Abstract
Background
Strong associations between early antibiotic exposure and increased risk of childhood allergies have been established. Antibiotics have the potential to induce microbial dysbiosis that may be linked to allergic conditions. This review examines the limited available evidence on the associations between adult antibiotic use, microbial dysbiosis and atopic conditions.
Methods
A systematic literature search was conducted using PubMed and Embase for relevant studies, published between 01–01–2000 and 08–17–2022. We searched for associations between antibiotic use, microbial dysbiosis, and allergic conditions in adults, defined as over 13 years of age for the purposes of this review.
Results
Twenty-one studies were analyzed, with the inclusion of four narrative reviews as scarce relevant literature was found when stricter selection criteria were employed. Relevant studies predominantly focused on asthma. Significant microbial differences were observed in most measures between healthy subjects and subjects with allergic conditions. However, no system-wise and strain-wise associations were evident. Notably, at the phyla level, the Bacillota and Pseudomonadota phyla were associated with asthmatics, while the Actinobacteria phylum was linked to healthy controls. Asthmatics tends to reflect upregulation in the Bacillota and Pseudomonadota phyla in both airway and gut microbiomes.
Conclusion
No compelling evidence could be found between adult antibiotic exposure, consequent microbial dysbiosis, and allergic conditions in adults. Our review is limited by scarce literature and therefore remains inconclusive. However, potential implications of antibiotic use impacting on allergic conditions justify additional research and heightened pharmacovigilance in this area.
Keywords:
Introduction
Antibiotics are indispensable to modern medicine and have contributed to extending average human lifespans.Citation1 However, the emergence of antimicrobial resistance (AMR) now poses a serious threat to global public health and underscores the importance of Appropriate Antibiotic Prescribing (AAP). In addition, antibiotics have been linked to an increased risk of allergic conditions in children.Citation2,Citation3 Whether antibiotics may potentiate or precipitate allergic conditions in adulthood is less clear, but the possibility of adverse consequences of antibiotics on allergic conditions deserves attention.
There is a growing appreciation that healthy microbiomes are critical in maintaining human health. Multiple communities of symbiotic microorganisms populate locations such as the skin, gut and airways and make diverse contributions from metabolic function to immune modulation. Treatment with antibiotics potentially disrupts the stability and composition of these microbiomes and could interfere with vital regulatory functions, leading to adverse effects on health, such as an increased risk of allergic responses.Citation4,Citation5
Research suggests that bidirectional interactions between the microbiome and the immune systemCitation6 may play a key role in mediating allergic conditions. The microbiome influences immune development, maintenance and action.Citation7 Extensive interactions exist between microbiota and the innate immune system in the gut and skin, influencing downstream activation of immune cells.Citation8 The adaptive immune system may also be altered by interactions with microbiota and their metabolites. Further complexity regarding these interactions exists, considering that the immune system itself can shape the microbial composition.Citation5
Substantial literature has described the impact of altered microbiota, commonly termed dysbiosis,Citation8 on the microbiome of children, specifically regarding the increased risk of allergies associated with antibiotic use.Citation4,Citation9 However, there appears to be less evidence regarding the impact of antibiotics on the adult microbiome and its relation to allergic conditions. Therefore, this review aims to examine the current understanding of the impact of antibiotics on the adult microbiome and its potential implications for allergic conditions through a systematic review.
Methods
Our Systematic Literature Review (SLR) was carried out based on the research methodology and standards defined by the Cochrane Collaboration in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.Citation10
Eligibility Criteria
Based on our research objectives, a set of inclusion and exclusion criteria was defined and used to select relevant studies, as shown in the PICOS Table ().
Table 1 PICOS Table Detailing Inclusion and Exclusion Criteria for the Studies Included in the SLR
Information Sources and Search Strategies
A systematic search for studies was conducted with specific search strings containing key search terms on the PubMed platform, including Medline and Embase, on August 17, 2022. A total of 230 full-text articles published between 2000 and 2022, written in English, were identified by the initial search.
Prior to screening, search filters were applied, removing 66 articles on the basis that they were not written in English (n=13); or that they were not conducted in human subjects (n=52), or both (n=1).
Selection Process
Content of the remaining 154 studies was screened by two reviewers independently for inclusion or exclusion based on the criteria listed in . Article screening was initially based on abstracts, followed by full texts, to confirm inclusion or exclusion. Lack of consensus between the two reviewers regarding screening results was resolved by a third reviewer. Exclusions were made only after screening full-text publications, with recorded reasons.
Included were studies or literature reviews done in adults and adolescents older than 13 years, which examined relationships between the microbiome, antibiotic use and allergic conditions. This cut-off for inclusion based on age of subjects was selected on consideration of the lack of studies that meet the conventional adult age cut-off of 18 years.
Excluded were studies done in children younger than 13 years or those that focused on clinical conditions not aligned with the aims of this paper.
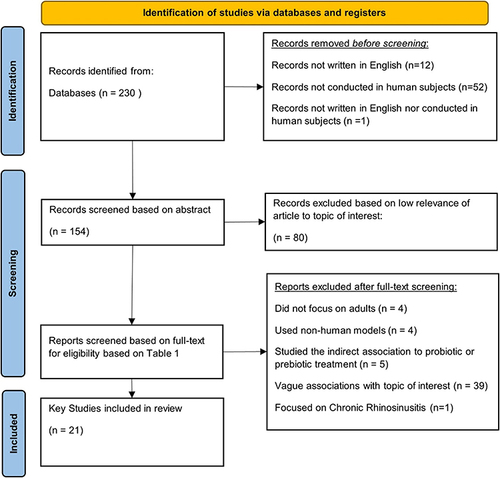
The selection process employed in this literature review has been summarized in a flowchart ().
Figure 1 PRISMA flow chart illustrating the study selection process from initial identification to final screening.
Data Collection Process
A Microsoft Excel template was used for data extraction, which was conducted by one reviewer and subsequently verified by a second reviewer.
The Good Research for Comparative Effectiveness (GRACE) checklist was utilized to assess the quality of included studies.Citation12 Two reviewers independently evaluated the included papers using the GRACE checklist, and any conflicts regarding evaluation results were resolved by discussion.
Data Items
The following parameters were extracted for all screened studies, based on article type () in the Excel spreadsheet for data extraction.
Table 2 Table Denoting Extraction Variables for Articles, Split by Study Type
Results
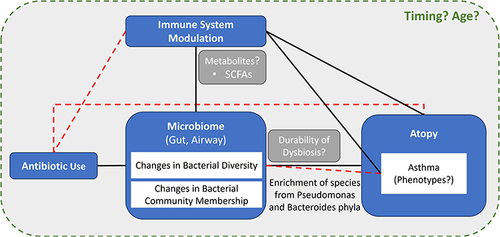
Key relationships discussed in this literature review have been summarized in a diagram ().
Figure 2 Summary figure denoting key relationships and influential factors uncovered in this SLR. Dashed lines denote an unclear relationship; Solid lines denote an established relationship.
Search Results
The initial PubMed database search identified 230 publications. Subsequent screening identified 21 key publications that met eligibility criteria outlined in the PICOS Table (). Evaluation using the GRACE Checklist found included studies to be of generally good quality.
Detailed results from data extraction conducted for clinical studies have been summarized in Table A of the Appendix.
Twenty-one studies were analyzed, with the inclusion of four narrative reviews given that scarce relevant literature was found when stricter selection criteria were employed. Most studies have focused on asthma. Significant microbial differences were observed in most measures between healthy subjects and subjects with allergic conditions.
However, no system-wise or strain-wise consensus was evident. Notably, at the phyla level, Bacillota and Pseudomonadota phyla were associated with asthmatics, while Actinobacteria phylum was linked to healthy controls. Asthmatics tended to reflect upregulation in Bacillota and Pseudomonadota in both the airway and gut. However, it is not clear whether antibiotic use in adults, with consequent microbial dysbiosis, is linked to allergic conditions.
Study Characteristics
Of 21 selected studies, four were literature reviews,Citation13–16 while 17 were clinical studies.Citation17–33
All the literature reviews (n=4) were non-systematic and narrative. Due to limited available data, the authors agreed to relax study inclusion criteria to include narrative reviews in addition to the studies that met PICO-S Table criteria.
Clinical studies (n=17) were mostly cross-sectional observational (n=14),Citation17,Citation19–25,Citation27–31,Citation33 randomized controlled trials (n=2),Citation18,Citation26 or non-randomized controlled trials (n=1).Citation25
The majority of the studies (n=12) investigated associations between the microbiome and allergic conditions, in combination with other factors.Citation17,Citation20–30 Only a small number of studies (n=3) investigated the effect of antibiotics on the microbiome and allergic disease,Citation18,Citation19,Citation33 of which only one randomized, placebo controlled trial was identified.Citation18
Study sample sizes varied widely from n=9 subjects to n=7389 subjects.Citation25,Citation33 Four studies had sample sizes of 20 subjects or less,Citation18,Citation25,Citation26,Citation32 and in one study the sample size was unknown.Citation31 Lack of statistical power was a limitation in some studies, but inclusion in this review was justified by the relative scarcity of relevant data.
Of the clinical studies (n=17), eleven were conducted in Western countries: United States of America (n=5),Citation17,Citation20,Citation24,Citation25,Citation27 Australia (n=3),Citation26,Citation28,Citation30 United Kingdom (n=2)Citation19,Citation23 and The Netherlands (n=1).Citation18 The remaining six studies came from South Korea (n=2),Citation22,Citation33 Colombia (n=1),Citation29 Egypt (n=1),Citation21 Israel (n=1)Citation32 and Japan (n=1).Citation31
We did not identify any multinational studies on adults.
The majority of the clinical studies were follow-up studies on sample populations from previous studies (n=11).Citation17–21,Citation23–29
Patient Characteristics
The age of subjects ranged from 13.9 years old to 79 years old.Citation22,Citation33 Gender balance varied from relatively even 44.4% to 59.5% females,Citation22,Citation32 to more skewed distributions such as 22.2% females,Citation30 76.4% females,Citation29 and up to 80% females.Citation18 Gender distribution was not reported in two studies.Citation23,Citation28
The role of gender as a possible confounder for microbiome and allergy could not be established. Key findings included no difference between asthmatics–non asthmatics and microbial diversity when categorized by gender.Citation22,Citation26,Citation27
Several clinical studies (n=8) did not report BMI.Citation19,Citation20,Citation23,Citation25,Citation27–30 Among clinical studies (n=9) that did report BMI,Citation17,Citation18,Citation22,Citation24,Citation26,Citation31–33 subjects fell within the BMI range of 18.5 to 27. One study suggested that BMI was associated with asthmatic status, and therefore a possible confounder of results.Citation23 However, another study found no significant differences in BMI between healthy controls and asthmatics.Citation26
No definitive conclusion was made regarding BMI as a confounding factor based on the reviewed studies.
Relationship Between Antibiotic Use, Microbiome and Allergic Conditions in Adults
Microbiome Site, Sampling and Sequencing
The majority of the included clinical studies (n=14) either reported on the airway (n=8),Citation17,Citation19,Citation20,Citation22,Citation25,Citation28,Citation30,Citation31 or gut microbiome (n=6).Citation18,Citation21,Citation23,Citation24,Citation29,Citation32
A wide spectrum of sampling methods was utilized in airway microbiome studies: induced sputum (n=4),Citation17,Citation20,Citation25,Citation30 oral washing (n=2),Citation17,Citation20 nasal brushing (n=2),Citation20,Citation22 bronchoalveolar lavage (n=2),Citation20,Citation25 bronchial brushing (n=1),Citation27 or oropharyngeal swab (n=1).Citation28
Fewer sampling methods have been reported for gut microbiome studies: most common was stool sampling (n=4),Citation18,Citation21,Citation29,Citation32 or microbial data sequenced from databases (n=2).Citation23,Citation24
A majority of studies used 16S rRNA sequencing for microbiome analysis (n=10),Citation17,Citation18,Citation20,Citation22,Citation24,Citation26–30 or other sequencing methods (n=2).Citation26,Citation32 One study looked at the microbiome sample by scaling up observed results on microscopy (n=1).Citation21
Relationship Between Microbiome and Allergic Conditions
Most studies have addressed the impact of the microbiome on asthma (n=15),Citation14,Citation15,Citation17,Citation18,Citation20–23,Citation25–31 with only one study on atopic dermatitis (n=1),Citation32 and one on allergic rhinitis (n=1).Citation33 The remaining studies examined allergic conditions in general (n=4).Citation13,Citation16,Citation19,Citation24
Influence on Bacterial Load
Bacterial load represents the number of bacteria living on a particular surface.Citation34 The analysis of microbial samples in terms of bacterial load was reported in only two studies and one literature review.Citation13,Citation17,Citation27
Notable divergence was observed in the directionality of bacterial load results. In bronchial brushings, an increased bacterial load was associated with asthmatics when compared to healthy controls,Citation27 while oral washing and induced sputum showed no difference.Citation17
Influence on Species Richness
Effects on Alpha Diversity
Alpha diversity evaluates diversity at a single site, making it useful for within-sample comparisons. Half of the clinical studies (n = 6) included this metric in their study of the microbiome, using alpha diversity to compare overall changes in microbiome between matched samples, often based on population averages.Citation17,Citation22,Citation25–27,Citation30
For the airway microbiome, there was no consensus regarding changes in diversity between asthmatics and non-asthmatics (). Either a lack of significant difference or an increase in diversity was observed for asthmatics.
Table 3 Table Summarizing the Effects Found in Selected Studies on Alpha Diversity Across Different Airway and Gut Microbiome Samples and Different Comparison Groups
The few studies on the gut microbiome did not enable us to identify clear trends.
Effects on Beta Diversity
Beta diversity reflects similarities or dissimilarities in microbial diversity between two sites and is therefore useful for between-sample comparisons, particularly regarding changes in microbiome composition.
Significant airway and gut microbiome differences were noted when comparing asthmatics with non-asthmatics ().
Table 4 Table Summarizing the Effects Found in Selected Studies on Beta Diversity Across Different Microbiome Samples and Different Comparison Groups
However, in contrast to trends reported in asthma, one study found significant gut microbiome differences between healthy subjects versus allergic subjects in all allergy traits (peanut, tree nut, shellfish, other food allergy, bee sting, dander, eczema, drug, seasonal) except asthma in their analysis of microbial dysbiosis.Citation24
Specific Microbes Associated with Allergic Conditions
Studies have employed different sampling methods of microbiome sites, limiting direct comparison. However, overall, the studies reflect different patterns of bacterial colonization in the microbiome in diseased individuals versus controls.
Bacteria of the Bacillota and Pseudomonadota phyla appear to be associated with asthma, while the Actinomycetota phyla appear to be linked to healthy controls ().
Table 5 Table Summarizing the Effects Found in Selected Studies on Bacterial Species Linked to Asthmatics or Healthy Controls
From the reviewed studies, it appears that there is no clear convergence in specific bacterial species implicated in allergic disease.
It also appears that some of these bacterial species are impacted by the effects of age or asthmatic phenotypes ().
Table 6 Table Summarizing the Effects Found in Selected Studies on Bacterial Species on Various Factors Linked to Allergic Conditions
Within similar human populations, divergence in bacterial composition patterns was also observed, as seen in two studies done on gut microbiome composition in Western cohorts.Citation23,Citation24
A study of 221 UK adults (36 asthmatics and 185 healthy controls) found that Bacteroides stercoris was enriched in healthy individuals, while Clostridium bolteae, Clostridium ramosum and Clostridium spiroforme were enriched in asthmatic individuals.Citation23
However, a study done on a cohort of 1879 American adults with a spectrum of allergies reported a different trend where microbial dysbiosis appeared to be characterized by enrichment of Bacteroidales and depletion of Clostridiales.Citation24
Effects of Antibiotics on the Microbiome and Allergic Conditions
Few studies (n=3) considered the potential impact of antibiotics on allergic conditions that may be precipitated or exacerbated by microbiome dysbiosis.Citation18,Citation19,Citation33
One randomized-controlled study exposed a cohort of healthy volunteers to a 7-day antibiotic regime of Ciprofloxacin, Vancomycin and Metronidazole.Citation18 Predictably, these antibiotics resulted in a loss in bacterial diversity, as well as a change in microbial community. This was characterized by the enrichment of Streptococcus species and depletion of Bacteroides species.
However, the study did not find a significant difference between the antibiotic and control groups in immune response measured by subsequent lung levels of eosinophil, neutrophils, lymphocyte, alveolar macrophage or neutrophil chemo-attractants following lung exposure to an allergen.
In another study, age was identified as a significant mediator of the relationship between asthma risk and antibiotics.Citation19 This was consistent across broad- and narrow-spectrum antibiotics and antibiotic classes. Greater risk of recurrent allergic rhinitis in middle school children was found with antibiotic use, especially when coupled with additional risks such as mold exposure, and IL-13 (rs20541) GA or AA genotypes.Citation33
In contrast, there appeared to be no independent association between adult antibiotic use and atopy when measured by skin prick testing with aeroallergens.Citation19
Links to the Immune System
There is growing interest in the effects of microbial dysbiosis on allergic conditions, which may be mediated by the immune system and inflammation.
A study investigating the influence of three different asthma-associated bacterial species (Staphylococcus aureus, Haemophilus influenzae and Prevotella species) on the action of eosinophils reported that each of these different bacterial species resulted in differential production of cytokines and chemokines ().Citation31
Table 7 Table Summarizing the Differential Effects of Different Bacterial Species on Cytokines or Chemokines Noted in Hosoki et al, 2013 Study
In addition, other researchers have suggested that cytokine levels are associated with the degree of airway obstruction implicated in asthma, specifically for IL-2 and MIP1β.Citation26
The study of the microbiome in atopic asthmatics and non-atopic asthmatics versus healthy controls also found that microbiome components correlated with various immune cells.Citation17 BAL, induced sputum and oral washing microbiome samples correlated with IL-7 levels, and IL-7 levels have been linked to the bacterial species Neisseria of the Pseudomonadota phylum.
In addition, differences in bacterial community memberships were also found to be related to different immune system components based on the microbiome sampling method. Bacterial community membership in induced sputum samples was found to be linked to blood eosinophils, whereas memberships from oral washing samples were linked to serum total IgE, BAL IL-8 cells, BAL memory T cells and TH17 cells. Bacterial abundance in both cases were linked to serum total IgE, BAL memory T cells, BAL CXCL11.Citation17
However, a study investigating the effect of antibiotics on the microbiome and allergic condition challenges whether these relationships exist or not.Citation18 Specifically, no significant difference between the antibiotic use group and control group was found regarding immune cells and immune cell mediators following local allergen challenge.
Other Factors (Age, Phenotype, Metabolism)
While the reviewed literature draws links between antibiotics, the microbiome, and the immune system, other factors may affect these relationships.
Age is a recurring factor cited in the literature and pertains to the microbiome not only in the difference between childhood and adult microbiomes but also in changes occurring in individuals from young adulthood into older age group adults.
The most striking illustration concerns the neonatal microbiome and childhood atopy. The absence of microbial colonization in the first 2 weeks of life was reported to exacerbate neonatal allergic reaction to human dust mites, which persisted into adulthood.Citation13 In Bisgaard et al review,Citation35 infants at high risk of developing asthma exhibited intestinal colonization by Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, or combination at 1 month of age. This was associated with an increased risk of asthma development by age 5, but the detection of the same species at 12 months apparently did not confer the same risk of developing asthma.
One study found that microbiota were less distinguishable for elderly subjects than in young adult subjects for comparisons between asthmatics and healthy controls within age groups.Citation22 The same study raised the possibility of links between the microbiome and metabolism, noting that certain metabolic processes like lysine degradation, N-glycan biosynthesis, caprolactam degradation, and peroxisome proliferator-activated receptor signaling pathways were downregulated in asthmatics and that these metabolic pathways are influenced by different groups of bacterial species depending on asthmatic status.
Specifically, Corynebacterium accolens and Propionibacterium acnes are associated with non-asthmatics, and Bacteroides caccae, Escherichia coli, Veillonella parvula, and Bifidobacterium longum are associated with asthmatics. Similar trends were observed with the upregulation of other metabolic processes like the pentose phosphate pathway, lipopolysaccharide biosynthesis, flagellar assembly, and bacterial chemotaxis.
Metabolites produced by bacteria such as short-chain fatty acids (SCFAs) may also play an important role in governing the relationship between microbiomes and allergic disease.Citation20
Genetic predisposition of subjects may be an additional factor to consider. Genes in the Type 2 inflammation (T2) asthma phenotype have been linked to inflammation, whereby an increase in lung inflammatory mediators for T2-low asthma phenotype has been observed.Citation20 With induced sputum samples, differences in the microbiome profiles of T2-low asthmatics were noted.
Another review also supports this point, proposing that genetic variation in innate immunity genes CD14 and TLR2 encode pattern recognition receptors that could modulate protective effects.Citation16
Discussion
To the best of the authors’ knowledge, this is the first systematic review to synthesize evidence of microbiome dysbiosis following antibiotic use, focused on whether there is an impact on allergic conditions in adults.
Demonstrating the potential impact of antibiotics on allergic conditions is inherently challenging. Factors such as dosage and duration of antibiotic course, antibiotic type, and different disease phenotypes create a multitude of variables.
We found limited evidence regarding the impact of antibiotics on allergic conditions in adults, in contrast to studies in children. The relatively few included studies were heterogeneous, with variation across multiple potential confounding factors with little consensus. However, these studies generally suggest significant links between microbial dysbiosis in airway or gut microbiomes and immune-related conditions.
Changes in Adult Airway and Gut Microbiomes Associated with Asthma
While we did not find consensus across system-wise and strain-wise measures of bacterial load, alpha diversity, beta diversity and key bacterial species, significant differences in airway and gut microbiomes are reported comparing healthy subjects and subjects with allergic conditions. This supports the view that microbial dysbiosis is linked to certain allergic conditions.Citation5,Citation36
Most of the reviewed studies only focused on asthma, and the association noted between microbial dysbiosis and allergic conditions might not apply to atopic conditions beyond specific allergic phenotypes of asthma. Other atopic conditions, such as allergic rhinitis and atopic dermatitis, are under-researched and merit more attention.
Differences between the upper and lower airway microbiomesCitation37 and distinct microbiome clusters from samples sourced by different methods, such as induced sputum, bronchial brushing and nasal brushing, add complexity to the subject.Citation38 For example, a study on nasopharyngeal microbiomes in asthmatic children compared nasal washing with nasal brushing and reported significant differences in both microbiome diversity and composition.Citation39
We found little consensus regarding specific bacterial species implicated in asthma. At best, a general trend was noted whereby distinct bacterial phyla were associated with asthmatics and healthy controls, respectively. Specifically, in asthmatics, bacteria within the Bacillota and Pseudomonadota phyla were upregulated, while the Actinobacteria phylum were linked to healthy controls ().
These phyla appear to be important in early stages of microbiome development, facilitating subsequent colonization by obligate anaerobes.Citation40 It may be that the upregulation of these species in adulthood could result in dysbiosis of other bacterial species.
Enrichment of Streptococcus and Staphylococcus species from the Bacillota phylum has been noted in the airways of adult and child asthmatics.Citation41 However, similar enrichment may not occur in the gut microbiome, where Lactobacillus from the Bacillota phylum has been found to be depleted in allergic conditions.Citation42
Enrichment of bacteria within the Pseudomonadota phylum, also termed Proteobacteria, has been well-established in multiple studies on lung microbial dysbiosis in asthma.Citation43 Higher prevalence of Proteobacteria, specifically Haemophilus, was observed in asthmatics using inhaled corticosteroids.Citation41 Among treatment-resistant asthmatics, members of the Moraxella catarrhalis or Haemophilus genera (both under the Pseudomonadota phylum) or of the Streptococcus genera (from Bacillota phylum) were apparently linked to increased measures of airway obstruction and greater neutrophil counts in sputum.Citation44 In addition, Ipci et al reported that Lactobacillus from the Bacillota phylum, as well as Pseudomonas and Rickettsia from Pseudomonadota phylum were enriched in asthmatics.Citation45
The most commonly cited mechanism linking microbial dysbiosis and downstream allergic conditions involves disrupted modulation of immune cells, which may be facilitated by certain bacterial species.Citation45,Citation46 Further discussion on these mechanisms is covered in Effect of Antibiotics on Allergic Conditions Mediated by the Immune System.
Antibiotic-Induced Transient or Long-Lasting Microbial Dysbiosis, with Potential Impact on Allergic Conditions
The therapeutic benefit of antibiotics in managing pathogenic bacteria risks simultaneously disrupting important functions enabled by commensal bacteria.
While some authors have suggested that the effect of short-course antibiotics on the microbiome is largely transient and quickly restored (citing unpublished literatureCitation15), individual responses will be different. For example, while oral ciprofloxacin was found to cause microbial changes persisting for several weeks, subsequent restoration of baseline microbiome was subject-dependent.Citation47,Citation48 In the case of Cefprozil, microbial dysbiosis itself was found to be subject-dependent.Citation47
Studies in children offer some important insights. Early-life antibiotic exposure has been associated with an increased likelihood of childhood asthma,Citation49,Citation50 and this could also pose a risk factor for atopic dermatitis and allergic rhinitis.Citation51
One pediatric study demonstrated that higher antibiotic dosage increased risk of childhood asthma, and use of broad-spectrum-only antibiotics over narrow-spectrum-only antibiotics also increased asthma risk.Citation52 However, in the same study, such a trend was not noted regarding timing of antibiotic administration nor antibiotic class.
In this review, three out of the 21 adult studies reported specifically on the effect of antibiotics on the microbiome and downstream allergic conditions,Citation18,Citation19,Citation33 but only one was a randomized controlled trial.Citation18 The other two studies were cohort-based observational studies that aimed to identify associations between a variety of factors, including antibiotic use and allergic conditions.Citation19,Citation33 Two of these three studies found an association between antibiotic use and asthma,Citation18,Citation33 while the third study found no significant association between antibiotic use and atopy.Citation19
The effect of antibiotic use on microbiomes and allergic conditions can vary widely depending on different combinations of various factors ().
Table 8 Table Summarizing the Varying Effects of Antibiotic Use Observed in Studies Based on Different Combinations of Factors
In addition, microbiomes in different anatomical locations may respond differently to antibiotic-induced microbial dysbiosis. For example, the gut restores its baseline microbiome more slowly than those in the throat and saliva.Citation57
There is also the possibility of reverse causation blurring the relationship between antibiotic use and allergy. Antibiotics are commonly prescribed in cases of respiratory infection and may be prescribed when there is diagnostic uncertainty with underlying allergic conditions. Studies that corrected for these confounding effects in children no longer observed an increase in allergy risk associated with antibiotics.Citation60,Citation61
Considering the apparent lack of substantive studies, more research would be helpful in determining whether similar risks to those identified in early childhood persist into adulthood.
Effect of Antibiotics on Allergic Conditions Mediated by the Immune System
Multiple studies have described ways in which the microbiome, immune system and allergies may be linked. This review found four studies that investigated the influence of antibiotics or microbiomes on immune system components.Citation17,Citation18,Citation26,Citation31
Specifically, bacterial community memberships and bacterial abundance were linked to immune system components such as eosinophils, immunoglobins (eg IgE), cytokines (eg IL-6, IL-8, IL-10, IL1β, TNFα and G-CSF), memory T cells, helper T cells (eg T helper 17 cells) and chemokines (eg, CXCL11).Citation18
Differences were found in the degree of induction of cytokines and chemokines between different asthma-associated bacterial species.Citation31 Levels of cytokines (eg IL-2 and MIP1β) were also linked to airway obstruction phenotypes in asthmatics.Citation26
While these results support the view that the effect of antibiotics on allergic conditions is facilitated by immune system modulation by bacterial species, this conclusion is not consistent among all authors. One study found no significant differences in levels of immune cells (eg eosinophils, lymphocytes, neutrophils and macrophages), cytokines (eg IL-1RA, IL-1B, IL-2, IL-6, IL-8, IL-33, TNFα, MPO) or chemokines (eg, Periostin and RANTES) between the control and antibiotic-treatment groups in a randomized controlled trial done in asthmatics.Citation18
Relationship Between the Immune Response and Allergic Conditions
The reviewed studies highlight that microbial dysbiosis and corresponding shifts in bacterial populations could influence the induction of cytokines and chemokines,Citation26,Citation31 as well as the levels of inflammatory cells.Citation17
The combination of numerous cytokines, chemokines and inflammatory cells with their respective mechanisms and pathways might explain a broad variety of clinical asthmatic phenotypes and other allergic conditions. The relationship between the microbiome and immune system is also likely to be bidirectional.Citation62 As mentioned previously, the microbiome plays a pivotal role in mediating the immune system in allergy. However, the immune system also plays a vital role in maintaining a non-pathogenic microbiome in different anatomical compartments.Citation6,Citation63 While there is a case to call for additional research, the possible permutations of the immune system are extensive and even greater when considering other factors like individual genomics and environmental factors.Citation64
Potential Mechanisms Underlying Immune System Modulation by Microbiomes
An important aspect of research into the underlying mechanisms of bacteria-mediated immune modulation has examined the role of bacteria-derived metabolites,Citation65 such as SCFAs described in children and mouse models.Citation66–68 Bacterial dysbiosis and loss of SCFA-generating species might result in the corresponding loss of SCFA-mediated protective effects against allergy following antibiotics that disrupt the microbiome.
Upregulation of specific bacterial species that generate pro-inflammatory metabolites after microbial dysbiosis could also influence the allergic response. A study in human neonates uncovered associations between specific metabolites and significantly different gut microbiome compositions, as well as an elevated risk for childhood atopy and asthma.Citation69 However, it remains to be seen whether such associations carry similar risk implications for atopy in adults.
Additional Factors That Might Impact the Relationship Between Antibiotics, Microbiome and Allergic Conditions
It is likely that the human microbiome is unique to each individual at some level. The microbiome is exposed to and shaped by life experiences, including diet, environmental factors and exercise, among others.Citation70 Additionally, metagenomic analyses have shown that individual-specific bacterial strains bear extremely high temporal stability.Citation71 Some of these factors may be prominent in determining the individual’s microbiome and immune system responses following antibiotic exposure.
Age
Several studies cite age as a potentially important variable in their results.Citation19,Citation21,Citation24,Citation30,Citation31,Citation33 In comparing healthy controls against asthmatics, significant microbiome differences were observed within different adult age groups, specifically between young adults versus elderly adults,Citation22 suggesting a natural evolution of the microbiome with age.
Developmental age appears to be a key determinant of compositional and functional differences within the intestinal microbiota.Citation70,Citation72,Citation73 In humans, lower gut microbiome diversity has been noted in the first week or month of life (but not first year) for asthmatic children compared to non-asthmatic children.Citation74 Murine studies have also demonstrated that early-life changes in microbiomes are linked to changes in immune responses and could be linked to protective effects on allergic asthma.Citation75 Nasopharyngeal microbiome diversities among asthmatic adolescents were found to change and become significantly different from those in asthmatic children,Citation76 indicating that microbiome development is not limited to infancy.
While studies propose that early-life changes in specific bacterial taxa could be important in determining childhood allergy risk, it is less evident how these relationships play out in adulthood.Citation73,Citation77–80 However, in a wider context, it would be prudent to raise and maintain awareness of microbiome dysbiosis as a potential risk to adult patients following antibiotic therapy.
Allergy-Associated Inhaled Corticosteroids Medication as a Possible Confounder
Patients using regular inhaled corticosteroids (ICS) for symptom control are at risk of potential immunosuppressant effects leading to bacterial overgrowth.Citation81 ICS could contribute to shifts in microbial composition and functional pathways,Citation82 as well as immune response.Citation83
While the short-term effect of ICS use can be controlled by exclusion criteria, the effect of long-term ICS use is unknown for asthma and largely unaccounted for in most studies.
Clinical Implications
The potential consequences of antibiotic therapy, including uncertain impact on allergic conditions, underscores the need for judicious prescribing.Citation84 In addition, close clinical monitoring and diligent pharmacovigilance in patients with atopic conditions who are treated with antibiotics may signal currently underappreciated risk. Based on a global point prevalence survey conducted in 53 countries on 29,891 adult in-patients regarding antibiotic consumption and resistance, a significant percentage (34.4%) of inpatients was prescribed at least one antibiotic.Citation85 This is a source of potentially substantial data for clinical research on antibiotic use and atopic conditions. While there is currently insufficient understanding of precise clinical interventions to steer the microbiome in a favorable direction, this line of research holds potential for future therapeutic applications.
Limitations
The scarcity of relevant studies in adults limits the insights and conclusions regarding this subject. Confining the review to human studies and English language literature potentially overlooks important scientific contributions, particularly for non-English publications.
Broadening inclusion criteria for studies to include non-systematic literature reviews pertaining to adults added heterogeneity and could have limited the objectivity of reviewed studies.
An additional limitation is that most studies focused on asthma, limiting insights regarding other allergic conditions.
Future Areas for Research
A lack of compelling and consistent evidence and a need for research is mentioned in several sections in this review. Additionally, a deeper understanding of the effects of substances that encourage microbial growth (prebiotics) or those that directly introduce micro-organisms (probiotics) in human microbiomes is worth investigating.
The downstream effects of micro-organisms induced or introduced into human microbiomes by vaccination, might also be of interest. For example, research to gain insight as to whether bacterial species targeted by vaccination, such as pneumococcal vaccines, are associated with microbiome changes, may also provide valuable insights.
The role of microbiomes in prevention of disease-causing bacterial pathogens developing antimicrobial resistance is an additional area with potential for future research.
The heterogeneous data in the reviewed studies reflect widely variable factors such as antibiotic type, dosage, timing and duration of treatment, microbiome sampling site and technique, environmental exposure, immune status, allergic condition and genomics. However, even with inherent complexity, more definitive insights are likely to emerge given advances in sequencing methods, which now allow for large-scale and rapid sequencing of the microbiome.Citation86
Conclusion
Our review could not conclusively determine links between antibiotics, microbial dysbiosis, and allergic conditions in adults, and was limited by the scarcity of relevant literature. Developmental age appears to be critically important in shaping the microbiome’s composition and function, and a significant determinant of subsequent atopic risk.
While research in children indicates important links between the developing microbiome, immune system maturation and risk of allergy, equivalent research in adults is limited.
Despite the lack of clear consensus found in this review, heightened awareness of appropriate antibiotic prescribing practices and the importance of pharmacovigilance are emphasized. Considering how commonly antibiotics are prescribed for allergy-related conditions, there is opportunity and a need for more research in this area.
Abbreviations
AMR, antimicrobial resistance; AAP, appropriate antibiotic prescribing, SLR, systematic literature review; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; GRACE, Good Research for Comparative Effectiveness; SCFA, short-chain fatty acids; T2, Type 2 Inflammation; PPAR, Peroxisome proliferator-activated receptor; ICS, inhaled corticosteroids.
Author Contributions
All listed authors for this SLR meet the authorship criteria as outlined by International Committee of Medical Journal Editors (ICMJE), bear accountability for the integrity of their written article, and have agreed to have this article published in this journal.
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Wan Zhen Janice Ng has no disclosures. Bhumika Aggarwal, James van Hasselt and Anand Manoharan are employees of GSK and hold stocks or shares in the company.
Additional information
Funding
References
- Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72–80. doi:10.1016/j.mib.2019.10.008
- Pacios O, Blasco L, Bleriot I, et al. Strategies to combat multidrug-resistant and persistent infectious diseases. Antibiot Basel Switz. 2020;9(2):E65. doi:10.3390/antibiotics9020065
- Renz H, Skevaki C. Early life microbial exposures and allergy risks: opportunities for prevention. Nat Rev Immunol. 2021;21(3):177–191. doi:10.1038/s41577-020-00420-y
- Duong QA, Pittet LF, Curtis N, Zimmermann P. Antibiotic exposure and adverse long-term health outcomes in children: a systematic review and meta-analysis. J Infect. 2022;85(3):213–300. doi:10.1016/j.jinf.2022.01.005
- Koidl L, Untersmayr E. The clinical implications of the microbiome in the development of allergy diseases. Expert Rev Clin Immunol. 2021;17(2):115–126. doi:10.1080/1744666x.2021.1874353
- Kogut MH, Lee A, Santin E. Microbiome and pathogen interaction with the immune system. Poult Sci. 2020;99(4):1906–1913. doi:10.1016/j.psj.2019.12.011
- Houghteling PD, Walker WA. From birth to “immunohealth”, allergies and enterocolitis. J Clin Gastroenterol. 2015;49(Suppl 1):S7–S12. doi:10.1097/mcg.0000000000000355
- Iebba V, Totino V, Gagliardi A, et al. Eubiosis and dysbiosis: the two sides of the microbiota. New Microbiol. 2016;39(1):1–12.
- Patrick DM, Sbihi H, Dai DLY, et al. Decreasing antibiotic use, the gut microbiota, and asthma incidence in children: evidence from population-based and prospective cohort studies. Lancet Respir Med. 2020;8(11):1094–1105. doi:10.1016/S2213-2600(20)30052-7
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi:10.7326/M14-2385
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
- Dreyer NA, Bryant A, Velentgas P. The GRACE checklist: a validated assessment tool for high quality observational studies of comparative effectiveness. J Manag Care Spec Pharm. 2016;22(10):1107–1113. doi:10.18553/jmcp.2016.22.10.1107
- Panzer AR, Lynch SV. Influence and effect of the human microbiome in allergy and asthma. Curr Opin Rheumatol. 2015;27(4):373–380. doi:10.1097/BOR.0000000000000191
- Kozik AJ, Huang YJ. The microbiome in asthma: role in pathogenesis, phenotype, and response to treatment. Ann Allergy Asthma Immunol. 2019;122(3):270–275. doi:10.1016/j.anai.2018.12.005
- Han MK, Huang YJ, Lipuma JJ, et al. Significance of the microbiome in obstructive lung disease. Thorax. 2012;67(5):456–463. doi:10.1136/thoraxjnl-2011-201183
- Martinez FD. The human microbiome. Early life determinant of health outcomes. Ann Am Thorac Soc. 2014;(Suppl 11):S7–12. doi:10.1513/AnnalsATS.201306-186MG
- Durack J, Christian LS, Nariya S, et al. Distinct associations of sputum and oral microbiota with atopic, immunologic, and clinical features in mild asthma. J Allergy Clin Immunol. 2020;146(5):1016–1026. doi:10.1513/AnnalsATS.201306-186MG
- Van Engelen TSR, Yang J, Haak BW, et al. Gut microbiome modulation by antibiotics in adult asthma: a human proof-of-concept intervention trial. Clin Gastroenterol Hepatol off Clin Pract J Am Gastroenterol Assoc. 2022;20(6):1404–1407.e4. doi:10.1016/j.cgh.2021.07.030
- Cullinan P, Harris J, Mills P, et al. Early prescriptions of antibiotics and the risk of allergic disease in adults: a cohort study. Thorax. 2004;59(1):11–15.
- Durack J, Huang YJ, Nariya S, et al. Bacterial biogeography of adult airways in atopic asthma. Microbiome. 2018;6(1):104. doi:10.1186/s40168-018-0487-3
- Okba AM, Saber SM, Abdel-Rehim AS, Amin MM, Mohamed DA. Fecal microbiota profile in atopic asthmatic adult patients. Eur Ann Allergy Clin Immunol. 2018;50(3):117–124. doi:10.23822/EurAnnACI.1764-1489.48
- Lee JJ, Kim SH, Lee MJ, et al. Different upper airway microbiome and their functional genes associated with asthma in young adults and elderly individuals. Allergy. 2019;74(4):709–719. doi:10.1111/all.13608
- Wang Q, Li F, Liang B, et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 2018;18(1):114. doi:10.1186/s12866-018-1257-x
- Hua X, Goedert JJ, Pu A, Yu G, Shi J. Allergy associations with the adult fecal microbiota: analysis of the American Gut Project. EBioMedicine. 2016;3:172–179. doi:10.1016/j.ebiom.2015.11.038
- Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013;131(2). doi:10.1016/j.jaci.2012.11.013
- Turturice BA, McGee HS, Oliver B, et al. Atopic asthmatic immune phenotypes associated with airway microbiota and airway obstruction. PLoS One. 2017;12(10):e0184566. doi:10.1371/journal.pone.0184566
- Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127(2):372–381.e1–3. doi:10.1016/j.jaci.2010.10.048
- Turek EM, Cox MJ, Hunter M, et al. Airway microbial communities, smoking and asthma in a general population sample. EBioMedicine. 2021;71:103538. doi:10.1016/j.ebiom.2021.103538
- Buendía E, Zakzuk J, San-Juan-Vergara H, Zurek E, Ajami NJ, Caraballo L. Gut microbiota components are associated with fixed airway obstruction in asthmatic patients living in the tropics. Sci Rep. 2018;8(1):9582. doi:10.1038/s41598-018-27964-3
- Simpson JL, Daly J, Baines KJ, et al. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47(3):792–800. doi:10.1183/13993003.00405-2015
- Hosoki K, Nakamura A, Kainuma K, et al. Differential activation of eosinophils by bacteria associated with asthma. Int Arch Allergy Immunol. 2013;161(Suppl 2):16–22. doi:10.1159/000350338
- Mashiah J, Fliss-Isakov N, Sprecher E, et al. Clinical efficacy of fecal microbial transplantation treatment in adults with moderate-to-severe atopic dermatitis. Immun Inflamm Dis. 2022;10(3). doi:10.1002/iid3.570
- Yang SI, Lee E, Jung YH, et al. Effect of antibiotic use and mold exposure in infancy on allergic rhinitis in susceptible adolescents. Ann Allergy Asthma Immunol. 2014;113(2):160–165.e1. doi:10.1016/j.anai.2014.05.019
- Collins English Dictionary. Bacterial Load. HarperCollins Publishers. Available from: https://www.collinsdictionary.com/us/dictionary/english/bacterial-load. Accessed December 26, 2022.
- Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357(15):1487–1495. doi:10.1056/nejmoa052632
- Pascal M, Perez-Gordo M, Caballero T, et al. Microbiome and allergic diseases. Front Immunol. 2018;9:1584. doi:10.3389/fimmu.2018.01584
- Ahmed B, Cox MJ, Cuthbertson L, et al. Comparison of the upper and lower airway microbiota in children with chronic lung diseases. PLoS One. 2018;13(8):e0201156. doi:10.1371/journal.pone.0201156
- An SQ, Warris A, Turner S. Microbiome characteristics of induced sputum compared to bronchial fluid and upper airway samples. Pediatr Pulmonol. 2018;53(7):921–928. doi:10.1002/ppul.24037
- Pérez-Losada M, Crandall KA, Freishtat RJ. Two sampling methods yield distinct microbial signatures in the nasopharynges of asthmatic children. Microbiome. 2016;4(1):25. doi:10.1186/s40168-016-0170-5
- Gómez-Martín M, Saturio S, Arboleya S, et al. Association between diet and fecal microbiota along the first year of life. Food Res Int. 2022;162:111994. doi:10.1016/j.foodres.2022.111994
- Hilty M, Burke C, Pedro H, et al. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5(1):e8578. doi:10.1371/journal.pone.0008578
- Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol. 2001;108(4):516–520. doi:10.1067/mai.2001.118130
- Chung KF. Airway microbial dysbiosis in asthmatic patients: a target for prevention and treatment? J Allergy Clin Immunol. 2017;139(4):1071–1081. doi:10.1016/j.jaci.2017.02.004
- Green BJ, Wiriyachaiporn S, Grainge C, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9(6):e100645. doi:10.1371/journal.pone.0100645
- Ipci K, Altıntoprak N, Muluk NB, Senturk M, Cingi C. The possible mechanisms of the human microbiome in allergic diseases. Head Neck Surg. 2017;274(2):617–626. doi:10.1007/s00405-016-4058-6
- Lunjani N, Satitsuksanoa P, Lukasik Z, Sokolowska M, Eiwegger T, O’Mahony L. Recent developments and highlights in mechanisms of allergic diseases: microbiome. Allergy. 2018;73(12):2314–2327. doi:10.1111/all.13634
- Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4554–4561. doi:10.1073/pnas.1000087107
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6(11):e280. doi:10.1371/journal.pbio.0060280
- Marra F, Lynd L, Coombes M, et al. Does antibiotic exposure during infancy lead to development of asthma?: a systematic review and meta analysis. Chest. 2006;129(3):610–618. doi:10.1378/chest.129.3.610
- Celedón JC, Fuhlbrigge A, Rifas-Shiman S, Weiss ST, Finkelstein JA. Antibiotic use in the first year of life and asthma in early childhood. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2004;34(7):1011–1016. doi:10.1111/j.1365-2222.2004.01994.x
- Yamamoto-Hanada K, Yang L, Narita M, Saito H, Ohya Y. Influence of antibiotic use in early childhood on asthma and allergic diseases at age 5. Ann Allergy Asthma Immunol off Publ Am Coll Allergy Asthma Immunol. 2017;119(1):54–58. doi:10.1016/j.anai.2017.05.013
- Donovan BM, Abreo A, Ding T, et al. Dose, Timing, and Type of Infant Antibiotic Use and the Risk of Childhood Asthma. Clin Infect Dis. 2020;70(8):1658–1665. doi:10.1093/cid/ciz448
- Eftekharivash L, Hamedi J, Zarrini G, Bakhtiari R. Acidophilic and acid tolerant actinobacteria as new sources of antimicrobial agents against Helicobacter pylori. Arch Razi Inst. 2021;76(2):261–272. doi:10.22092/ari.2019.128039.1401
- Stewardson AJ, Gaïa N, François P, et al. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin Microbiol Infect off Publ Eur Soc Clin Microbiol Infect Dis. 2015;21(4):344.e1–11. doi:10.1016/j.cmi.2014.11.016
- Slater M, Rivett DW, Williams L, et al. The impact of azithromycin therapy on the airway microbiota in asthma. Thorax. 2014;69(7):673–674. doi:10.1136/thoraxjnl-2013-204517
- Taylor SL, Leong LEX, Mobegi FM, et al. Long-term azithromycin reduces Haemophilus influenzae and increases antibiotic resistance in severe asthma. Am J Respir Crit Care Med. 2019;200(3):309–317. doi:10.1164/rccm.201809-1739OC
- Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. PLoS One. 2010;5(3):e9836. doi:10.1371/journal.pone.0009836
- De La Cochetière MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Doré J. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol. 2005;43(11):5588–5592. doi:10.1128/JCM.43.11.5588-5592.2005
- Zimmermann P, Curtis N. The effect of antibiotics on the composition of the intestinal microbiota - A systematic review. J Infect. 2019;79(6):471–489. doi:10.1016/j.jinf.2019.10.008
- Legatzki A, Rösler B, von Mutius E. Microbiome diversity and asthma and allergy risk. Curr Allergy Asthma Rep. 2014;14(10):466. doi:10.1007/s11882-014-0466-0
- Kuo CH, Kuo HF, Huang CH, Yang SN, Lee MS, Hung CH. Early life exposure to antibiotics and the risk of childhood allergic diseases: an update from the perspective of the hygiene hypothesis. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. 2013;46(5):320–329. doi:10.1016/j.jmii.2013.04.005
- Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity. 2017;46(4):562. doi:10.1016/j.immuni.2017.04.008
- Parkin J, Cohen B. An overview of the immune system. Lancet Lond Engl. 2001;357:9270. doi:10.1016/S0140-6736(00)04904-7
- Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi:10.1136/gutjnl-2015-309990
- Levy M, Blacher E, Elinav E. Microbiome, metabolites and host immunity. Curr Opin Microbiol. 2017;35:8–15. doi:10.1016/j.mib.2016.10.003
- Rackaityte E, Lynch SV. The human microbiome in the 21st century. Nat Commun. 2020;11(1):5256. doi:10.1038/s41467-020-18983-8
- Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev off J Int Assoc Study Obes. 2017;18(1):18–31. doi:10.1111/obr.12484
- Roduit C, Frei R, Depner M, et al. Increased food diversity in the first year of life is inversely associated with allergic diseases. J Allergy Clin Immunol. 2014;133(4):1056–1064. doi:10.1016/j.jaci.2013.12.1044
- Fujimura KE, Sitarik AR, Havstad S, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–1191. doi:10.1038/nm.4176
- Lozupone CA, Stombaugh J, Gonzalez A, et al. Meta-analyses of studies of the human microbiota. Genome Res. 2013;23(10):1704–1714. doi:10.1101/gr.151803.112
- Sunagawa S, Schloissnig S, Arumugam M, et al. Individuality and temporal stability of the human gut microbiome. Cent Asian J Glob Health. 2013;2(Suppl):120. doi:10.5195/cajgh.2013.120
- Stiemsma LT, Turvey SE. Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin Immunol off J Can Soc Allergy Clin Immunol. 2017;13:3. doi:10.1186/s13223-016-0173-6
- Arrieta MC, Stiemsma LT, Dimitriu PA, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015;7(307):307ra152. doi:10.1126/scitranslmed.aab2271
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2014;44(6):842–850. doi:10.1111/cea.12253
- Cahenzli J, Köller Y, Wyss M, Geuking MB, McCoy KD. Intestinal microbial diversity during early-life colonization shapes long-term IgE levels. Cell Host Microbe. 2013;14(5):559–570. doi:10.1016/j.chom.2013.10.004
- Pérez-Losada M, Alamri L, Crandall KA, Freishtat RJ. Nasopharyngeal microbiome diversity changes over time in children with asthma. PLoS One. 2017;12(1):e0170543. doi:10.1371/journal.pone.0170543
- Stiemsma LT, Arrieta MC, Dimitriu PA, et al. Shifts in Lachnospira and Clostridium sp. in the 3-month stool microbiome are associated with preschool age asthma. Clin Sci Lond Engl. 2016;130(23):2199–2207. doi:10.1042/CS20160349
- Santacroce L, Man A, Charitos IA, Haxhirexha K, Topi S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front Biosci Landmark Ed. 2021;26(6):135–148. doi:10.52586/4930
- Nash MJ, Frank DN, Friedman JE. Early microbes modify immune system development and metabolic homeostasis—the “restaurant” hypothesis revisited. Front Endocrinol. 2017;8:349. doi:10.3389/fendo.2017.00349
- Ouwehand A, Isolauri E, Salminen S. The role of the intestinal microflora for the development of the immune system in early childhood. Eur J Nutr. 2002;41(Suppl 1):I32–37. doi:10.1007/s00394-002-1105-4
- Durack J, Lynch SV, Nariya S, et al. Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol. 2017;140(1):63–75. doi:10.1016/j.jaci.2016.08.055
- Huang C, Yu Y, Du W, et al. Insights into gut microbiome and its functional pathways in asthma patients through high-throughput sequencing. Future Microbiol. 2021;16:421–438. doi:10.2217/fmb-2020-0101
- Toraldo DM, Rizzo E, Conte L. Effects of inhaled corticosteroids (ICS) on lung microbiota and local immune response in long-term treatment of chronic obstructive pulmonary disease (COPD): utility of titration and therapeutic index. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(7):849–858. doi:10.1007/s00210-022-02237-z
- Anderson M, Clift C, Schulze K, et al. Averting the AMR crisis: what are the avenues for policy action for countries in Europe? European Observatory on Health Systems and Policies; 2019. Available from: http://www.ncbi.nlm.nih.gov/books/NBK543406/. Accessed November 9, 2022.
- Versporten A, Zarb P, Caniaux I, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619–e629. doi:10.1016/S2214-109X(18)30186-4
- Wensel CR, Pluznick JL, Salzberg SL, Sears CL. Next-generation sequencing: insights to advance clinical investigations of the microbiome. J Clin Invest. 2022;132(7):e154944. doi:10.1172/JCI154944
