Abstract
Purpose
Tezepelumab, a human monoclonal antibody, blocks thymic stromal lymphopoietin. In the phase 3 NAVIGATOR study (NCT03347279), tezepelumab reduced annualized asthma exacerbation rates (AAERs) versus placebo, irrespective of baseline disease characteristics, and improved lung function and symptom control versus placebo in adults and adolescents with severe, uncontrolled asthma. We assessed the efficacy of tezepelumab in patients with severe asthma with or without nasal polyps (NPs) in the 2 years before randomization in NAVIGATOR.
Methods
Patients with severe asthma (N=1059) were randomized (1:1) and received tezepelumab 210 mg or placebo every 4 weeks subcutaneously for 52 weeks. Prespecified exploratory analyses included: AAER over 52 weeks and changes from baseline to week 52 in pre-bronchodilator forced expiratory volume in 1 second, Sino-Nasal Outcome Test (SNOT)-22 scores, and asthma control and health-related quality life (HRQoL) outcomes in NP subgroups. Changes from baseline in fractional exhaled nitric oxide (FeNO), blood eosinophil counts, total immunoglobulin E (IgE), eosinophil-derived neurotoxin (EDN), matrix metalloproteinase-10 (MMP-10), and serum interleukin (IL)-5, IL-6, IL-8 and IL-13 were assessed (post hoc).
Results
Tezepelumab reduced the AAER over 52 weeks versus placebo by 85% (95% confidence interval [CI]: 72, 92; n=118) and 51% (95% CI: 40, 60; n=941) in patients with and without NPs, respectively. At week 52, tezepelumab improved lung function, asthma control and HRQoL versus placebo in patients with and without NPs. Tezepelumab reduced SNOT-22 total scores (least-squares mean difference versus placebo [95% CI]) in patients with NPs at 28 weeks (–12.57 points [–19.40, –5.73]) and 52 weeks (–10.58 points [–17.75, –3.41]). At week 52, tezepelumab reduced blood eosinophil counts and FeNO, IgE, IL-5, IL-13, EDN and MMP-10 levels versus placebo, irrespective of NP status.
Conclusion
Tezepelumab resulted in clinically meaningful improvements in sino-nasal symptoms and asthma outcomes in patients with severe asthma with comorbid NPs.
Plain Language Summary
Asthma is a long-term condition caused by ongoing inflammation of the lower airways. The main symptoms are difficulty breathing, coughing, wheezing and shortness of breath. Approximately 41% of patients with severe asthma also have chronic rhinosinusitis with nasal polyps, a condition that affects the upper airways and sinuses. Nasal polyps are painless soft growths inside your nose that can keep growing if not treated. Symptoms include nasal congestion with mucus, facial pain and a reduced sense of smell or taste. People with both severe asthma and nasal polyps often have severe symptoms.
Thymic stromal lymphopoietin (TSLP) is a signaling molecule released by cells lining the airways in response to airborne triggers, such as smoke, pollen and viruses. TSLP activates several pathways that cause inflammation in the airways, leading to asthma symptoms. Tezepelumab is a biologic treatment that targets the very start of these inflammatory pathways by blocking TSLP.
The 1-year-long clinical trial called “NAVIGATOR” reported that tezepelumab reduced asthma attacks and improved lung function and asthma symptom control compared with placebo in patients with severe asthma that was not controlled with their current medicines. This analysis of data from NAVIGATOR looked at patients with both severe asthma and nasal polyps, showing that tezepelumab treatment improved sino-nasal symptoms compared with placebo. Tezepelumab also reduced asthma attacks and improved asthma symptoms, regardless of a patient’s medical history of nasal polyps. The effects of tezepelumab in patients with severe nasal polyps are being investigated in another clinical trial called “WAYPOINT”.
Introduction
Asthma and chronic rhinosinusitis are common comorbidities, and the frequency of asthma is higher in patients with chronic rhinosinusitis with nasal polyps (CRSwNP) than in those without nasal polyps.Citation1–4 It has been estimated that approximately 41% of patients with severe asthma also have CRSwNP.Citation5 Patients with both conditions tend to have more severe sino-nasal symptoms, more extensive lower airway inflammation, impaired lung function and a reduced health-related quality of life (HRQoL) than those with asthma or CRSwNP alone.Citation6 The clinical and economic burden of the disease is well documented.Citation7
Although the mechanisms underlying the relationship between severe asthma and CRSwNP have not been fully elucidated, it has been suggested that the production of type 2 (T2) inflammatory cytokines, primarily interleukin (IL)-4, IL-5 and IL-13,Citation8 induces the migration of eosinophils, mast cells and basophils to the airway mucosa, causing a reactive inflammatory response.Citation9–11 Pronounced eosinophilic infiltration is a feature in both asthma and CRSwNP.Citation6,Citation12 Increased levels of extracellular matrix proteins and some matrix metalloproteinases (MMPs) have also been observed in nasal tissues in patients with CRSwNP (with or without asthma),Citation13,Citation14 as well as increased serum periostin levels in patients with asthma,Citation15 supporting an underlying T2 inflammatory pathology.Citation16–20 Indeed, an association between comorbid asthma and CRSwNP has been reported among patients with a T2 endotype. However, patients with type 1 (T1, related to interferon gamma), type 3 (T3, related to IL-17) and mixed T1/T2 or T2/T3 CRSwNP endotypes have also been identified.Citation1
Targeted Treatment for the Management of Severe Uncontrolled Asthma with Comorbid CRSwNP
Asthma with comorbid CRSwNP is difficult to control owing to its association with high rates of exacerbations and glucocorticoid dependence.Citation6 Several studies have investigated the efficacy of T2-targeted biologic treatments in patients with severe asthma with comorbid nasal polyps. Benralizumab, dupilumab, mepolizumab and omalizumab have been reported to improve both sino-nasal and asthma outcomes in these patients.Citation21–29 Treatment with dupilumab has also been associated with improvements in upper and lower airway outcome measures in a different study population of patients with severe CRSwNP with comorbid asthma (of any severity).Citation30 However, real-world evidence has shown that some patients with severe asthma do not respond to treatment with these biologics.Citation31–33 Furthermore, not all patients with asthma with comorbid CRSwNP show evidence of T2 inflammation.Citation1 The role of eosinophils in airway inflammation has been questionedCitation34 and there is evidence of non-T2 inflammation in the pathophysiology of CRSwNP.Citation35 Overall, there remains an unmet need for an effective medical treatment for patients with severe asthma with comorbid CRSwNP. Targeting the epithelium at a higher, upstream level of the inflammatory cascade may provide a broader, more effective approach by encompassing T2 and non-T2 pathways.
TSLP is an epithelial cytokine that is an upstream activator of pro-inflammatory pathways.Citation36,Citation37 It has been implicated in shared pathophysiological processes underlying both severe asthma and CRSwNP, and is associated with both T2 and non-T2 inflammation.Citation38 TSLP is an alarmin released by airway epithelial cells in response to a variety of stimuli including microbes, allergens, irritants and physical trauma.Citation39–42 TSLP gene polymorphisms have been associated with the risk for development of chronic rhinosinusitis in Chinese patients and CRSwNP in Japanese patients.Citation43–45 TSLP mRNA and protein levels are elevated in the nasal polyps of patients with CRSwNP, specifically eosinophilic nasal polypsCitation46–52 in which increased TSLP bioactivity, as assessed in bioassay studies, is associated with the initiation of T2 inflammatory signaling pathways.Citation47,Citation53,Citation54 The downstream impact of TSLP-derived signaling in CRSwNP includes effects relevant to both T2 and non-T2 inflammation, including promoting differentiation of naïve cluster of differentiation 4 (CD4+) T helper cells into pro-inflammatory T helper 2 (Th2) cells that produce IL-4, IL-5, IL-13 and tumor necrosis factor,Citation36 as well as driving the release of pro-inflammatory molecules by eosinophils, mast cells and macrophages.Citation55–58 TSLP potentiates eosinophilic inflammation by promoting eosinophil recruitment, survival and degranulation,Citation59–62 and activation of group 2 innate lymphoid cells.Citation63 In addition, the presence of Charcot–Leyden crystals is a hallmark of eosinophilic airway inflammation and has been identified in eosinophilic nasal polyps.Citation64 These crystals may actively promote T2 inflammatory processes and may trigger non-T2 pathways including neutrophilic inflammation via IL-8, independently of IL-17 in nasal polyps.Citation64,Citation65
Tezepelumab is a mAb (IgG2λ) that binds specifically to TSLP, blocking it from interacting with its heterodimeric receptor.Citation66,Citation67 Tezepelumab treatment significantly reduced exacerbations and improved lung function, asthma control and health-related quality of life compared with placebo, irrespective of baseline disease characteristics, in patients with severe, uncontrolled asthma in the phase 2b PATHWAY study (NCT02054130) and the phase 3 NAVIGATOR study (NCT03347279).Citation68,Citation69 In PATHWAY and NAVIGATOR, tezepelumab reduced levels of T2 inflammatory biomarkers (ie blood eosinophils, fractional exhaled nitric oxide [FeNO] and immunoglobulin E [IgE]).Citation69,Citation70 Similarly, in the phase 2 CASCADE study (NCT03688074), tezepelumab reduced the number of airway submucosal eosinophilic inflammatory cells and improved asthma clinical outcomes compared with placebo in patients with moderate-to-severe, uncontrolled asthma.Citation71 In contrast, a significant reduction in eosinophil cell count in the bronchial submucosa was not observed in patients with asthma who received dupilumab in the phase 2 EXPEDITION study (NCT02573233).Citation72
In a post hoc analysis of PATHWAY, patients with asthma and nasal polyps (n = 23) had higher baseline levels of T2 inflammatory biomarkers than patients without nasal polyps (n = 112). Tezepelumab treatment reduced exacerbations and T2 inflammatory biomarker levels, irrespective of nasal polyp status; however, the effect of tezepelumab on sino-nasal symptoms associated with CRSwNP was not evaluated.Citation73 This exploratory subgroup analysis from the NAVIGATOR study evaluated the effect of tezepelumab treatment on clinical outcomes and biomarker levels in patients with severe asthma with and without a history of comorbid nasal polyps in the 2 years before randomization, including an assessment of sino-nasal symptoms among those with nasal polyps.
Materials and Methods
Study Design
NAVIGATOR was a phase 3, multicenter, randomized, double-blind, placebo-controlled study. Full study design and inclusion and exclusion criteria have been described previously.Citation69 Eligible patients were non-smokers, 12–80 years of age, with physician-diagnosed asthma. Before the date of informed consent, patients had been receiving medium- or high‑dose inhaled glucocorticoids (daily dose of at least 500μg fluticasone propionate or equivalent) for at least 12 months and at least one additional controller medication, with or without oral glucocorticoids, for at least 3 months. Patients were required to have a morning pre-bronchodilator forced expiratory volume in 1 second (FEV1) below 80% of the predicted normal value (< 90% for patients 12–17 years old) during the run-in period. Furthermore, a post-bronchodilator (albuterol/salbutamol) FEV1 reversibility of at least 12% and at least 200 mL must have been documented during the 12 months before screening or during the run-in period. Included patients were required to have experienced at least two documented asthma exacerbations (defined as a worsening of asthma symptoms that led to hospitalization, an emergency room visit that resulted in the use of systemic glucocorticoids for ≥ 3 consecutive days, or the use of systemic glucocorticoids for ≥ 3 consecutive days) in the 12 months before the date of informed consent. Patients were randomized 1:1 to receive tezepelumab 210 mg or placebo every 4 weeks subcutaneously for 52 weeks.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines and applicable regulatory requirements. Approvals from the Copernicus central Institutional Review Board (Cary, NC, USA) and local independent ethics committees were obtained, and all patients or their guardians provided written informed consent in accordance with local requirements.
Outcomes
The presence of a diagnosis of nasal polyps (of any severity) and either rhinitis or chronic sinusitis in a patient’s medical record determined whether they were deemed to have CRSwNP (hereon referred to as “nasal polyps”) at baseline. The exploratory subgroup analysis evaluated the efficacy of tezepelumab in patients with and without a history of nasal polyps in the 2 years before randomization in NAVIGATOR. The primary efficacy endpoint in NAVIGATOR was the annualized asthma exacaerbation rate (AAER; events per patient-year) over the 52-week treatment period in the overall population. The AAER was assessed in patients with and without a history of nasal polyps in the 2 years before randomization in NAVIGATOR. Secondary asthma-related outcomes assessed by nasal polyp status included changes from baseline in pre-bronchodilator FEV1 (minimum clinically important difference [MCID], 0.1 L),Citation74 Asthma Control Questionnaire-6 (ACQ-6) score (range, 0 [no impairment] to 6 [maximum impairment]; MCID, 0.5 points),Citation75 Asthma Quality of Life Questionnaire (standardized) for patients aged 12 years and older (AQLQ[S]+12) overall score (range, 1 [maximum impairment] to 7 [no impairment]; MCID, 0.5 points)Citation76 and Asthma Symptom Diary (ASD) score (range, 0 [no symptoms] to 4 [worst possible symptoms]; MCID, 0.5 points).Citation77 Changes from baseline in Sino-Nasal Outcome Test (SNOT)-22 total and domain scores were assessed only in those patients with a history of nasal polyps (MCID, 8.9 points).Citation78,Citation79 The SNOT-22 questionnaire comprises 22 items, each scored from 0 (no problem) to 5 (problem as bad as can be) and categorized into five domains: nasal (8 items), ear/facial (4 items), sleep (4 items), function (3 items) and emotion (3 items). SNOT-22 domain scores were calculated as the mean score of items in a given domain (range, 0 to 5). All item scores were summed to determine the SNOT-22 total score, ranging from 0 to 110.
The following exploratory biomarkers were assessed at baseline and at post-baseline time points up to week 52: blood eosinophil counts; FeNO levels; serum levels of total IgE, quantified using an immunoassay (Phadia, Thermo Fisher Scientific, Waltham, MA, USA); serum levels of cytokines (IL-5, IL-6, IL-8 and IL-13), quantified using a high-sensitivity sandwiched immunoassay (Simoa, Quanterix, Lexington, MA, USA); eosinophil-derived neurotoxin (EDN) levels, quantified using an ELISA assay (ALPCO, Salem, NH, USA); and MMP-10 levels, quantified using the Myriad Rules-Based Medicine xMAP assay (Rules-Based Medicine, Austin, TX, USA). Serum levels of TSLP were measured using a highly sensitive, fit-for-purpose electrochemiluminescence S-PLEX assay (Meso Scale Diagnostics, Gaithersburg, MD, USA).Citation80 This assay specifically detects long-form TSLP and not short-form TSLP. The lower limit of quantification is 2.9 pg/mL. The intra- and inter-assay coefficients of variation in serum were 7.9–8.7% and 4.2–10%, respectively. No interference was observed with IL-2, -3, -4, -5, -7, -9, -12p70, -13, -15, -18, -21, -33 or -17E/-25.
Analyses of AAER and changes from baseline in FEV1, ACQ-6, AQLQ(S)+12 and ASD, as well as SNOT-22 total scores in those with nasal polyps were prespecified; all other analyses, including SNOT-22 domain scores, were post hoc.
Statistical Analyses
The AAER over 52 weeks was estimated using a negative binomial regression model, with treatment group, region, age group, history of exacerbations, nasal polyps subgroup (yes or no) and treatment-by-subgroup interaction included as covariates.
Changes from baseline to week 52 in pre-bronchodilator FEV1, ACQ-6 score, AQLQ(S)+12 overall score and ASD score were assessed in patients with and without nasal polyps using a repeated measures model. Treatment, visit, region, age group, nasal polyps subgroup (yes or no), and treatment-by-visit, treatment-by-subgroup, visit-by-subgroup and treatment-by-visit-by-subgroup interactions were included as covariates, and the baseline value for the relevant outcome measure was included as a continuous linear covariate. The changes from baseline to week 52 in SNOT-22 total and domain scores were assessed in patients with nasal polyps using the repeated measures model described above, excluding subgroup and subgroup interaction terms.
Changes from baseline to week 52 in levels of exploratory biomarkers were assessed in patients with and without nasal polyps using the repeated measures model described above. Estimates for the biomarker analyses were geometric mean ratio changes from baseline. Treatment group, region, age group, visit, log of the baseline level of the corresponding biomarker, nasal polyps subgroup (yes or no), and treatment-by-visit, treatment-by-subgroup, visit-by-subgroup and treatment-by-visit-by-subgroup interactions were included as covariates. Geometric least-squares (LS) mean ratio changes from baseline with 95% confidence intervals (CIs) and geometric LS mean ratio differences between tezepelumab and placebo with 95% CIs were generated for each parameter over time by antilog transformation of LS means for log-transformed ratio changes from baseline.
All analyses were conducted without control of type 1 error; as such, p values are not presented.
Results
Baseline Demographics and Clinical Characteristics
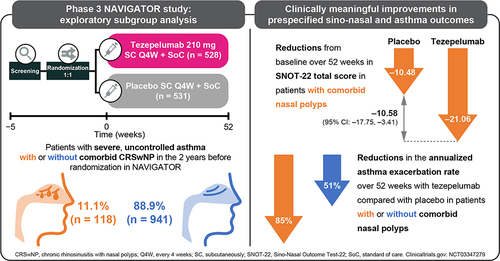
Of the 1059 patients who received study treatment, 528 received tezepelumab 210 mg Q4W and 531 received placebo. Of these, 118 patients (11.1%) had a history of nasal polyps in the 2 years before randomization in NAVIGATOR (tezepelumab, n = 62; placebo, n = 56) and 941 (88.9%) did not (tezepelumab, n = 466; placebo, n = 475). Baseline demographics and clinical characteristics were generally similar between patients with and without a history of nasal polyps (). As expected, compared with those without nasal polyps, patients with nasal polyps had higher blood eosinophil counts (median: 425 cells/μL versus 240 cells/μL), higher FeNO levels (median: 45.0 ppb versus 29.0 ppb) and a higher number of exacerbations in the past year (> 2 exacerbations: 53.4% versus 38.4%). The proportion of patients who were atopic (FEIA positive for any perennial aeroallergen) was lower for those with nasal polyps than for those without (46.6% versus 66.4%). Patients with nasal polyps had higher baseline exploratory T2 biomarker (TSLP, IL-5, IL-13 and EDN), MMP-10 and IL-8 levels than patients without nasal polyps. There was no difference in baseline IL-6 levels between patients with and without nasal polyps.
Table 1 Baseline Demographics and Clinical Characteristics of Patients with and without Nasal Polyps in the 2 Years Before Randomization in the NAVIGATOR Study
Exacerbation Rates
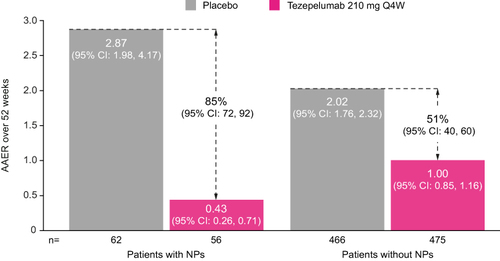
In the placebo group, the AAER over 52 weeks was numerically higher in patients with nasal polyps (2.87 [95% CI: 1.98, 4.17]) than in those without nasal polyps (2.02 [95% CI: 1.76, 2.32]) (). Compared with placebo, tezepelumab treatment reduced the AAER over 52 weeks by 85% (95% CI: 72, 92) in patients with nasal polyps and by 51% (95% CI: 40, 60) in those without. See Supplementary Material for results in patients with and without any history of chronic sinusitis, another asthma-relevant comorbidity (Supplementary Figure 1).
Figure 1 AAER over 52 weeks in patients with or without nasal polyps in the 2 years before randomization in the NAVIGATOR study.
Pre-Bronchodilator FEV1
The LS mean difference between tezepelumab and placebo in pre-bronchodilator FEV1 at week 52 was 0.21 L (95% CI: 0.06, 0.36) in patients with nasal polyps and 0.12 L (95% CI: 0.07, 0.18) in patients without nasal polyps (). Improvements in pre-bronchodilator FEV1 were observed as early as week 2 and were largely sustained over 52 weeks in both subgroups (Supplementary Figure 2).
Table 2 Change from Baseline to Week 52 in Pre-Bronchodilator FEV1 and ACQ-6, AQLQ(S)+12 and ASD Scores in Patients with and without Nasal Polyps in the 2 Years Before Randomization in the NAVIGATOR Study
ACQ-6, AQLQ(S)+12 and ASD Scores
Tezepelumab treatment improved ACQ-6 and AQLQ(S)+12 scores compared with placebo in patients with and without a history of nasal polyps (). Tezepelumab treatment improved ASD scores compared with placebo in patients with nasal polyps (). The LS mean difference between tezepelumab and placebo in ACQ-6 score at week 52 was −0.80 points (95% CI: −1.18, −0.42) in patients with nasal polyps and −0.27 points (95% CI: −0.40, −0.13) in patients without nasal polyps. Improvements from baseline in ACQ-6 score were observed as early as week 2 and were sustained over 52 weeks in both subgroups (Supplementary Figure 3). The LS mean difference between tezepelumab and placebo in AQLQ(S)+12 score at week 52 was 0.99 points (95% CI: 0.59, 1.40) in patients with nasal polyps and 0.25 points (95% CI: 0.11, 0.39) in patients without nasal polyps. The LS mean difference between tezepelumab and placebo in ASD score at week 52 was –0.45 points (95% CI: –0.67, –0.22) in patients with nasal polyps and –0.07 points (95% CI: –0.15, 0.01) in those without.
SNOT-22 Total and Domain Scores
In patients with a history of nasal polyps in the previous 2 years, the mean (standard deviation [SD]) SNOT-22 total score at baseline was 49.6 points (20.8) for tezepelumab and 49.1 points (19.9) for placebo (). Mean baseline SNOT-22 domain scores (SD) were also similar between the treatment arms. The nasal domain had the highest score (greatest impairment) at baseline, followed by the sleep, function, emotion and ear/facial domains (). At week 52, the LS mean change (standard error) from baseline in SNOT-22 total score was −21.06 points (2.50) for tezepelumab and −10.48 points (2.56) for placebo (LS mean difference: –10.58 points [95% CI: –17.75, –3.41] for tezepelumab compared with placebo) (). A clinically meaningful improvement from baseline in SNOT-22 total score was observed from the earliest post-baseline time point assessed (week 28; LS mean difference: –12.57 points [–19.40, –5.73]) and was sustained over 52 weeks (Supplementary Figure 4). Tezepelumab treatment resulted in a reduction in all SNOT-22 domain scores at week 52 compared with placebo; the greatest improvement was observed in the sleep domain, followed by the function and nasal domains ().
Figure 2 Change from baseline in SNOT-22 total score over 52 weeks in patients with nasal polyps in the 2 years before randomization in the NAVIGATOR study.
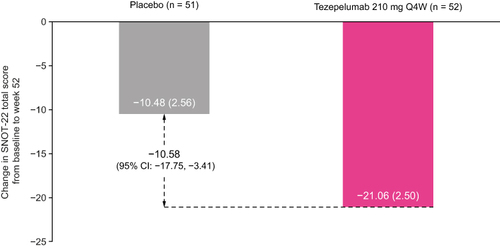
Figure 3 Change from baseline in SNOT-22 nasal (A), ear/facial (B), sleep (C), function (D) and emotion (E) domain scores over 52 weeks in patients with nasal polyps in the 2 years before randomization in the NAVIGATOR study.
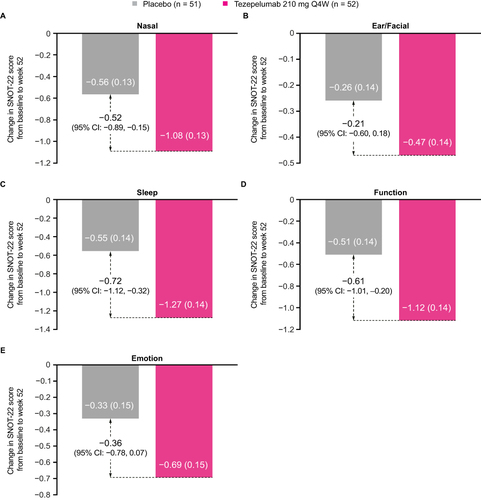
Table 3 Baseline SNOT-22 Total and Domain Scores in Patients with Nasal Polyps in the 2 Years Before Randomization in the NAVIGATOR Study
Biomarker Changes
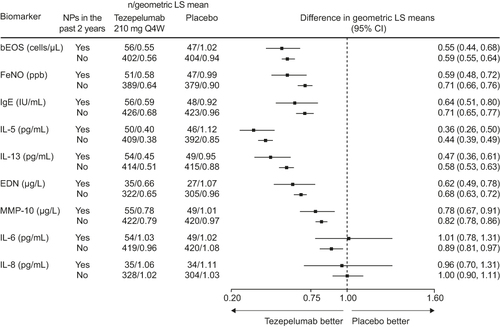
Blood eosinophil counts and levels of FeNO, serum IgE, IL-5, IL-13, EDN and MMP-10 were decreased in patients who received tezepelumab compared with placebo, irrespective of nasal polyp status (). Findings for IL-6 and IL-8 were not affected by tezepelumab treatment, irrespective of nasal polyp status (). The magnitudes of the reductions in T2 biomarkers were generally similar between patients with and without a history of nasal polyps.
Figure 4 Change from baseline in exploratory biomarkers over 52 weeks with tezepelumab compared with placebo in patients with or without NPs in the 2 years before randomization in the NAVIGATOR study.
Discussion
This exploratory analysis investigated the efficacy of tezepelumab in patients with severe, uncontrolled asthma with or without a history of nasal polyps of any severity in the 2 years before randomization in the NAVIGATOR study. The findings extend our understanding of the efficacy of anti-TSLP treatment with tezepelumab in patients with these lower and upper airway comorbidities. Consistent with findings from the phase 2b PATHWAY study, tezepelumab treatment substantially reduced asthma exacerbations and improved lung function, asthma control and health-related quality of life over 52 weeks compared with placebo, irrespective of nasal polyp status.Citation73 The effect of tezepelumab on sino-nasal symptoms associated with CRSwNP was previously unknown. Compared with placebo, tezepelumab treatment resulted in clinically meaningful improvements in sino-nasal symptoms in patients with nasal polyps, as assessed by the change from baseline in SNOT-22 total score over 52 weeks.
The validated SNOT-22 questionnaire is a widely utilized, disease-specific tool that evaluates patient-reported chronic rhinosinusitis sino-nasal symptoms in general, including nasal polyps, and the impact of chronic rhinosinusitis on health-related quality of life.Citation78,Citation79,Citation81 Patients treated with tezepelumab showed clinically meaningful improvements in SNOT-22 total score that were numerically greater than for placebo from the first post-baseline time point assessed (week 28) and that were sustained over the 52-week study treatment period. The magnitude of the change in SNOT-22 total score from baseline to week 52 (LS mean difference versus placebo: –10.58 points) exceeded the MCID (8.9 points)Citation78,Citation79 and was comparable to that reported in post hoc analyses from studies for other T2-targeted biologics. In the LIBERTY ASTHMA QUEST study, dupilumab improved SNOT-22 total scores in patients with moderate-to-severe, uncontrolled asthma with comorbid chronic rhinitis (with or without nasal polyps) (LS mean difference versus placebo: –10.32).Citation27,Citation82 In the ANDHI study, benralizumab improved nasal polyp outcomes in patients with severe eosinophilic uncontrolled asthma with ongoing nasal polyps and a high SNOT-22 total score at baseline (> 30 points), as measured by a clinically meaningful change in SNOT-22 total score from baseline to week 24 (LS mean difference versus placebo: –10.44 points).Citation21 Finally, in the MUSCA study, mepolizumab improved SNOT-22 total scores from baseline to week 24 in patients with severe, eosinophilic asthma with comorbid nasal polyps (LS mean difference versus placebo: –11.8).Citation24 In the POLYP 1 and 2 studies, omalizumab significantly improved SNOT-22 total scores from baseline to week 24, although this was in a different study population of patients with nasal polyps with an inadequate response to intranasal corticosteroids (LS mean difference versus placebo: –10.43 and –8.84 points, respectively).Citation83 Differences in study populations should be taken into consideration when comparing the results from different trials.
In the present study, analysis of the changes from baseline in SNOT-22 domain scores highlighted the factors driving the overall improvement in SNOT-22 total score. Tezepelumab treatment improved all five SNOT-22 domain scores at week 52, with the greatest improvements seen for the sleep, function and nasal domains. The beneficial effect of tezepelumab on sino-nasal symptoms may contribute to the improvement in sleep scores, which in turn has a positive impact on functioning.Citation84
The findings from the present analysis provide an insight into the underlying pathophysiology and anti-TSLP mechanism in patients with severe, uncontrolled asthma with comorbid nasal polyps. The patients with comorbid nasal polyps had higher baseline levels of T2 inflammatory biomarkers than those without nasal polyps, as observed in PATHWAYCitation73 and reported previously.Citation18 Patients with nasal polyps typically have high eosinophil levels, higher than those reported in asthma alone.Citation6 In agreement, in NAVIGATOR, 67% of patients with nasal polyps had a baseline blood eosinophil count of at least 300 cells/μL compared with 39% of patients in the subgroup without nasal polyps. Although the small sample size should be taken into consideration, 33% (n = 39) of the patients with nasal polyps had a baseline blood eosinophil count of less than 300 cells/μL, supporting the involvement of non-T2 driven pathways in severe asthma with comorbid nasal polyps. Tezepelumab treatment was associated with reductions in the levels of a broad spectrum of T2-associated exploratory biomarkers (blood eosinophils, FeNO, IgE, IL-5, IL-13 and EDN) and the tissue remodeling protein MMP-10. The greatest reductions were observed for the pro-inflammatory cytokines IL-5 and IL-13. IL-5 promotes the maturation, proliferation, activation and migration of eosinophils, while the biological activity of IL-13 elevates FeNO levels.Citation85,Citation86 Accordingly, in the present study, it is unsurprising that the effects of tezepelumab on IL-5 and IL-13 were accompanied by reductions in blood eosinophil counts and FeNO levels. Targeting both IL-5 and IL-13 may help to target mucosal inflammation, a key driving factor in the pathophysiology of nasal polyps. IL-8, an inflammatory biomarker often associated with neutrophilic inflammationCitation87 and often found elevated locally in patients with CRSwNP,Citation88 was elevated in the serum of patients with nasal polyps in this analysis. Tezepelumab treatment was not associated with any change in serum IL-6 or IL-8 levels. It is important to note that the patients included in the analysis were enriched for T2 inflammation. In addition, the evaluation of serum biomarkers may not fully reflect the mechanism of action of tezepelumab at the lung or nasal tissue level.
Consistent with the findings reported for the PATHWAY study,Citation73 the results from this exploratory analysis suggest that patients with comorbid nasal polyps had more severe asthma at baseline than those without. Firstly, patients with nasal polyps had experienced more exacerbations in the 12 months before entering the study than those without nasal polyps. Similarly, among patients receiving placebo, those with nasal polyps were more likely to have experienced exacerbations over the 52-week study period than those without. However, the small number of patients with nasal polyps in NAVIGATOR limits the ability to draw conclusions around disease severity in the two subgroups. A greater reduction in the AAER was observed in patients with nasal polyps who were treated with tezepelumab compared with placebo (85% [95% CI: 72, 92]), than in those without nasal polyps (51% [95% CI: 40, 60]). The reduction in the AAER observed with tezepelumab in patients with nasal polyps in NAVIGATOR was greater than that observed in a post hoc pooled analysis of mepolizumab in patients with severe asthma and any history of nasal polyps (68%).Citation89 Dupilumab reduced the AAER by up to 63% in a similar cohort of patients in the LIBERTY ASTHMA QUEST study.Citation90 These comparisons should be interpreted with caution owing to differences in study population numbers and study design.
Asthma control and health-related quality of life were assessed in this exploratory analysis using well-recognized tools. The study findings demonstrate that, compared with placebo, tezepelumab treatment resulted in improvements in ACQ-6 and AQLQ(S)+12 scores in patients with nasal polyps that were clinically meaningful and numerically higher than those observed in patients without nasal polyps; improvements in ASD score were numerically higher in those with nasal polyps.
Limitations of this study include the exploratory nature of the analysis and limited sample size; the study was not powered to evaluate the impact of tezepelumab treatment in patients with nasal polyps. For this analysis, patients were required to have a history of nasal polyps in the 2 years before randomization in the NAVIGATOR study, as determined from patients’ medical records. As no formal diagnosis was completed, the study findings may not necessarily be based on the clinical situation at the time of the study. Severe asthma with comorbid nasal polyps is frequently associated with T2 inflammation, and the specific contribution of non-T2 inflammation in disease pathogenesis in this subgroup is unknown. Analysis of exploratory biomarkers in nasal polyp tissue and/or nasal lining fluid would enable further understanding of the mechanism of action underlying the efficacy of tezepelumab in patients with nasal polyps, in whom T2 and non-T2 inflammatory mechanisms may be more prominent.
Conclusions
Findings from this study support the potential benefits of tezepelumab in patients with severe, uncontrolled asthma with comorbid nasal polyps, further demonstrating the efficacy of this anti-TSLP therapy in a broad patient population of patients with severe asthma. Tezepelumab treatment resulted in improvements in the SNOT-22 total score for sino-nasal symptoms, including nasal polyps, that were clinically meaningful and greater than those for placebo. Tezepelumab also reduced exacerbations and improved lung function, asthma control and health-related quality of life over 52 weeks compared with placebo in adults and adolescents, irrespective of nasal polyp status. A phase 3, randomized, placebo-controlled trial (WAYPOINT) is evaluating the efficacy and safety of tezepelumab in patients with severe CRSwNP.
Data Sharing Statement
This study is registered at ClinicalTrials.gov with the identifier NCT03347279 (registration date: 20 November 2017). Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines and applicable regulatory requirements. All patients or their guardians provided written informed consent before participation in the study.
Author Contributions
All authors made significant contributions to the work reported, whether to the study conception, design and execution; data acquisition, analysis and interpretation; or all these areas. All authors contributed to drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article was submitted; and agreed to be accountable for all aspects of the work.
Disclosure
Tanya M Laidlaw has served on scientific advisory boards for Amgen, GSK, Regeneron and Sanofi. Andrew Menzies-Gow is an employee of AstraZeneca and may own stock or stock options in AstraZeneca; has attended advisory board meetings for AstraZeneca, GSK, Novartis, Regeneron, Sanofi and Teva Pharmaceuticals; has received speaker fees from AstraZeneca, Novartis, Sanofi and Teva Pharmaceuticals; has participated in research with AstraZeneca, for which his institution has been remunerated; has attended international conferences with Teva Pharmaceuticals; and has consultancy agreements with AstraZeneca and Sanofi. Joseph K Han has received consultancy fees from AstraZeneca, Genentech, Gossamer Bio, GSK, Novartis, Regeneron and Sanofi-Genzyme. Elliot Israel has served as a consultant to and received personal fees from 4D Pharma, AB Science, the Allergy and Asthma Network, Amgen, AstraZeneca, Avillion, Biometry, Cowen, Equillium, Genentech, GSK, Merck, National Heart, Lung and Blood Institute, Novartis, Pneuma Respiratory, PPS Health, Regeneron Pharmaceuticals, Sanofi, Sienna Biopharmaceuticals, Teva Pharmaceuticalsand Westchester Medical Center; has received non-financial support from Circassia, Teva Pharmaceuticals and Vorso Corp; and has received clinical research grants from AstraZeneca, Avillion, Genentech, Gossamer Bio, National Institutes of Health (NIH), Novartis, Patient-Centered Outcomes Research Institute and Sanofi; royalties or licenses from UpToDate – Wolters Kluwer; stock options from Nesos Corp; study drug for NIH PrecISE trial for CSL Behring, Laurel Pharmaceuticals, Om Pharmaceuticals, Nestlé and Sun Pharmaceuticals; study drug for NIH IDEA for Sanofi-Regeneron. Jason K Lee has received research support from ALK, AstraZeneca Bausch Health, Genentech, GSK, Meda, Medexus, Miravo, Novartis, Regeneron, Roche, Sanofi-Genzyme and Takeda; has received fees for speakers’ bureau from Aralez, AstraZeneca, GSK, Medexus, Merck, Mylan, Novartis and Sanofi-Genzyme; and has received consultancy fees from and is an advisory committee member of AstraZeneca, GSK, Medexus, Novartis, Regeneron and Sanofi-Genzyme. Tobias Welte has received fees for lectures and/or advisory board meetings from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, GSK, MSD, Novartis, Roche, Pfizer and Sanofi Aventis; grants from the German Ministry of Research and Education, AstraZeneca and GSK. Jonathan Corren has received grants and personal fees from AstraZeneca, Genentech, RAPT, Regeneron and Vectura; and has received grants from Optinose, Pulmatrix, Sanofi and Teva Pharmaceuticals. Scott Caveney and Jean-Pierre Llanos are employees of Amgen and own stock in Amgen. Nicole Martin, Neil Martin, Ayman Megally, Bhavini Parikh and Sylvia Vong are employees of AstraZeneca and may own stock or stock options in AstraZeneca. The authors report no other conflicts of interest in this work.
Acknowledgments
The authors thank Christopher S Ambrose, MD, Gene Colice, MD, and Bill Cook, PhD, of AstraZeneca, Gaithersburg, MD, USA, for their contributions to the NAVIGATOR study. Andrew Menzies-Gow has a new and additional affiliation of Respiratory and Immunology, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK. Medical writing support for this manuscript was provided by Elizabeth Gandhi, PhD, of PharmaGenesis Cambridge, Cambridge, UK, with funding from AstraZeneca and Amgen Inc. The findings reported in this manuscript have been previously presented (in part) at the European Respiratory Society International Congress, September 5–8, 2021, virtual meeting (Menzies-Gow A, et al. Eur Respir J. 2021;58:PA876) and at the American Academy of Allergy Asthma and Immunology Annual Meeting February 25–28, 2022, Phoenix, AZ, USA (Carr T, et al. J Allergy Clin Immunol. 2022;149:Suppl AB152).
Additional information
Funding
References
- Stevens WW, Peters AT, Tan BK, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2019;7(8):2812–2820 e2813. doi:10.1016/j.jaip.2019.05.009
- Tint D, Kubala S, Toskala E. Risk factors and comorbidities in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2016;16(2):16. doi:10.1007/s11882-015-0589-y
- Jarvis D, Newson R, Lotvall J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67(1):91–98. doi:10.1111/j.1398-9995.2011.02709.x
- Seybt MW, McMains KC, Kountakis SE. The prevalence and effect of asthma on adults with chronic rhinosinusitis. Ear Nose Throat J. 2007;86(7):409–411. doi:10.1177/014556130708600719
- Canonica GW, Malvezzi L, Blasi F, et al. Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: evidences from the Severe Asthma Network Italy (SANI) registry. Respir Med. 2020;166:105947. doi:10.1016/j.rmed.2020.105947
- Laidlaw TM, Mullol J, Woessner KM, Amin N, Mannent LP. Chronic rhinosinusitis with nasal polyps and asthma. J Allergy Clin Immunol Pract. 2021;9(3):1133–1141. doi:10.1016/j.jaip.2020.09.063
- Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2021;14:127–134. doi:10.2147/JAA.S290424
- Vanderhaegen T, Gengler I, Dendooven A, Chenivesse C, Lefèvre G, Mortuaire G. Eosinophils in the field of nasal polyposis: towards a better understanding of biologic therapies. Clin Rev Allergy Immunol. 2022;62(1):90–102. doi:10.1007/s12016-021-08844-7
- Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23(2):145–148. doi:10.2500/ajra.2009.23.3284
- Han JK. Subclassification of chronic rhinosinusitis. Laryngoscope. 2013;123 Suppl 2:S15–S27. doi:10.1002/lary.23979
- Radabaugh JP, Han JK, Moebus RG, Somers E, Lam K. Analysis of histopathological endotyping for chronic rhinosinusitis phenotypes based on comorbid asthma and allergic rhinitis. Am J Rhinol Allergy. 2019;33(5):507–512. doi:10.1177/1945892419846263
- Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. doi:10.4193/Rhin20.600
- Du K, Wang M, Zhang N, et al. Involvement of the extracellular matrix proteins periostin and tenascin C in nasal polyp remodeling by regulating the expression of MMPs. Clin Transl Allergy. 2021;11(7):e12059. doi:10.1002/clt2.12059
- Katainen E, Kostamo K, Virkkula P, et al. Local and systemic proteolytic responses in chronic rhinosinusitis with nasal polyposis and asthma. Int Forum Allergy Rhinol. 2015;5(4):294–302. doi:10.1002/alr.21486
- Kanemitsu Y, Matsumoto H, Izuhara K, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013;132(2):305–312 e303. doi:10.1016/j.jaci.2013.04.050
- Håkansson K, Bachert C, Konge L, et al. Airway inflammation in chronic rhinosinusitis with nasal polyps and asthma: the united airways concept further supported. PLoS One. 2015;10(7):e0127228. doi:10.1371/journal.pone.0127228
- Chaaban MR, Walsh EM, Woodworth BA. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy. 2013;27(6):473–478. doi:10.2500/ajra.2013.27.3981
- Wang M, Bu X, Luan G, et al. Distinct type 2-high inflammation associated molecular signatures of chronic rhinosinusitis with nasal polyps with comorbid asthma. Clin Transl Allergy. 2020;10(1):26. doi:10.1186/s13601-020-00332-z
- Yan B, Lou H, Wang Y, et al. Epithelium-derived cystatin SN enhances eosinophil activation and infiltration through IL-5 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2019;144(2):455–469. doi:10.1016/j.jaci.2019.03.026
- Ediger D, Sin BA, Heper A, Anadolu Y, Misirligil Z. Airway inflammation in nasal polyposis: immunopathological aspects of relation to asthma. Clin Exp Allergy. 2005;35(3):319–326. doi:10.1111/j.1365-2222.2005.02194.x
- Canonica GW, Harrison TW, Chanez P, et al. Benralizumab improves symptoms of patients with severe, eosinophilic asthma with a diagnosis of nasal polyposis. Allergy. 2022;77(1):150–161. doi:10.1111/all.14902
- Tiotiu A, Oster JP, Roux PR, et al. Effectiveness of omalizumab in severe allergic asthma and nasal polyposis: a real-life study. J Investig Allergol Clin Immunol. 2020;30(1):49–57. doi:10.18176/jiaci.0391
- Harrison TW, Chanez P, Menzella F, et al. Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med. 2021;9(3):260–274. doi:10.1016/S2213-2600(20)30414-8
- Howarth P, Chupp G, Nelsen LM, et al. Severe eosinophilic asthma with nasal polyposis: a phenotype for improved sinonasal and asthma outcomes with mepolizumab therapy. J Allergy Clin Immunol. 2020;145(6):1713–1715. doi:10.1016/j.jaci.2020.02.002
- Nolasco S, Crimi C, Pelaia C, et al. Benralizumab effectiveness in severe eosinophilic asthma with and without chronic rhinosinusitis with nasal polyps: a real-world multicenter study. J Allergy Clin Immunol Pract. 2021;9(12):4371–4380 e4374. doi:10.1016/j.jaip.2021.08.004
- Bagnasco D, Brussino L, Bonavia M, et al. Efficacy of benralizumab in severe asthma in real life and focus on nasal polyposis. Respir Med. 2020;171:106080. doi:10.1016/j.rmed.2020.106080
- Hopkins C, Buchheit KM, Heffler E, et al. Improvement in health-related quality of life with dupilumab in patients with moderate-to-severe asthma with comorbid chronic rhinosinusitis with/without nasal polyps: an analysis of the QUEST study. J Asthma Allergy. 2022;15:767–773. doi:10.2147/JAA.S363527
- Berger P, Menzies-Gow A, Peters AT, et al. Long-term efficacy of dupilumab in asthma with or without chronic rhinosinusitis and nasal polyps. Ann Allergy Asthma Immunol. 2023;130(2):215–224. doi:10.1016/j.anai.2022.11.006
- Gallo S, Castelnuovo P, Spirito L, et al. Mepolizumab improves outcomes of chronic rhinosinusitis with nasal polyps in severe asthmatic patients: a multicentric real-life study. J Pers Med. 2022;12(8):1304. doi:10.3390/jpm12081304
- Laidlaw TM, Bachert C, Amin N, et al. Dupilumab improves upper and lower airway disease control in chronic rhinosinusitis with nasal polyps and asthma. Ann Allergy Asthma Immunol. 2021;126(5):584–592 e581. doi:10.1016/j.anai.2021.01.012
- Kavanagh JE, d’Ancona G, Elstad M, et al. Real-world effectiveness and the characteristics of a “super-responder” to mepolizumab in severe eosinophilic asthma. Chest. 2020;158(2):491–500. doi:10.1016/j.chest.2020.03.042
- Eger K, Kroes JA, Ten Brinke A, Bel EH. Long-term therapy response to anti-IL-5 biologics in severe asthma - a real-life evaluation. J Allergy Clin Immunol Pract. 2021;9(3):1194–1200. doi:10.1016/j.jaip.2020.10.010
- Harvey ES, Langton D, Katelaris C, et al. Mepolizumab effectiveness and identification of super-responders in severe asthma. Eur Respir J. 2020;55(5):1902420. doi:10.1183/13993003.02420-2019
- Laidlaw TM, Prussin C, Panettieri RA, et al. Dexpramipexole depletes blood and tissue eosinophils in nasal polyps with no change in polyp size. Laryngoscope. 2019;129(2):E61–E66. doi:10.1002/lary.27564
- Ahern S, Cervin A. Inflammation and endotyping in chronic rhinosinusitis - a paradigm shift. Medicina. 2019;55(4):95. doi:10.3390/medicina55040095
- Ebina-Shibuya R, Leonard WJ. Role of thymic stromal lymphopoietin in allergy and beyond. Nat Rev Immunol. 2022;23:24–37. doi:10.1038/s41577-022-00735-y
- Varricchi G, Pecoraro A, Marone G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol. 2018;9:1595. doi:10.3389/fimmu.2018.01595
- Klimek L, Hagemann J, Welkoborsky HJ, et al. Epithelial immune regulation of inflammatory airway diseases: chronic rhinosinusitis with nasal polyps (CRSwNP). Allergol Select. 2022;6:148–166. doi:10.5414/alx02296e
- Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204(2):253–258. doi:10.1084/jem.20062211
- Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi:10.1080/14728222.2020.1783242
- Golebski K, van Tongeren J, van Egmond D, de Groot EJ, Fokkens WJ, van Drunen CM. Specific induction of TSLP by the viral RNA analogue poly(I:C) in primary epithelial cells derived from nasal polyps. PLoS One. 2016;11(4):e0152808. doi:10.1371/journal.pone.0152808
- Lan F, Zhang N, Holtappels G, et al. Staphylococcus aureus induces a Th2 response via TSLP and IL-33 release in human airway mucosa. J Allergy Clin Immunol. 2015;135(2):AB81. doi:10.1016/j.jaci.2014.12.1196
- Hui CC, Yu A, Heroux D, et al. Thymic stromal lymphopoietin (TSLP) secretion from human nasal epithelium is a function of TSLP genotype. Mucosal Immunol. 2015;8(5):993–999. doi:10.1038/mi.2014.126
- Zhang Y, Wang X, Zhang W, Han D, Zhang L, Bachert C. Polymorphisms in thymic stromal lymphopoietin gene demonstrate a gender and nasal polyposis-dependent association with chronic rhinosinusitis. Hum Immunol. 2013;74(2):241–248. doi:10.1016/j.humimm.2012.11.004
- Nakayama T, Hirota T, Asaka D, et al. A genetic variant near TSLP is associated with chronic rhinosinusitis with nasal polyps and aspirin-exacerbated respiratory disease in Japanese populations. Allergol Int. 2020;69(1):138–140. doi:10.1016/j.alit.2019.06.007
- Dogan M, Sahin M, Yenisey C. Increased TSLP, IL-33, IL-25, IL-19, IL 21 and amphiregulin (AREG) levels in chronic rhinosinusitis with nasal polyp. Eur Arch Otorhinolaryngol. 2019;276(6):1685–1691. doi:10.1007/s00405-019-05379-8
- Kimura S, Pawankar R, Mori S, et al. Increased expression and role of thymic stromal lymphopoietin in nasal polyposis. Allergy Asthma Immunol Res. 2011;3(3):186–193. doi:10.4168/aair.2011.3.3.186
- Nagarkar DR, Poposki JA, Tan BK, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132(3):593–600.e512. doi:10.1016/j.jaci.2013.04.005
- Ogasawara N, Klingler AI, Tan BK, et al. Epithelial activators of type 2 inflammation: elevation of thymic stromal lymphopoietin, but not IL-25 or IL-33, in chronic rhinosinusitis with nasal polyps in Chicago, Illinois. Allergy. 2018;73(11):2251–2254. doi:10.1111/all.13552
- Ouyang Y, Fan E, Li Y, Wang X, Zhang L. Clinical characteristics and expression of thymic stromal lymphopoetin in eosinophilic and non-eosinophilic chronic rhinosinusitis. ORL J Otorhinolaryngol Relat Spec. 2013;75(1):37–45. doi:10.1159/000346929
- Cao P, Wang BF, Zhang Y, et al. TSLP signaling and TH2-type inflammation is enhanced in eosinophilic but not noneosinophilic nasal polyps. J Allergy Clin Immunol. 2011;127(2):AB140. doi:10.1016/j.jaci.2010.12.557
- Kato A, Poposki JA, Nagarkar DR, et al. Role of thymic stromal lymphopoietin (TSLP) in chronic rhinosinusitis. J Allergy Clin Immunol. 2012;129(2):AB70. doi:10.1016/j.jaci.2011.12.755
- Poposki JA, Klingler AI, Stevens WW, et al. Proprotein convertases generate a highly functional heterodimeric form of thymic stromal lymphopoietin in humans. J Allergy Clin Immunol. 2017;139(5):1559–1567.e1558. doi:10.1016/j.jaci.2016.08.040
- Kato A, Schleimer RP, Bleier BS. Mechanisms and pathogenesis of chronic rhinosinusitis. J Allergy Clin Immunol. 2022;149(5):1491–1503. doi:10.1016/j.jaci.2022.02.016
- Cao PP, Zhang YN, Liao B, et al. Increased local IgE production induced by common aeroallergens and phenotypic alteration of mast cells in Chinese eosinophilic, but not non-eosinophilic, chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2014;44(5):690–700. doi:10.1111/cea.12304
- Dwyer DF, Ordovas-Montanes J, Allon SJ, et al. Human airway mast cells proliferate and acquire distinct inflammation-driven phenotypes during type 2 inflammation. Sci Immunol. 2021;6(56):eabb7221. doi:10.1126/sciimmunol.abb7221
- Buchheit KM, Cahill KN, Katz HR, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016;137(5):1566–1576.e1565. doi:10.1016/j.jaci.2015.10.020
- Braile M, Fiorelli A, Sorriento D, et al. Human lung-resident macrophages express and are targets of thymic stromal lymphopoietin in the tumor microenvironment. Cells. 2021;10(8):2012. doi:10.3390/cells10082012
- Wong CK, Hu S, Cheung PFY, Lam CWK. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43(3):305–315. doi:10.1165/rcmb.2009-0168OC
- Kato Y, Takabayashi T, Sakashita M, et al. Expression and functional analysis of CST1 in intractable nasal polyps. Am J Respir Cell Mol Biol. 2018;59(4):448–457. doi:10.1165/rcmb.2017-0325OC
- Nocera AL, Mueller SK, Workman AD, et al. Cystatin SN is a potent upstream initiator of epithelial-derived type 2 inflammation in chronic rhinosinusitis. J Allergy Clin Immunol. 2022;150(4):872–881. doi:10.1016/j.jaci.2022.04.034
- Wang WW, Lu DM, Zheng M, Zhang JG, Zhang B. TSLP regulates eotaxin-1 production by nasal epithelial cells from patients with eosinophilic CRSwNP. Rhinology. 2018;56(4):370–377. doi:10.4193/Rhin17.045
- Poposki JA, Klingler AI, Tan BK, et al. Group 2 innate lymphoid cells are elevated and activated in chronic rhinosinusitis with nasal polyps. Immun Inflamm Dis. 2017;5(3):233–243. doi:10.1002/iid3.161
- Gevaert E, Delemarre T, De Volder J, et al. Charcot-Leyden crystals promote neutrophilic inflammation in patients with nasal polyposis. J Allergy Clin Immunol. 2020;145(1):427–430.e424. doi:10.1016/j.jaci.2019.08.027
- Persson EK, Verstraete K, Heyndrickx I, et al. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science. 2019;364(6442):eaaw4295. doi:10.1126/science.aaw4295
- Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–2110. doi:10.1056/NEJMoa1402895
- Marone G, Spadaro G, Braile M, et al. Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs. 2019;28(11):931–940. doi:10.1080/13543784.2019.1672657
- Corren J, Parnes JR, Wang L, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017;377(10):936–946. doi:10.1056/NEJMoa1704064
- Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi:10.1056/NEJMoa2034975
- Corren J, Pham TH, Garcia Gil E, et al. Baseline type 2 biomarker levels and response to tezepelumab in severe asthma. Allergy. 2022;77(6):1786–1796. doi:10.1111/all.15197
- Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312. doi:10.1016/S2213-2600(21)00226-5
- ClinicalTrials.gov (NCT02573233). Evaluation of dupilumab’s effects on airway inflammation in patients with asthma (EXPEDITION). Available from: https://clinicaltrials.gov/ct2/show/study/NCT02573233. Accessed July 05, 2023.
- Emson C, Corren J, Sałapa K, Hellqvist Å, Parnes JR, Colice G. Efficacy of tezepelumab in patients with severe, uncontrolled asthma with and without nasal polyposis: a post hoc analysis of the phase 2b PATHWAY study. J Asthma Allergy. 2021;14:91–99. doi:10.2147/jaa.S288260
- Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3 Suppl):S65–S87. doi:10.1016/j.jaci.2011.12.986
- Asthma Control Questionnaire. American thoracic society. Available from: http://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/acq.php. Accessed July 05, 2023.
- Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the asthma quality of life questionnaire. Chest. 1999;115(5):1265–1270. doi:10.1378/chest.115.5.1265
- Globe G, Wiklund I, Mattera M, Zhang H, Revicki DA. Evaluating minimal important differences and responder definitions for the asthma symptom diary in patients with moderate to severe asthma. J Patient Rep Outcomes. 2019;3(1):22. doi:10.1186/s41687-019-0109-2
- Chowdhury NI, Mace JC, Bodner TE, et al. Investigating the minimal clinically important difference for SNOT-22 symptom domains in surgically managed chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7(12):1149–1155. doi:10.1002/alr.22028
- Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item sinonasal outcome test. Clin Otolaryngol. 2009;34(5):447–454. doi:10.1111/j.1749-4486.2009.01995.x
- Pham T, Kearley J, Parnes JR, Leung D, Goleva E, Griffiths J. Development of a highly sensitive assay to quantitate circulating thymic stromal lymphopoietin (TSLP) levels in blood. J Allergy Clin Immunol. 2020;145(2):AB30. doi:10.1016/j.jaci.2019.12.820
- Khan AH, Reaney M, Guillemin I, et al. Development of Sinonasal Outcome Test (SNOT-22) domains in chronic rhinosinusitis with nasal polyps. Laryngoscope. 2022;132(5):933–941. doi:10.1002/lary.29766
- Busse W, Maspero JF, Katelaris CH, et al. Dupilumab improves SNOT-22 scores in asthma patients with chronic rhinosinusitis or nasal polypsosis (CRS/NP) in LIBERTY ASTHMA QUEST. Eur Resp J. 2018;52:PA1125. doi:10.1183/13993003.congress-2018.PA1125
- Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol. 2020;146(3):595–605. doi:10.1016/j.jaci.2020.05.032
- Busse WW, Wellman A, Diamant Z, et al. Impact of dupilumab on SNOT-22 sleep and function scores in CRSwNP. J Allergy Clin Immunol Pract. 2022;10(9):2479–2482 e2473. doi:10.1016/j.jaip.2022.05.013
- Pelaia C, Paoletti G, Puggioni F, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol. 2019;10:1514. doi:10.3389/fphys.2019.01514
- Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol. 2016;170(2):122–131. doi:10.1159/000447692
- Bickel M. The role of interleukin-8 in inflammation and mechanisms of regulation. J Periodontol. 1993;64(5 Suppl):456–460.
- Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138(5):1344–1353. doi:10.1016/j.jaci.2016.05.041
- Jain N, Siri D, Yancey S, Price R, Wenzel S. Mepolizumab reduces exacerbations and improves health-related quality of life in patients with severe asthma and nasal polyps, sinusitis, or allergic rhinitis. J Allergy Clin Immunol. 2020;145(2):AB26. doi:10.1016/j.jaci.2019.12.808
- Maspero JF, Katelaris CH, Busse WW, et al. Dupilumab efficacy in uncontrolled, moderate-to-severe asthma with self-reported chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(2):527–539 e529. doi:10.1016/j.jaip.2019.07.016