Abstract
Background
The purpose of this study was to investigate macrolides as an adjunct to an asthma controller regimen in children with asthma.
Methods
Prospective clinical trials of macrolide therapy in children with asthma using outcome measures of change in forced expiratory volume in one second (FEV1) and/or oral corticosteroid requirement were searched for in PubMed up to December 2009. The reference lists of studies were also included in the analysis, as well as those listed in published meta-analyses.
Results
The literature search yielded 116 studies, six of which were included in this meta-analysis. The change in FEV1 from baseline with adjunctive use of macrolide therapy in all children was not significant (0.25% predicted; 95% confidence interval [CI] −0.37, 0.86 predicted, P = 0.43); however, the change in FEV1 among children receiving daily oral corticosteroids was significant (3.89% predicted; 95% CI −0.01, 7.79, P = 0.05). Addition of macrolide therapy to the treatment of children with oral corticosteroid-dependent asthma resulted in a statistically significant decrease in daily corticosteroid dosage (−3.45 mg/day; 95% CI −5.79, −1.09 mg/day, P = 0.004). This reduction in daily corticosteroid dosage was directly proportional to the duration of macrolide therapy (−0.17 mg methylprednisolone per week of macrolide therapy; 95% CI −0.33, −0.021, P = 0.025).
Conclusion
Addition of macrolides to the treatment regimen of children with oral corticosteroid-dependent asthma improves FEV1 and decreases the daily dosage of corticosteroids required for control in these children. The degree of dose reduction is directly related to the duration of macrolide therapy. Additional large, randomized, placebo-controlled trials of adjunctive macrolide use in children with oral corticosteroid-dependent asthma are required to verify this observation.
Introduction
Asthma is a respiratory disease characterized by airway inflammation, airway hyper-responsiveness, and resultant reversible airway obstruction. Approximately 12% of children are afflicted with the disease, and it is the most common cause of childhood hospitalizations.Citation1 Asthma therapy revolves around managing respiratory symptoms, airway inflammation, and airway hyper-responsiveness.Citation1 Commonly used agents include bronchodilators and inhaled corticosteroids.Citation1 Patients with more severe asthma require daily oral steroid therapy and occasionally immunosuppressive agents.
When managing chronic asthma in children, any side effects of the treatment modality become important. Recently, long-acting beta-agonists have received a black-box warning for monotherapy.Citation2 Furthermore, corticosteroids are notorious for a wide range of detrimental effects, such as growth retardation, decreased bone mineral density, skin thinning, cataracts, glucose intolerance, hypertension, central obesity, and suppression of the hypothalamic-pituitary-adrenal axis.Citation2,Citation3 The 2008 Childhood Asthma Management Program study of long-term oral corticosteroid use in children with asthma found a dose-dependent increase in risk of osteopenia and a decrease in bone mineral accrual.Citation4 Significant improvement in chronic treatment of asthma in children is necessary to address the morbidity and mortality associated with current approaches.
Antibiotics, especially macrolides, have been extensively investigated as a potential treatment in asthma, chronic obstructive pulmonary disease, and cystic fibrosis.Citation5,Citation6 Although many clinicians prescribe antibiotics in the setting of chronic asthma and its exacerbations, current national guidelines discourage such use.Citation7,Citation8 Nevertheless, due to their anti-inflammatory and antibacterial effects, macrolides show great potential as an adjunct treatment for asthma.Citation9 Since the late 1950s, macrolide therapy has been recognized to improve outcomes in children and adults with asthma.Citation10–Citation16 In 1970, Itkin and MenzelCitation17 reported a steroid-sparing effect of macrolides. In agreement with Itkin and Menzel, in a randomized controlled trial designed to investigate the effects of macrolide therapy on childhood asthma, Kamada et alCitation14 showed a statistically significant steroid-sparing effect and an improvement in forced expiratory volume in one second (FEV1). In contrast, a Cochrane meta-analysis of macrolide use for chronic asthma in children and adults showed insufficient evidence to support or refute use of macrolides.Citation18 The present systematic review and meta-analysis was undertaken to determine the effect of macrolide therapy on management of asthma in children.
Materials and methods
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria when performing this study. The MEDLINE and EBM (Evidence-Based Medicine) Reviews/Cochrane Central Register of Controlled Trials databases were searched using both the Ovid and PubMed search engines, covering the period from 1950 to 2009. Search terms included “macrolides”, “asthma”, and “children”, with a focus on the subtopics “treatment” and “therapy”. A review of the bibliographies of selected references was also performed.
Study selection and data extraction
Inclusion criteria were a clinical diagnosis of asthma, age 2–18 years, a macrolide antibiotic used as an adjunctive controller, oral corticosteroid use with dosage determined before and after initiation of macrolide therapy, and FEV1 as percent predicted measured before and after initiation of macrolide therapy. Studies were excluded if presented as a review article or meta-analysis or if patients with chronic respiratory conditions other than asthma, such as cystic fibrosis, were included. The studies recovered from the database searches were screened by title, abstract, and article type. The methods sections from those studies that survived the initial cull were blindly reviewed for compliance with our inclusion criteria by MD and RL.
Statistical analysis
Differences in mean FEV1 and daily oral dosage of corticosteroids before and after therapy were computed for each study and for all studies combined. Heterogeneity among studies was determined by Cochrane’s Q statistic and by calculating the resulting P value. A P < 0.05 was suggestive of significant heterogeneity. I2 was calculated to determine the extent of heterogeneity across studies. In cases where heterogeneity was significant, the studies were combined using the random-effects model. The effect of duration of macrolide therapy on daily oral corticosteroid dosage was determined by meta-regression; a P value < 0.05 for the slope of the regression line was considered to be statistically significant. All analyses were performed with meta-analysis software (Comprehensive Meta-Analysis version 2.0, Biostat Inc, Englewood, NJ, http://www.meta-analysis.com).
Results
Literature flow
The Ovid, MEDLINE, and PubMed searches yielded 116 references for review. From the 116 titles and abstracts, 22 references were excluded. The full text of 94 references was then reviewed and another 79 references were excluded, resulting in a total of 15 studies that were then reviewed by blinded team members (MD and RL). Of the 15 references, sixCitation11–Citation16 were included in the final report (, ). Because only twoCitation11,Citation14 of the six included studies used a placebo group, the present study considered the pre and post-treatment groups only to maintain homogeneity.
Figure 1 Flow chart of publications included in systematic review.
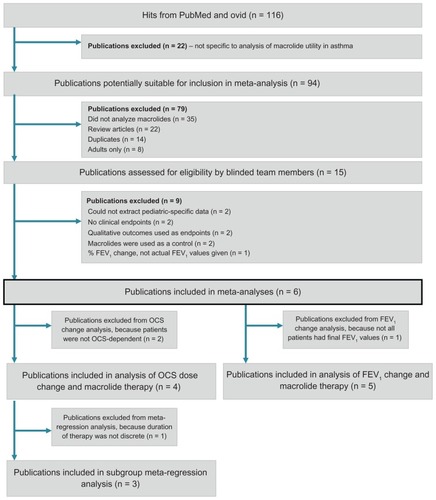
Table 1 Characteristics of studies included in meta-analysis of macrolide use in childhood asthma
The total pediatric sample size of the combined studies was 69. Five prospective studiesCitation11–Citation15 were identified and included in the analysis of macrolide therapy and FEV1 change (, ). Four studiesCitation13–Citation16 were included in the analysis of macrolide therapy and oral corticosteroid dose change (, ), three of which were included in the subgroup meta-regression analysis.Citation13–Citation15
Figure 2 Random effects model analysis of adjunctive macrolide use and FEV1 change in children with asthma.
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in one second.
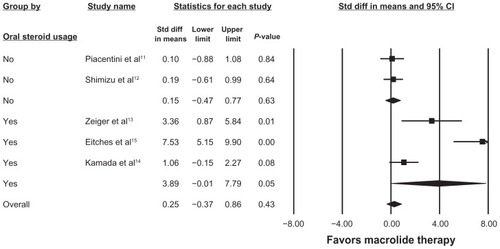
Figure 3 Random effects model analysis of adjunctive macrolide use and oral steroid dose change in children with asthma.
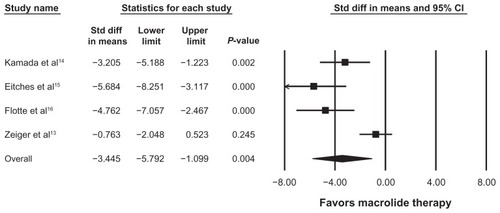
Table 2 Changes in FEV1 with addition of macrolide
Table 3 Changes in OCS dose with addition of macrolide
Of note, four of sixCitation11–Citation14 studies specifically excluded patients with evidence of upper respiratory illness or viral infection. Piacentini et al reported that none of the patients in their study had clinical signs or symptoms or airway infections in the months before or during the study.Citation11 Shimizu et al required that their subjects were free from upper respiratory tract infections for 4 weeks prior to study entry.Citation12 Zieger et al noted that no patients had evidence of infection at the start of the study.Citation13 Kamada et al excluded all patients who had evidence of respiratory infection within 4 weeks of enrollment.Citation14 Eitches et alCitation15 and Flotte et alCitation16 did not specify viral illness as an exclusion criterion.
FEV1 change
presents the five studies included in the analysis of the effects of macrolide on FEV1 in children with asthma. Due to significant heterogeneity across studies (I2 = 89.80, Q = 39.23, P = 0.002), the random-effects model was used. As seen in , macrolide therapy had no impact overall on changes in FEV1. When subsets of studies were investigated, macrolide therapy failed to impact FEV1 in patients who were not oral corticosteroid-dependent (standard difference in mean = 0.15% predicted; 95% CI −0.47, 0.77, P = 0.63), but had a significant impact on oral corticosteroid-dependent patients (standard difference in mean = 3.89% predicted; 95% CI −0.01, 7.79, P = 0.05).
Change in oral corticosteroid dose
and present the four studies included in the analysis of the effects of macrolide on oral corticosteroid dosing in children with asthma. The random-effects model was used due to significant heterogeneity across studies (I2 = 82.49, Q = 17.14, P < 0.05). Addition of macrolide therapy in children with oral corticosteroid-dependent asthma resulted in a statistically significant decrease in daily corticosteroid dosage (−3.45 mg/day; 95% CI −5.79, −1.09, P = 0.004). The effect of duration of macrolide therapy on daily oral corticosteroid dosage was determined by meta-regression. As the duration of macrolide therapy increased, the daily oral corticosteroid dosage was reduced by 0.17 mg per week (95% CI = 0.021 −0.33 mg of Methylprednisolone per week of macrolide therapy, P = 0.025).
Discussion
The present study found that while adjunctive macrolide therapy did not affect FEV1 in children without oral corticosteroid-dependent asthma, FEV1 improved in children with oral corticosteroid-dependent asthma. Moreover, among children requiring daily oral corticosteroids for control of asthma, addition of a macrolide agent decreased daily oral corticosteroid dosage requirements, and the degree of this reduction was directly proportional to duration of macrolide therapy.
While the utility of macrolide therapy in asthma has been well studied in the general population, it has been poorly studied in childhood asthma. Richeldi et alCitation18 reviewed randomized controlled trials of macrolide administration in patients with asthma, and identified a sample size of 398 adults from six studies, and 18 children from one study. This meta-analysis found insufficient evidence to recommend macrolide therapy in patients with chronic asthma. In particular, the authors failed to find that macrolide therapy had a significant impact on daily oral corticosteroid dosage. This last conclusion was based on two studies. Nelson et alCitation19 studied 75 adults and did not find a difference in daily oral corticosteroid dosage between their treatment and placebo groups. Kamada et alCitation14 studied 11 children, and found a statistically significant reduction in daily oral corticosteroid dosage for children receiving macrolide therapy when compared with a placebo group. The present study, which found an effect of macrolide therapy on daily oral corticosteroid dosage, included parallel and crossover clinical trials involving only children. These observations suggest that macrolide therapy may have a steroid-sparing effect in children but not in adults. Further studies are needed to resolve this issue.
Potential mechanisms of macrolide effects are likely related to modulation of inflammatory cytokines, such as interleukin (IL)-5, IL-8, and vascular endothelial growth factor (VEGF). In a prospective cohort study from 2010, Korematsu et alCitation20 showed a correlation between children with severe asthma and high levels of IL-8 and VEGF. Furthermore, that study showed that low-dose erythromycin significantly improved clinical symptoms and decreased levels of both IL8 and VEGF in children with severe asthma, but not in children with mild to moderate asthma.Citation20 In a cross-sectional controlled study performed in 2011, Lin et alCitation21 showed that children with asthma produced significantly higher levels of IL-5 compared with children without asthma, and addition of azithromycin significantly decreased IL-5 levels in a dose-dependent fashion. In the present study, the most severely affected children, ie, those who were dependent on oral corticosteroids, saw the most benefit from macrolide therapy, a result that is likely related to the pathophysiology observed in the Korematsu et alCitation20 study. It is unclear why children but not adults (as determined by a Cochrane reviewCitation18) show significant steroid-sparing effects from adjunctive macrolide therapy. The common saying may be appropriate here, ie, that “children are not simply young adults”.
The present study has several limitations. First, troleandomycin and methylprednisolone were used in all studies where a steroid-sparing effect was observed. Prior studies have suggested a pharmacokinetic interaction between these two agents that directly increases steroid concentrations.Citation13,Citation22 It is not known whether this same effect exists between other members of the macrolide and corticosteroid classes. Another significant limitation is sample size: our aggregate data analyzed a total of 69 patients from six studies, and four of these (n = 28) were analyzed for steroid-sparing effect. Nevertheless, to the authors’ knowledge, this is the largest sample size to date to have evaluated the effect of macrolides in children with corticosteroid-dependent asthma. Further, the studies were performed many years earlier, in some cases 30 years ago, when definitions and standard of care protocols were different from those used today. Furthermore, although the improvement in FEV1 was statistically significant, it may not necessarily be clinically significant, and future studies should include outcomes, such as responses to an asthma symptom questionnaire and numbers of acute care visits.
In summary, this study demonstrates that macrolides have a steroid-sparing effect in children with steroid-dependent asthma. In addition, we found a direct association between duration of macrolide therapy and magnitude of change in oral corticosteroid dosage before and after therapy. The present study supports the need for a large, randomized, placebo-controlled, crossover trial of a modern macrolide in children with steroid-dependent asthma.
Disclosure
The authors report no conflicts of interest in this work.
References
- BehrmanREKliegmanRMJensonHBNelson Textbook of Pediatrics17th edPhiladelphia, PASaunders Elsevier Science2004
- KramerJMBalancing the benefits and risks of inhaled long-acting beta-agonists – the influence of valuesN Engl J Med20093601592159519369665
- PetersSPSafety of inhaled corticosteroids in the treatment of persistent asthmaJ Natl Med Assoc20069885186116775906
- KellyHWVan NattaMLCovarRATonsaciaJGreenRPStrunkRCEffect of long-term corticosteroid use on bone mineral density in children: a prospective longitudinal assessment in the Childhood Asthma Management Program (CAMP) studyPediatrics2008122e53e6118595975
- RubinBKHenkeMOImmunomodulatory activity and effectiveness of macrolides in chronic airway diseaseChest200412570S78S14872003
- EquiABalfour-LynnIMBushARosenthalMLong term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trialLancet200236097898412383667
- PaulIMMaselliJHHershALBousheyHANielsonDWCabanaMDAntibiotic prescribing during pediatric ambulatory care visits for asthmaPediatrics20111271014102121606155
- De BoeckKVermeulenFMeytsIHutsebautLFrackaertDProesmansMCoprescription of antibiotics and asthma drugs in childrenPediatrics20111271022102621606144
- ShinkaiMRubinBKMacrolides and airway inflammation in childrenPaediatr Respir Rev2005622723516153572
- KaplanMAGoldinMThe use of triacetyl-oleandomycin in chronic infectious asthmaAntibiot Annu1959627327613637749
- PiacentiniGLPeroniDGBodiniAAzithromycin reduces bronchial hyperresponsiveness and neutrophilic airway inflammation in asthmatic children: a preliminary reportAllergy Asthma Proc20072819419817479604
- ShimizuTKatoMMochizukiHTokuyamaKMorikawaAKuroumeTRoxithromycin reduces the degree of bronchial hyperresponsiveness in children with asthmaChest19941064584617774320
- ZeigerRSSchartzMSperlingWSimonRAStevensonDDEfficacy of troleandomycin in outpatients with severe corticosteroid dependent asthmaJ Allergy Clin Immunol1980664384466968762
- KamadaAKHillMRIkleDNBrennerAMSzeflerSJEfficacy and safety of low-dose troleandomycin therapy in children with severe, steroid-requiring asthmaJ Allergy Clin Immunol1993918738828473676
- EitchesRWRachelefskyGSKatzRMMendozaGRSiegelSCMethylprednisolone and troleandomycin in treatment of steroid dependent asthmatic childrenAm J Dis Child19851392642693872062
- FlotteTRLoughlinGMBenefits and complications of troleandomycin (TAO) in young children with steroid-dependent asthmaPediatr Pulmonol1991101781821852515
- ItkinIHMenzelMLThe use of macrolide antibiotic substances in the treatment of asthmaJ Allergy1970451461625309312
- RicheldiLFerraraGFabbriLLassersonTJGibsonPGMacrolides for chronic asthmaCochrane Database Syst Rev20054CD00299716235309
- NelsonHSHamilosDLCorselloPRLevesqueNVBuchmeierADBuckerBLA double-blind study of troleandomycin and methylprednisolone in asthmatic subjects who require daily corticosteroidsAm Rev Respir Dis19931473984048430965
- KorematsuSYamamotoKTomokazuNThe indication and effectiveness of low-dose erythromycin therapy in pediatric patients with bronchial asthmaPediatr Allergy Immunol20102148949220546525
- LinSJLeeWJLiangYWYanDCChengPJKuoMLAzithromycin inhibits IL-5 production of T helper type 2 cells from asthmatic childrenInt Arch Allergy Immunol20115617918621597298
- SpectorSLKatzFHFarrRSTroleandomycin effectiveness in steroid-dependent asthma and bronchitisJ Allergy Clin Immunol197454367