Abstract
Asthma is a complex disorder frequently associated with a poor symptom control, concomitant morbidity, mortality, and significant health care costs due to lack of compliance or inadequate therapeutic options. Interleukin-5 (IL-5) plays a key role in the pathogenesis of eosinophilic disorders, and in the latest years has become a definite target for treatment. Besides asthma, other hypereosinophilic disorders include the hypereosinophilic syndrome, eosinophilic granulomatosis with polyangiitis, sinonasal polyposis, COPD with eosinophilic airway inflammation, allergic rhinitis, atopic dermatitis, eosinophilic esophagitis. The introduction of mepolizumab, a fully humanized monoclonal antibody that binds to IL-5, may represent a useful therapeutic option to control exacerbations and improve asthma-related quality of life in a subgroup of patients with persistent airway eosinophilia and moderate to severe asthma. Several studies carried out in recent years allow, at present, a careful patient selection for appropriate individualized treatment in severe asthma. Further research is anyway needed in order to better understand the pathogenetic mechanisms of asthma and to find new biomarkers. The high costs of biological agents as compared with standard drugs may be largely offset by increased clinical efficacy and good safety profile in selected patients.
Introduction
Severe asthma is frequently associated with a suboptimal symptom and exacerbation control,Citation1 concomitant morbidity, mortality, and significant health care costsCitation2 due to inadequate therapeutic options and poor adherence, despite the availability of several effective drugs at present. As a consequence, there is an increasing need to identify new molecules able to overcome treatment limitations. Asthma is a complex disorder with many different phenotypes and endotypesCitation3 whose definition is based on clinical, inflammatory, or causative factors. The most important pathophysiological mechanism of asthma is represented by airways inflammation, predisposing to exacerbations and bronchial remodeling especially in the absence of regular adequate therapy.Citation4–Citation6 A typical characteristic of cellular inflammation in asthma is the, sometimes, concomitant presence of eosinophils and neutrophils and their distinct roles in the pathogenesis of the disease.Citation7 Eosinophilic asthma is a specific phenotype associated with thickening of the basement membrane and with corticosteroid responsiveness. On the contrary, noneosinophilic asthma, which includes patients with severe disease, is not characterized by basement membrane thickening, and appears to be corticosteroid resistant.Citation8
Many researchers have been trying to find drugs to treat bronchial inflammation and prevent the allergic and nonallergic inflammatory cascade responsible for acute relapses of asthma.
The introduction of omalizumab has represented an innovative tool for therapy, allowing to deal with some unmet needs, in particular for the immunoglobulin E (IgE)-driven phenotype of asthma.Citation9,Citation10 Some patients with severe atopic and nonatopic asthma have frequent exacerbations associated with persistent eosinophilic inflammation despite continuous treatment with high-dose inhaled glucocorticoids, with or without oral glucocorticoids. Up to 60% of the patients present tissue eosinophilia,Citation11 whose level is correlated with asthma severity.Citation12 Most exacerbations are eosinophilic, while a neutrophilic infiltrate is less common.Citation13 Mepolizumab, a humanized monoclonal antibody against interleukin 5 (IL-5) (anti-IL-5 hMoAb), selectively inhibits eosinophilic airway inflammation and induces a significant reduction in severe asthma exacerbations, regardless of IgE levels in blood and atopy. Early studies have demonstrated that mepolizumab does not modify pulmonary function.Citation14,Citation15
The aim of this article was to review current literature on mepolizumab, focusing on its applications in the treatment of severe asthma and other hypereosinophilic diseases.
IL-5 and eosinophil role in the inflammatory cascade
Recruitment, granule maturation, and survival of eosinophils in the airways are promoted by IL-3, granulocyte–macrophage colony stimulating factor (GM-CSF), and especially IL-5, which represents the most important interleukin responsible for eosinophilic airway inflammation in asthmatics.Citation16 IL-5 is a 134-amino acid protein that forms a 52 kDa homodimer related to both GM-CSF and IL-3.Citation17–Citation19 IL-5 is produced by CD4+ Th2 cells, mast cells, eosinophils, and basophils and is involved in several steps of eosinophil development and function.Citation20 It stimulates the final differentiation of activated B cells into antibody-forming cells and enhances the proliferation and differentiation of eosinophils precursors.Citation21
According to some recent experimental studies on murine models, IL-5 seems also to be involved in airway remodeling, since administration of anti-IL-5 in mice blocks almost completely peribronchial and sub-epithelial fibrosis induced by allergen inhalation.Citation22
Mature eosinophils circulate in the blood for 6–10 hoursCitation23 and then migrate to connective tissues to end their life cycle after 8–12 days. The main function of eosinophils is to activate inflammatory immune reactions and to protect the organism from parasites and worms through a number of mechanisms, such as antigen–antibody internalization and presentation, release of proinflammatory mediators, and microrganism killing. These cells express on their surface-specific receptors for the Fc fragment of IgE and are thus able to recognize and bind microorganisms (mainly helminths) opsonized by these antibodies.
Their granules contain not only cytotoxic primary cationic proteins (eosinophil peroxidase, eosinophil cationic protein, major basic protein, eosinophil-derived neurotoxin) but also mediators of allergic reactions, such as histaminase and arylsulfatase. Eosinophils also release IL-5 and leukotrienes that are involved in the pathophysiology of asthma by increasing the secretion of mucus and inducing bronchoconstriction.Citation24 The eosinophilia is associated with a wide variety of conditions besides asthma, such as atopic diseases, helminthic infestation, hypersensitivity to drugs, and cancer.
Eosinophils express in particular type I receptor IL-5Rα, so they respond primarily to IL-5, whose gene is located on chromosome 5 and is close to the genes encoding for IL-3, IL-4, and GM-CSF. The IL-5 receptor includes an α chain and a βc chain; the α chain is specific for IL-5, while the βc subunit is also recognized by IL-3 and GM-CSF.
IL-5 plays an important role also in basophil and mast cell activity. These cells express several cytokine receptors, such as IL-5R, IL-3R, IL-4R, IL-2Ra, and GM-CSFRa,Citation25–Citation28 and produce IL-4 and IL-13.Citation27 In addition, after IL-5 and GM-CSF stimulation, an increased production of mediators has been observed. Moreover, IL-5 amplifies allergen-induced histamine release from basophils in patients with allergic rhinitis.Citation28
The expression of IL-5 is regulated by several transcription factors including GATA3, and its clinical significance has long been associated with numerous diseases including allergic rhinitis and asthma, characterized by a significant increase of eosinophils in blood and airways.
Produced by Th2 lymphocytes and activated mast cells, the main biological role of IL-5 is to control proliferation, differentiation, and activation of eosinophils, representing a functional link between T-cell activation and inflammatory responses mediated by eosinophils.
Eosinophils express a wide spectrum of surface receptors, at least ten different classes, such as CC-chemokine receptor 3 (CCR3), that mediates eosinophil chemotaxis in response to the eotaxins, CCL11, CCL24, and CCL26.Citation29 CCR3 can also be activated by CCL5/RANTES, CCL7/MCP3, CCL/MCP2, and CCL12/MCP5. Eosinophils also express CCR1, the primary receptor for CCL3/MIP1α, CCL5, and the platelet-activating factor receptor.
Other receptors are SIGLEC-8 and SIGLEC-F, cell-surface Ig-like lectins that are members of structurally related carbohydrate-binding protein family.Citation30 The functions of these proteins are still unknown, but antibodies specific for SIGLEC-8 induce apoptosis of eosinophils.
Pattern-recognition receptors and toll-like receptors are expressed by both human and mouse eosinophils,Citation31 although at lower levels than neutrophils and macrophages. These proteins promote eosinophil function in vivo and regulate chemotaxis, adhesion, and survival.Citation32
Neutrophils are frequently present in the airways of patients with severe asthma and/or fixed airflow obstruction, and can be detected in airway secretions during severe asthma exacerbations. IL-8 is an important chemoattractant for neutrophils, and its expression by airway smooth muscle cells is upregulated in severe asthmatics.Citation27 In the absence of eosinophil chemoattractants, IL-8-stimulated neutrophils help eosinophils to accumulate in asthmatic airways by releasing O2–, MMP-9, LTB4, and PAF.Citation33
Therapeutic approach to eosinophilic disorders
Given the critical role of IL-5 in influencing the biological activities of eosinophils, this molecule and its receptor are a clear target in the treatment of hypereosinophilic diseases. The spectrum of eosinophilic disorders is broad, and includes the hypereosinophilic syndrome (HES), nasal polyposis (NP), COPD with eosinophilic airway inflammation,Citation34 allergic rhinitis, atopic dermatitis, eosinophilic esophagitis, eosinophilic granulomatosis with polyangiitis, but especially bronchial asthma ().Citation35
Table 1 Efficacy and safety of mepolizumab in asthma and other eosinophilic diseases
Several randomized clinical trials (RCTs) have been performed, and many others are currently in various stages of development with regard to anti-IL-5 (mepolizumab, reslizumab) or IL-5Rα (benralizumab) monoclonal antibodies. All the clinical trials conducted so far showed a reduction of eosinophils in peripheral blood and induced sputum but a large variability has been found in clinical results ().Citation14,Citation36–Citation41
Early studies and controversies on mepolizumab for asthma
Mepolizumab (SB-240563, Bosatria®; GlaxoSmithKline, Brentford, UK) was the first anti-IL-5 molecule designed and tested in RCTs for eosinophilic allergic asthma. It is a humanized monoclonalCitation42 N-glycosylated IgG1 kappa antibody able to inhibit IL-5 with both high specificity (half maximal inhibitory concentration <1 nM) and affinity (kilodalton =4.2 pM), thus preventing its binding to the alpha chain of the IL-5 receptor complex expressed on the eosinophil cell surface.Citation43 Mepolizumab is formed by two light chains and two heavy chains connected by a disulfide bridge with a molecular weight of 49 kDa ().Citation44–Citation47
Figure 1 Interaction between Th2 lymphocytes and eosinophils, and molecular structure of mepolizumab with its therapeutic target.
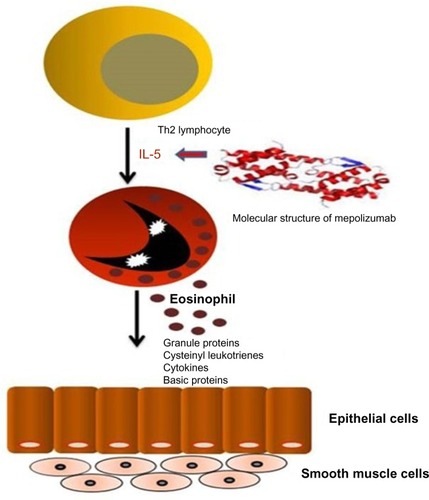
Hart et alCitation47 carried out a preclinical study on the pharmacological activity and safety of mepolizumab in Naive and Ascaris suum-sensitive cynomolgus monkeys. Single intravenous (IV) dose of mepolizumab significantly reduced blood eosinophilia, eosinophil migration into airways, and levels of RANTES and IL-6 in lungs for 6 weeks. However, mepolizumab did not affect acute bronchoconstrictive responses. Monthly doses of mepolizumab >5 mg/kg caused an 80%–100% decrease in blood and bronchoalveolar lavage eosinophils lasting 2 months after administration; there was no effect on eosinophil precursors in bone marrow after 6 months of treatment. The study demonstrated the safety of this drug in monkeys with a long-term reduction in circulating and tissue-resident eosinophils, which correlated with mepolizumab plasma concentrations. In one of the first studies on humans, Leckie et al tested mepolizumab in a small group of 24 patients with mild atopic asthma.Citation14 The study had the aim of evaluating efficacy of a single IV dose of mepolizumab in the reduction of eosinophilia in sputum, airway hyperresponsiveness (AHR), and late response to inhaled allergens. Study results showed a reduction of both blood and sputum eosinophils after 4 and 16 weeks of treatment: blood eosinophil level decreased from 0.25×109/L in the placebo group to 0.04×109/L in the 10 mg/kg group (P<0.0001). After inhaled allergen challenge, sputum eosinophils were 12.2% in the placebo group and 0.9% (P=0.0076) in the 10 mg/kg group, with a persistent effect at day 30 after the dose. There were no effects on AHR and late response to inhaled allergens.
Flood-Page et alCitation15 evaluated in a fashion randomized, double-blind, parallel-group study the effect of three 750 mg IV doses of mepolizumab or placebo in a group of 24 mild asthma patients. Mepolizumab produced a median decrease from baseline of 55% for airway eosinophils, 52% for bone marrow eosinophils, and 100% for blood eosinophils, without appreciable effect on bronchial mucosa staining for eosinophil major basic protein. There were no significant changes in clinical outcomes (AHR, forced expiratory volume in 1 second [FEV1], and peak expiratory flow [PEF] recordings) between the mepolizumab and placebo groups. Anti-IL-5 treatment reduced but did not deplete airway or bone marrow eosinophils.
Anti-IL-5 mAbs for asthma: from early clinical failure to evidence of efficacy
The first mepolizumab study produced contradictory results, and several authors questioned the efficacy of this new molecule on asthma treatment.Citation15,Citation48 As a matter of fact, no significant data were found in terms of AHR, PEF, and FEV1 despite a remarkable reduction of airway and blood eosinophiliaCitation14,Citation15 in fact, anti-IL-5 produced a median decrease of 55% for airway eosinophils, 52% for bone marrow eosinophils (P=0.003), and 100% for blood eosinophils (P=0.02). Another RCT had the aim of investigating the effect of three IV infusions of mepolizumab, 250 or 750 mg at monthly intervals, on clinical outcome measures in 362 patients with asthma experiencing persistent symptoms despite inhaled corticosteroid therapy. Mepolizumab was associated with a significant reduction in blood and sputum eosinophils in both treatment groups, but an incorrect selection of the sample did not take into due consideration the level of airways eosinophilic inflammation and did not allow statistically significant changes to be found in any of the clinical end points measured. There was a trend for decrease in exacerbation rates in the mepolizumab 750 mg treatment group, but it was not statistically significant (P=0.0065); therefore, the treatment did not appear to add clinical benefit.Citation36
On the contrary, two subsequent studies demonstrated mepolizumab efficacy in the control of asthma. The first one was a study on 61 subjects who had refractory eosinophilic asthma and a history of recurrent severe exacerbations.Citation37 Patients received IV infusions of either mepolizumab or placebo at monthly intervals for 1 year. Mepolizumab was associated with significantly fewer severe exacerbations than placebo (2.0 vs 3.4 mean exacerbations per patient), with a significant improvement in the Asthma Quality of Life Questionnaire AQLQ score and a decrease of eosinophil counts in the blood and sputum, P<0.001 and P=0.002, respectively. However, there were no significant differences between the two groups with respect to symptoms, postbronchodilator FEV1, or AHR. The second report was another study involving patients with persistent sputum eosinophilia and symptoms despite prednisone treatment.Citation38 Nine patients were assigned to receive mepolizumab, administered in five monthly IV infusions of 750 mg, and eleven patients to placebo. There were 12 asthma exacerbations in ten patients who received placebo, while only one patient who received mepolizumab had an asthma exacerbation. The use of mepolizumab was associated with a significant decrease in the number of sputum and blood eosinophils (P=0.002). Moreover, subjects who received mepolizumab were able to reduce their prednisone dose and improvements in eosinophil numbers, asthma control, and FEV1 were maintained for 8 weeks after the latest infusion.
A conclusive research was the DREAM trial,Citation39 a large multicentre, double-blind, placebo-controlled study recruiting 621 patients with a history of recurrent severe asthma exacerbations and signs of eosinophilic inflammation. They were randomly assigned to receive one of three doses of IV mepolizumab (75 mg, 250 mg, or 750 mg) or matched placebo (100 mL 0.9% NaCl). Mepolizumab was demonstrated to significantly reduce the number of asthma exacerbations in patients with severe eosinophilic asthma compared with placebo. It was observed that 75 mg, 250 mg, and 750 mg mepolizumab reduced the rate of clinically significant exacerbations per patient per year by 48% (P<0.001), 39% (P=0.005), and 52% (P <0.001), respectively. Additionally, each dose of treatment lowered blood and sputum eosinophil counts (P<0.0001) and was well tolerated for 12 months, despite a small effect on FEV1, AQLQ, and Asthma Control Questionnaire (ACQ) scores compared with the placebo group.
These studies have represented an important progress in the selection of the most appropriate treatment for the subgroup(s) of patients affected by severe asthma with frequent exacerbations and persistent eosinophilia, which may be approximately 40% of severe asthmatics.Citation49 A post hoc analysis of the DREAM trial tried to assess the effect of treatment with mepolizumab on the frequency of exacerbations in the atopic and non-atopic subgroups, seasonal patterns of response by subgroup, and the changes in lung function and exhaled nitric oxide fraction according to subgroup.Citation50 Interestingly, the reduction in exacerbations with mepolizumab treatment was unaffected by season or atopy/IgE levels. Later, a supervised cluster analysis with recursive partitioning approach was applied to data of DREAM study to identify characteristics that maximized the differences among subgroups.Citation51 Three predictors were identified in four primary clusters: blood eosinophils, airway reversibility, and body mass index. The reduction in exacerbations and significant therapeutic benefit was larger in patients with eosinophilic inflammation who received mepolizumab, confirming the necessity of this condition as a response predictor.
Another recent work compared the efficacy responses in the oral corticosteroids (OCS)-dependent group from the DREAM study with the non-OCS-dependent subgroup. Mepolizumab reduced the peripheral eosinophils and was effective at reducing exacerbation rates in the non-OCS and OCS groups during the 52-week treatment period with a greater reduction noted in the OCS group. At study entry, peripheral eosinophils level was ≥300 cells/mL in the previous 12 months; after active treatment, a reduction of 71% in non-OCS-dependent group and 65% in OCS-dependent group was observed; P=0.136 for non-OCS versus OCS groups. The exacerbation rate/year was 1.90 for placebo and 1.07 for mepolizumab in the non-OCS-dependent group, and 3.12 for placebo and 1.54 for mepolizumab in the OCS-dependent group, with a rate ratio of 0.56 and 0.49, respectively (P=0.503). These results showed that mepolizumab treatment reduces peripheral eosinophils and exacerbation risk both in OCS-dependent and non-OCS-dependent group.Citation52
In the subsequent MENSA trial, 576 patients were selected with recurrent asthma exacerbations and eosinophilic inflammation despite high doses of inhaled glucocorticoids in one of three study groups.Citation53 Patients were assigned to receive treatment with mepolizumab, administered as either a 75 mg IV dose or a 100 mg subcutaneous (SC) dose, or placebo every 4 weeks for 32 weeks. The primary end point was the rate of exacerbation, secondary outcomes were FEV1, scores on the St George’s Respiratory Questionnaire (SGRQ), and the five-item Asthma Control Questionnaire (ACQ-5). The rate of exacerbations was reduced by 47% among patients receiving IV dose and by 53% among those receiving SC dose. At the end of the study, the mean increase from baseline in FEV1 was 100 mL greater in patients receiving IV mepolizumab than in those receiving placebo and 98 mL greater in patients receiving SC mepolizumab than in placebo group. There were also significant improvements in the SGRQ and ACQ-5 scores in the IV and SC mepolizumab groups than in the placebo group, with a safety profile of mepolizumab comparable to placebo. Mepolizumab decreased the eosinophil counts by week 4 of treatment (with reductions of 83% in the IV group and 86% in the SC group), and variations were maintained during the study. All these findings confirmed the efficacy of mepolizumab administered either intravenously or subcutaneously in terms of reduction of asthma exacerbations but, unlike other studies, all the clinical measures of asthma control were also improved.
In a 12-month follow-up analysis following the study of Haldar et al,Citation37 the authors reported the changes that occurred after stopping mepolizumab therapy. Cessation of treatment was associated with an early rise in the blood eosinophils continuing to baseline levels over 6 months. The frequency of severe exacerbations increased significantly at 3 to 6 months after stopping mepolizumab and the worsening of asthma control was anticipated by a rise in sputum and blood eosinophils.Citation54
A Phase III, multicenter, placebo-controlled, double-blind, parallel-group study is currently underway and is recruiting 780 patients with severe eosinophilic asthma to evaluate the safety and efficacy of mepolizumab adjunctive therapyCitation55 on markers of disease control. Primary outcome measure is mean change from baseline in SGRQ score at week 24. Secondary outcome measures are the mean change from baseline in pre-bronchodilator FEV1, percentage of participants achieving a 4-point or greater reduction from baseline in SGRQ score, and mean change from baseline in ACQ-5 score at week 24. The overall intent of this RCT is to fully explore the impact of mepolizumab on health-related quality of life and other measures of asthma control, including lung function ().
Table 2 Main studies and results for mepolizumab in asthma
Despite controversial evidence of clinical efficacy, the first trials in particular being less favorable than the latest more positive ones, all the studies quoted earlier demonstrated a positive effect of mepolizumab on blood eosinophil counts.Citation14,Citation36–Citation41,Citation53 As a matter of fact, a pooled analysis from seven studies on a total of 1,131 patients showed that IV or SC administration of mepolizumab was associated with a significant reduction in blood eosinophils compared to placebo (mean deviation: −0.29×109/L, 95% confidence interval [CI]: −0.44 to −0.14×109/L, P=0.0001). Also, data demonstrated a significant reduction for the percentage of sputum eosinophils, with a mean difference of −6.05% (P=0.0003).Citation56
Finally, an unusual Phase II, double-blind, placebo-controlled study evaluated the effect of one IV dose of benralizumab, added to current standard asthma medication, on recurrence (asthma exacerbations and/or hospitalization for acute asthma) after an emergency department (ED) visit for asthma exacerbation.Citation57 Patients were stratified by baseline eosinophil count of less than or equal to 450 or greater than 450 cells/μL. Administration of one dose of benralizumab resulted in a long-lasting reduction of eosinophils and in the rate and severity of exacerbations in subjects who presented to the ED with severe asthma exacerbation and were poorly responsive to standard therapy. This study may represent a further step to identify patients suitable for anti-IL-5 mAbs treatment, even in an emergency setting so far not considered.
Mepolizumab in other hypereosinophilic diseases
Whenever the presence of systemic eosinophilic inflammation is a clinically relevant finding, it is intuitive to think that blocking IL-5 or its receptor could be a promising strategy for treatment. Therefore, not only asthma but also other, sometimes very severe, eosinophil-related disorders may represent a potential target for IL-5 inhibition. These conditions include HES and atopic dermatitis (AD), eosinophilic granulomatosis with polyangiitis, eosinophilic esophagitis (EoE), and NP. In 2004, Garrett et alCitation58 published an open-label trial of anti-IL-5 in which three IV doses were administered at 4-week intervals to four patients with HES (defined by peripheral blood and/or tissue eosinophilia). The effects of treatment on safety, eosinophil levels (in peripheral blood and/or tissue), pulmonary function, and quality of life were measured over a 28-week period. Anti-IL-5 was well tolerated in all patients and lowered peripheral blood eosinophil counts, which had remained persistently high despite systemic glucocorticoid therapy. The decline in circulating eosinophil counts was sustained for at least 12 weeks after the last dose of anti-IL-5. In addition, anti-IL-5 improved clinical and quality of life measurements. This study suggested a significant therapeutic potential of mepolizumab for HES. A study by Rothenberg et alCitation59 showed that treatment with mepolizumab had a positive corticosteroid-sparing effect in patients negative for Fip1-like1–platelet-derived growth factor receptor (FIP1L1-PDGFRA) fusion gene affected by the HES, confirming the effectiveness of the drug for this disease.
Since tissue and blood eosinophils in AD are more activated than in other eosinophilic disorders,Citation60 and peripheral blood eosinophils of AD patients show a significantly delayed programmed cell death.Citation61 Mepolizumab was earlier considered a potential treatment for this disease.Citation62 A randomized double-blind, placebo-controlled, parallel group study enrolled 18 patients with AD to receive two single IV doses of 750 mg mepolizumab and 22 to receive placebo treatment. Peripheral blood eosinophil counts were significantly reduced in the treatment group compared with placebo but no clinical success was reached by the physician’s global assessment, objective scoring atopic dermatitis (SCORAD), and itch scoring.Citation63
In EGPA, formerly known as the Churg–Strauss Syndrome, mepolizumab was used for remission induction in an open-label study of ten patients with refractory or relapsing EGPA; in eight of these subjects a remission was observed, allowing a reduction of OCS.Citation64 A later case series that studied seven patients with EGBPA, treated with only four IV doses of mepolizumab, showed a reduction of OCS dose in all patients with a stability of symptoms and an optimal safety profile during the study.Citation65
In an interesting study, authors investigated whether mepolizumab treatment reduced esophageal mast cell accumulation in 43 pediatric EoE biopsy specimens from a previous randomized anti-IL-5 trial. Forty percent of the patients responded to anti-IL-5 (defined as <15 eosinophils per high-power field following mepolizumab therapy), and 77% of all subjects had significantly fewer mast cells, IL-9+ cells, and mast cell–eosinophil couplets in the esophageal epithelium.Citation66
Anti-IL-5 was studied in two subsequent RCTs, ie, in pediatric and adult patients, respectively.Citation67–Citation68 A mild-to-moderate improvement in eosinophils counts was observed, but mepolizumab did not improve symptoms compared to placebo.
Finally, 30 patients with severe NP (grade 3 or 4 or recurrent after surgery) refractory to corticosteroids were randomized to receive either two single IV injections of 750 mg mepolizumab or placebo.Citation69 The primary outcome of this study was the reduction in NP score at 8 weeks after the first dosing and secondary outcomes included changes in CT scan scores and functional assessments, such as nasal peak inspiratory flow or symptom scores. Mepolizumab produced a significant reduction in total polyp score in 12 of 20 patients, as confirmed by computed tomography (CT) scan evaluations.
Future developments: COPD
In severe COPD patients, the eosinophil-predominant phenotype is associated with an increased risk of exacerbations and oral corticosteroid treatment. Two Phase III RCTs are currently in progress to evaluate the efficacy and safety of mepolizumab as an adjunctive therapy for adults affected by COPD with eosinophil-predominant phenotype.Citation70,Citation71 These studies will enroll approximately 1,500 patients at high risk of exacerbations despite standard care. The primary end points of the two studies are the frequency of exacerbations and the decrease of sputum eosinophils from baseline.
Safety profile
Available literature data demonstrate that anti-IL-5 treatment is safe for clinical use. It was very well tolerated in all clinical trials. Anti-mepolizumab neutralizing antibodies were detected in fewer than 2% of cases in two studies.Citation36,Citation72 In large trials, the number of adverse events were superimposable to placebo.Citation39,Citation50
Conclusion
In recent years, a remarkable progress in the scientific knowledge about pathogenetic mechanisms and therapeutic strategies for asthma has had only a partial impact on our ability to improve clinical outcomes. Asthma in fact is a very heterogeneous disease and only an accurate identification of the “phenotype”, through anatomical, clinical, functional, and biological evaluations can allow a correct definition of the clinical and therapeutic patient profile. The present lack of validated biomarkers makes the physician’s job even harder, since IL-5 may not be the only player of persistent airway eosinophilia and may also involve other immune cells and Th2- cytokine such as Il-13. Despite high doses of inhaled corticosteroids or oral corticosteroids, 5% of asthmatics have severe symptoms, enhanced airway remodeling, frequent exacerbations, and a greater risk to die from asthma. A subgroup (20%–40%) of these patients has persistent airway eosinophilia and frequent exacerbations.Citation73 A neutrophilic inflammation, which persists even under full corticosteroid therapy, may contribute in part to the manifestations of severe asthma, through the control of eosinophilic inflammation.Citation74 The identification of an “eosinophilic phenotype”, supported by several and sometimes controversial studies, would allow to select a subgroup of potential mepolizumab responders, which may overcome the problem of poor response to OCS. Several studies did not show a significant improvement in lung function, probably due to limited efficacy in removing tissue eosinophils and proinflammatory mediators;Citation15 however, mepolizumab can be a promising therapeutic option in selected subjects with persistent airway eosinophilia for the positive results obtained in clinical outcome measures, such as decrease in exacerbation rate, quality of life improvement, and good safety profile. An appropriate patient selection is therefore essential to avoid treatment failure, because several studies confirm that in a specific subgroup of patients, eosinophils play an important role in exacerbations and mepolizumab therapy may have clinical benefit. However, when eosinophilia is not present, or even in some patients with eosinophilic asthma, mepolizumab has no clinical effects.
Biological therapies in asthma are far more expensive than standard drugs.Citation75 While manufacturer has not given any hint as to the price of mepolizumab, analysts estimate a cost from ≥10,000 to ≥15,000 per patient per year of treatment.Citation76 Owing to its clinical efficacy and safety profile in selected subsets of severe patients at high risk for hospitalization and fatal asthma, the treatment by using this drug might prove to be a cost-effective one.
Disclosure
The authors report no conflicts of interest in this work.
References
- AccordiniSCorsicoAGBraggionMThe cost of persistent asthma in Europe: an international population-based study in adultsInt Arch Allergy Immunol201316019310122948386
- RabeKFAdachiMLaiCKWorldwide severity and control of asthma in children and adults: the global asthma insights and reality surveysJ Allergy Clin Immunol20041141404715241342
- WenzelSSevere asthma: from characteristics to phenotypes to endotypesClin Exp Allergy201242565065822251060
- ManuyakornWHowarthPHHolgateSTAirway remodelling in asthma and novel therapyAsian Pac J Allergy Immunol2013311310823517388
- SchuijsMJWillartMAHammadHLambrechtBNCytokine targets in airway inflammation. Lung damage and airway remodelling in severe asthmaCurr Opin Pharmacol201313335136123643194
- BrightlingCEGuptaSGonemSSiddiquiSLung damage and airway remodelling in severe asthmaClin Exp Allergy201242563864922192725
- FahyJVEosinophilic and neutrophilic inflammation in asthma: insights from clinical studiesProc Am Thorac Soc200916325625919387026
- PepeCFoleySShannonJDifferences in airway remodeling between subjects with severe and moderate asthmaJ Allergy Clin Immunol200511654454916159622
- HumbertMBeasleyRAyresJBenefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATEAllergy200560330931615679715
- NormansellRWalkerSMilanSJOmalizumab for asthma in adults and childrenCochrane Database Syst Rev20141CD00355924414989
- ZhangJYWenzelSETissue and BAL based biomarkers in asthmaImmunol Allergy Clin North Am20072762363217996580
- BousquetJChanezPLacosteJYEosinophilic inflammation in asthmaN Engl J Med1990323103310392215562
- TurnerMOHussackPSearsMRExacerbations of asthma without sputum eosinophiliaThorax199550105710617491553
- LeckieMJten BrinkeAKhanJEffects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic responseLancet20003562144214811191542
- Flood-PagePTMenzies-GowANKayABRobinsonDSEosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airwayAm J Respir Crit Care Med2003167219920412406833
- UhmTGKimBSChungIYEosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthmaAllergy Asthma Immunol Res201242687922379601
- McKinnonMBankMSolariRRobinsonGInterleukin-5 and the interleukin receptor: targets for drug discovery in asthmaSandersonCJInterleukin-5: From molecule to Drug Target for AsthmaNew YorkMarcel Dekker, Inc1999299320
- Menzies-GowAFlood-PagePSehmiRAnti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmaticsJ Allergy Clin Immunol2003111471471912704348
- WellsTNGraberPProudfootAEThe three-dimensional structure of human interleukin-5 at 2.4-angstroms resolution: implication for the structures of other cytokinesAnn N Y Acad Sci1994287251181278030983
- TakatsuKNakajimaHIL-5 and eosinophiliaCurr Opin Immunol20082028829418511250
- KouroTTakatsuKIL-5- and eosinophil-mediated inflammation: from discovery to therapyInt Immunol200921121303130919819937
- TanakaHKomaiMNagaoKRole of interleukin-5 and eosinophils in allergen-induced airway remodeling in miceAm J Respir Cell Mol Biol2004316214975941
- BecchettiEBaniDBaroniTIstologia UmanaNapoliIdelson-Gnocchi20113933949788879475419
- RosenbergHFDyerKDFosterPSEosinophils: changing perspectives in health and diseaseNat Rev Immunol201313192223154224
- ValentPThe phenotype of human eosinophils, basophils, and mast cellsJ Allergy Clin Immunol199494117711837798557
- TobaKKoikeTShibataANovel technique for the direct flow cytofluorometric analysis of human basophils in unseparated blood and bone marrow, and the characterization of phenotype and peroxidase of human basophilsCytometry19993524925910082306
- DahinenCARihsSOchsensbergerBRegulation of cytokine expression by human blood basophilsInt Arch Allergy Immunol19971131341379130503
- KowalKNolteHSkovPSEffect of allergen-specific immuno-therapy on recombinant human interleukin 3-mediated amplification of allergen-induced basophil histamine releaseAllergy Asthma Proc20052645646216541969
- LloydCMRankinSMChemokines in allergic airway diseaseCurr Opin Pharmacol2003344344812901955
- BochnerBSSiglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptorsClin Exp Allergy20093931732419178537
- KvarnhammarAMCardellLOPattern recognition receptors in human eosinophilsImmunology2012136112022242941
- MånssonACardellLORole of atopic status in Toll-like receptor (TLR)7- and TLR9-mediated activation of human eosinophilsJ Leukoc Biol20098571972719129482
- NakagomeKMatsushitaSNagataMNeutrophilic inflammation in severe asthmaInt Arch Allergy Immunol201215819610222627375
- BafadhelMMcCormickMSahaSProfiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary diseaseRespiration2012831364421912093
- MenzellaFLusuardiMGaleoneCZucchiLTailored therapy for severe asthmaMultidiscip Respir Med2015101125671117
- Flood-PagePSwensonCFaifermanIA study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthmaAm J Respir Crit Care Med20071761062107117872493
- HaldarPBrightlingCEHargadonBMepolizumab and exacerbations of refractory eosinophilic asthmaN Engl J Med200936097398419264686
- NairPPizzichiniMMKjarsgaardMMepolizumab for prednisone-dependent asthma with sputum eosinophiliaN Engl J Med200936098599319264687
- PavordIDKornSHowarthPMepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trialLancet201238065165922901886
- CastroMZangrilliJWechslerMEReslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trialsLancet Respir Med20153535536625736990
- LimHFNairPEfficacy and safety of reslizumab in patients with moderate to severe eosinophilic asthmaExpert Rev Respir Med20159213514225578680
- Zia-AmirhosseiniPMinthornEBenincosaLJPharmacokinetics and pharmacodynamics of SB-240563, a humanized monoclonal antibody directed to human interleukin-5, in monkeysJ Pharmacol Exp Ther199929131060106710565825
- European Medicines AgencyWithdrawal Assessment Report for BosatriaLondon, UKEuropean Medicines Agency2009 Doc. Ref: EMEA/454803/2009
- LopezAFSandersonCJGambleJRCampbellHDYoungIGVadasMARecombinant human interleukin 5 is a selective activator of human eosinophil functionJ Exp Med198816712192242826636
- OhnishiTSurSCollinsDSFishJEGleichGJPetersSPEosinophil survival activity identified as interleukin-5 is associated with eosinophil recruitment and degranulation and lung injury twenty-four hours after segmental antigen lung challengeJ Allergy Clin Immunol19939246076158409120
- ClutterbuckEJSandersonCJRegulation of human eosinophil precursor production by cytokines: a comparison of recombinant human interleukin-1 (rhIL-1), rhIL-3, rhIL-5, rhIL-6, and rh granulocyte- macrophage colony-stimulating factorBlood1990759177417792184902
- HartTKCookRMZia-AmirhosseiniPPreclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeysJ Allergy Clin Immunol2001108225025711496242
- WenzelSEEosinophils in asthma – closing the loop or opening the door?N Engl J Med2009360101026102819264692
- SimpsonJLScottRBoyleMJGibsonPGInflammatory subtypes in asthma: assessment and identification using induced sputumRespirology200611546116423202
- OrtegaHChuppGBardinPThe role of mepolizumab in atopic and nonatopic severe asthma with persistent eosinophiliaEur Respir J201444123924124659543
- OrtegaHLiHSurukiRAlbersFGordonDYanceySCluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthmaAnn Am Thorac Soc20141171011101724983709
- PrazmaCMWenzelSBarnesNDouglassJAHartleyBFOrtegaHCharacterisation of an OCS-dependent severe asthma population treated with mepolizumabThorax201469121141114224834924
- OrtegaHGLiuMCPavordIDMepolizumab treatment in patients with severe eosinophilic asthmaN Engl J Med2014371131198120725199059
- HaldarPBrightlingCESingapuriAOutcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12-month follow-up analysisJ Allergy Clin Immunol2014133392192324418480
- GlaxoSmithKlineEfficacy and Safety Study of Mepolizumab Adjunctive Therapy in Participants with Severe Eosinophilic Asthma on Markers of Asthma Control2014 Available from: https://clinicaltrials.gov/ct2/show/NCT02281318Accessed June 1, 2015 NLM Identifier: NCT02281318
- LiuYZhangSLiDWJiangSJEfficacy of anti-interleukin-5 therapy with mepolizumab in patients with asthma: a meta-analysis of randomized placebo-controlled trialsPLoS One201383e5987223544105
- NowakRMParkerJMSilvermanRAA randomized trial of benralizumab, an antiinterleukin 5 receptor α monoclonal antibody, after acute asthmaAm J Emerg Med2015331142025445859
- GarrettJKJamesonSCThomsonBAnti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromesJ Allergy Clin Immunol2004113111511914699394
- RothenbergMEKlionADRoufosseFEMepolizumab HES Study GroupTreatment of patients with the hypereosinophilic syndrome with mepolizumabN Engl J Med200820358(12)1215122818344568
- LeifermanKMA role for eosinophils in atopic dermatitisJ Am Acad Dermatol2001451S21S2411423867
- WediBRaapULewrickHKappADelayed eosinophil programmed cell death in vitro: a common feature of inhalant allergy and extrinsic and intrinsic atopic dermatitisJ Allergy Clin Immunol19971005365439338549
- GnanakumaranGBabuKSTechnology evaluation: mepolizumab, GlaxoSmithKlineCurr Opin Mol Ther20035332132512870444
- OldhoffJMDarsowUWerfelTAnti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitisAllergy200560569369615813818
- MoosigFGrossWLHerrmannKBremerJPHellmichBTargeting interleukin-5 in refractory and relapsing Churg–Strauss syndromeAnn Intern Med201115534134321893636
- KimSMarigowdaGOrenEIsraelEWechslerMEMepolizumab as a steroid-sparing treatment option in patients with Churg–Strauss syndromeJ Allergy Clin Immunol201012561336134320513524
- OtaniIMAnilkumarAANewburyROAnti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitisJ Allergy Clin Immunol201313161576158223623266
- StraumannAConusSGrzonkaPAnti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trialGut201059213019828470
- SpergelJMRothenbergMECollinsMHReslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trialJ Allergy Clin Immunol201212945646322206777
- GevaertPVan BruaeneNCattaertTMepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposisJ Allergy Clin Immunol2011128598999521958585
- GlaxoSmithKlineEfficacy and Safety of Mepolizumab as an Add-On Treatment in Chronic Obstructive Pulmonary Disease (COPD)2014 Available from: https://clinicaltrials.gov/ct2/show/NCT02105961Accessed May 26, 2015 NLM Identifier: NCT02105961
- McMaster UniversityMepolizumab in Chronic Obstructive Pulmonary Diseases (COPD) with Eosinophilic Bronchitis2011 Available from: https://clinicaltrials.gov/ct2/show/NCT01463644Accessed May 26, 2015 NLM Identifier: NCT01463644
- RoufosseFEKahnJEGleichGJLong-term safety of mepolizumab for the treatment of hypereosinophilic syndromesJ Allergy Clin Immunol2013131246146723040887
- RobinsonDSMepolizumab treatment for asthmaExpert Opin Biol Ther201313229530222998420
- KikuchiSNagataMKikuchiIHagiwaraKKanazawaMAssociation between neutrophilic and eosinophilic inflammation in patients with severe persistent asthmaInt Arch Allergy Immunol2005137171115947478
- MenzellaFFacciolongoNPiroRClinical and pharmacoeconomic aspects of omalizumab: a 4-year follow-upTher Adv Respir Dis201262879522323442
- Cost a focus as GSK takes lead in new wave of biotech lung drugs Reuters Edition UK. Mon September 8, 2014 1:45 pm BST. Available from: http://uk.reuters.com/article/2014/09/08/health-respiratory-gsk-idUKL5N0R921Y20140908Accessed July 31, 2015
