In fact, an asthma model of mice immunized with culture supernatant of mycobacteria, M. vaccae, showed Th1 and Treg responses.Citation16 The results of the current study suggest that the administration of Ag85B DNA has several potential advantages due to the activation of Th1 and Treg cells for the prevention and treatment of asthma.
Immunization with mycobacteria or mycobacteria products has been reported to inhibit the development of allergic disease.Citation29–Citation32 However, various causative factors affect immune responses by mycobacteria. It was reported as a notable point that the efficacy of mycobacteria in preventing allergic inflammation of asthma was strongly affected by Nramp1 alleles.Citation33 Several host genetic factors, including natural resistance-associated macrophage protein 1 (NRAMP1),Citation34 vitamin D receptor (VDR),Citation35,Citation36 and Mendelian susceptibility to mycobacterial disease,Citation37 have been reported to be involved in responses to mycobacteria (reviewed in Casanova and Abel).Citation38 Differences in immune responses induced by different mycobacteria strains have also been reported. The differential immune responses were mediated by lipid-extracted molecules of mycobacteria.Citation39 Moreover, environmental factors are important for immune responses induced by mycobacteria in therapy for atopic diseases.Citation40,Citation41 Presensitization of mycobacteria in the natural environment affects the induction of Th1-type immune responses by mycobacteria vaccination.Citation9,Citation42,Citation43 However, the specific components of mycobacteria that inhibit the development of allergic responses have not been reported. Ag85B is a single component of mycobacteria, and this product might not be affected by various other mycobacteria factors involved in immune responses. In fact, Th1-type immune responses induced by Ag85B are not affected by Nramp in mice.Citation44,Citation45
Wu et al demonstrated the effects of intranasal administration of Ag85B in a mouse model of asthma.Citation46 It was previously reported that Ag85B has strong adjuvant activities involving Th1 immune responses.Citation14 Intranasal administration of a plasmid DNA (DNA vaccine) with adjuvant activities has been considered to be inappropriate for human use. Intranasal inactivated influenza vaccine, with adjuvant, induced Bell’s palsy in humans. Therefore, intranasal inactivated influenza vaccine with adjuvant is no longer in clinical use.Citation47 Systemic administration of a plasmid DNA (DNA vaccine) is better than intranasal administration if the same effects of the plasmid DNA can be induced. The current study demonstrated the usefulness of Ag85B DNA vaccine and provided evidence of the potential utility of Ag85B DNA vaccine for the prevention and treatment of asthma, even with only a single systemic administration before or after antigen sensitization.
Conclusion
The correction of immune response should be considered in the prevention and treatment of asthma. Ag85B has potential utility for the prevention and treatment of asthma even with only a single administration.
Acknowledgments
This work was supported by Health Science Research Grants from the Ministry of Health, Labor, and Welfare of Japan and the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
- UmetsuDTDeKruyffRHTH1 and TH2 CD4+ cells in human allergic diseasesJ Allergy Clin Immunol19971001169257779
- Wills-KarpMLuyimbaziJXuXInterleukin-13: central mediator of allergic asthmaScience19982825397225822619856949
- LambrechtBNHammadHThe airway epithelium in asthmaNat Med201218568469222561832
- BentleyAMMengQRobinsonDSHamidQKayABDurhamSRIncreases in activated T lymphocytes, eosinophils, and cytokine mRNA expression for interleukin-5 and granulocyte/macrophage colony-stimulating factor in bronchial biopsies after allergen inhalation challenge in atopic asthmaticsAm J Respir Cell Mol Biol19938135428417755
- BossleyCJFlemingLGuptaAPediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokinesJ Allergy Clin Immunol20121294974982e1322385633
- AkdisCAAkdisMMechanisms and treatment of allergic disease in the big picture of regulatory T cellsJ Allergy Clin Immunol2009123473574619348912
- HawrylowiczCMO’GarraAPotential role of interleukin-10-secreting regulatory T cells in allergy and asthmaNat Rev Immunol20055427128315775993
- RacilaDMKlineJNPerspectives in asthma: molecular use of microbial products in asthma prevention and treatmentJ Allergy Clin Immunol200511661202120516337446
- KrishnaMTSalviSSCould administration of bacille Calmette-Guerin vaccination at birth protect from the development of asthma and allergic diseases in the western world? Has this question been adequately investigated?Pediatr Allergy Immunol200213317217612144638
- HaradaMMagara-KoyanagiKWataraiHIL-21-induced Bepsilon cell apoptosis mediated by natural killer T cells suppresses IgE responsesJ Exp Med2006203132929293717178921
- ArnoldussenDLLinehanMSheikhABCG vaccination and allergy: a systematic review and meta-analysisJ Allergy Clin Immunol2011127124625320933258
- TamuraTArigaHKinashiTThe role of antigenic peptide in CD4+ T helper phenotype development in a T cell receptor transgenic modelInt Immunol200416121691169915477229
- KuromatsuIMatsuoKTakamuraSInduction of effective antitumor immune responses in a mouse bladder tumor model by using DNA of an alpha antigen from mycobacteriaCancer Gene Ther20018748349011498769
- TakamuraSMatsuoKTakebeYYasutomiYAg85B of mycobacteria elicits effective CTL responses through activation of robust Th1 immunity as a novel adjuvant in DNA vaccineJ Immunol200517542541254716081827
- NishikuboKMurataYTamakiSA single administration of interleukin- 4 antagonistic mutant DNA inhibits allergic airway inflammation in a mouse model of asthmaGene Ther200310262119212514625566
- Zuany-AmorimCSawickaEManliusCSuppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cellsNat Med20028662562912042815
- KornTBettelliEOukkaMKuchrooVKIL-17 and Th17 cellsAnnu Rev Immunol20092748551719132915
- TakedaKKaishoTAkiraSToll-like receptorsAnnu Rev Immunol20032133537612524386
- TutingTStorkusWJFaloLDJrDNA immunization targeting the skin: molecular control of adaptive immunityJ Invest Dermatol199811121831889699714
- CondonCWatkinsSCCelluzziCMThompsonKFaloLDJrDNA-based immunization by in vivo transfection of dendritic cellsNat Med1996210112211288837611
- PorgadorAIrvineKRIwasakiABarberBHRestifoNPGermainRNPredominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunizationJ Exp Med19981886107510829743526
- TajiriKImanaka-YoshidaKMatsubaraASuppressor of cytokine signaling 1 DNA administration inhibits inflammatory and pathogenic responses in autoimmune myocarditisJ Immunol201218942043205322798678
- LloydCMHawrylowiczCMRegulatory T cells in asthmaImmunity200931343844919766086
- JinBShunTYuXHYangYXYeoAEThe effects of TLR activation on T-cell development and differentiationClin Dev Immunol2012201283648522737174
- OuabedAHubertFXChabannesDGautreauLHeslanMJosienRDifferential control of T regulatory cell proliferation and suppressive activity by mature plasmacytoid versus conventional spleen dendritic cellsJ Immunol200818095862587018424705
- JarnickiAGConroyHBreretonCAttenuating regulatory T cell induction by TLR agonists through inhibition of p38 MAPK signaling in dendritic cells enhances their efficacy as vaccine adjuvants and cancer immunotherapeuticsJ Immunol200818063797380618322186
- BellMPSvingenPARahmanMKXiongYFaubionWAJrFOXP3 regulates TLR10 expression in human T regulatory cellsJ Immunol200717931893190017641056
- RutzSJankeMKassnerNHohnsteinTKruegerMScheffoldANotch regulates IL-10 production by T helper 1 cellsProc Natl Acad Sci U S A200810593497350218292228
- HerzUGerholdKGruberCBCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal modelJ Allergy Clin Immunol199810258678749819307
- WangCCRookGAInhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccaeImmunology19989333073139640239
- YangXWangSFanYZhuLSystemic mycobacterial infection inhibits antigen-specific immunoglobulin E production, bronchial mucus production and eosinophilic inflammation induced by allergenImmunology199998332933710583590
- CavalloGPEliaMGiordanoDBaldiCCammarotaRDecrease of specific and total IgE levels in allergic patients after BCG vaccination: preliminary reportArch Otolaryngol Head Neck Surg200212891058106012220212
- BellamyRSusceptibility to mycobacterial infections: the importance of host geneticsGenes Immun20034141112595896
- SmitJJVan LoverenHHoekstraMOKarimiKFolkertsGNijkampFPThe Slc11a1 (Nramp1) gene controls efficacy of mycobacterial treatment of allergic asthmaJ Immunol2003171275476012847242
- BellamyRRuwendeCCorrahTTuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor geneJ Infect Dis199917937217249952386
- YangHFZhangZHChangZQTangKLLinDZXuJZVitamin D deficiency affects the immunity against Mycobacterium tuberculosis infection in miceClin Exp Med8102012 [Epub ahead of print.]
- ReichenbachJRosenzweigSDoffingerRDupuisSHollandSMCasanovaJLMycobacterial diseases in primary immunodeficienciesCurr Opin Allergy Clin Immunol20011650351111964733
- CasanovaJLAbelLGenetic dissection of immunity to mycobacteria: the human modelAnnu Rev Immunol20022058162011861613
- MancaCReedMBFreemanSDifferential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesisInfect Immun20047295511551415322056
- ArkwrightPDDavidTJEffect of Mycobacterium vaccae on atopic dermatitis in children of different agesBr J Dermatol200314951029103414632810
- ArkwrightPDDavidTJIntradermal administration of a killed Mycobacterium vaccae suspension (SRL 172) is associated with improvement in atopic dermatitis in children with moderate-to-severe diseaseJ Allergy Clin Immunol2001107353153411240956
- MartignonGOryszczynMPAnnesi-MaesanoIDoes childhood immunization against infectious diseases protect from the development of atopic disease?Pediatr Allergy Immunol200516319320015853947
- ChoiISKohYIEffects of BCG revaccination on asthmaAllergy200358111114111614616120
- LozesEHuygenKContentJImmunogenicity and efficacy of a tuberculosis DNA vaccine encoding the components of the secreted antigen 85 complexVaccine19971588308339234526
- BaldwinSLD’SouzaCDOrmeIMImmunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85ATuber Lung Dis199979425125910692994
- WuJXuJCaiCGaoXLiLZhongNAg85B DNA vaccine suppresses airway inflammation in a murine model of asthmaRespir Res2009105119531238
- MutschMZhouWRhodesPUse of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in SwitzerlandN Engl J Med2004350989690314985487