Abstract
Purpose
The iPREDICT program aimed to develop an integrated digital health solution capable of continuous data streaming, predicting changes in asthma control, and enabling early intervention.
Patients and Methods
As part of the iPREDICT program, asthma triggers were characterized by surveying 221 patients (aged ≥18 years) with self-reported asthma for a risk–benefit analysis of parameters predictive of changes in disease control. Seventeen healthy volunteers (age 25–65 years) tested 13 devices to measure these parameters and assessed their usability attributes.
Results
Patients identified irritants such as chemicals, allergens, weather changes, and physical activity as triggers that were the most relevant to deteriorating asthma control. Device testing in healthy volunteers revealed variable data formats/units and quality issues, such as missing data and low signal-to-noise ratio. Based on user preference and data capture validity, a spirometer, vital sign monitor, and sleep monitor formed the iPREDICT integrated system for continuous data streaming to develop a personalized/predictive algorithm for asthma control.
Conclusion
These findings emphasize the need to systematically compare devices based on several parameters, including usability and data quality, to develop integrated digital technology programs for asthma care.
Introduction
Asthma affects approximately 25 million Americans and imposes a considerable burden on patients and the United States (US) healthcare system.Citation1 Nearly half of all patients with asthma experience ≥1 asthma attack per year.Citation2 On an annual basis, this chronic respiratory disease costs the US more than $80 billion dollars.Citation3 Furthermore, the intrusive symptoms of asthma limit physical and social activities and impair the mental and emotional wellbeing of patients.Citation4,Citation5
The burden of chronic inflammatory respiratory diseases, such as asthma, may be mitigated by providing patients with effective self-management options,Citation6 as outlined in current evidence-based strategies.Citation7 Self-management of asthma requires appreciation of symptom onset and deterioration of disease control.Citation8 However, patients may not always recognize personal asthma triggers and/or may lack sufficient knowledge to identify them.Citation9,Citation10 For example, the agreement between triggers perceived by patients and their respective allergens confirmed by sensitization testing is only moderate.Citation10 Thus, patients would benefit from the ability to monitor their disease and to identify triggers to control symptoms and reduce the risk of future asthma attacks or exacerbation events.
Digital self-management solutions are increasingly providing new sources of data for patients to better self-manage their asthma.Citation9,Citation11–15 Given the interplay of genetics and the environment in the progression of this heterogeneous disease, to improve its clinical outcomes, the evolving era of precision medicine relies on novel real-time data sources in addition to historical evidence.Citation16 Many wearable sensors and digital devices capable of capturing biological and environmental data are currently under development and have been integrated into digital health solutions.Citation13,Citation15,Citation17–20 These devices promise to further the advancements in precision medicine and patient-centric care. However, challenges to the wider adoption of digital solutions include patient compliance, a lack of approved integrated systems, and unequal access to medical technology.Citation9,Citation21,Citation22 Thus, the selection of the right device(s) and data analysis strategies can improve the success of digital solutions in chronic disease management.
The individualized PREdiction of DIsease Control using digital sensor Technology (iPREDICT) program was conceptualized as a system of wearable biosensors and connected devices to characterize the individual experience of a patient with asthma and establish a prognostic model of disease control by measuring changes from baseline data. To accomplish this, the identification and selection of high-quality digital devices integrated with minimal burden on the daily lives of patients with asthma was required. Here, we describe the technology of the iPREDICT system, including the characterization of asthma triggers and measurable parameters, and the assessment and selection of optimal wearable devices and digital biosensors for inclusion in the iPREDICT system.
Materials and Methods
Measurable Parameter Analysis in Patients with Asthma
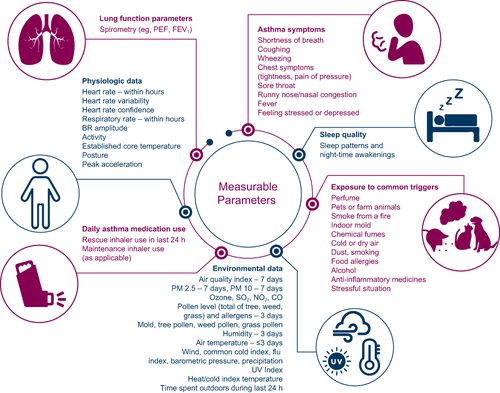
The development of the iPREDICT system required the initial establishment of a set of measurable parameters that correlated with changes in asthma control. This involved qualitative and quantitative assessments to characterize prodromal symptomology and physiology in the period between initial appearance of symptoms and onset of disease worsening, such as heart rate, respiratory rate, and asthma triggers. To accomplish this, 221 patients aged ≥18 years with self-reported asthma (Supplementary Figure 1) completed an online survey to characterize their subjective perception of asthma control, impact of asthma on their daily lives, personal asthma triggers, and value of a predictive tool designed to detect changes in disease control. Asthma triggers were categorized into nine clusters. For each identified trigger cluster, characteristics such as digital detection potential, participant self-report, time to early detection (2–24 h), percentage of participants affected, and predictive power were analyzed. A risk–benefit analysis of measurable parameters, validated in the literature as potentially predictive of changes in asthma control, was conducted separately to identify those that were important to patients and could be recorded with minimal patient burden.
Device Usability Testing in Healthy Volunteers
The conclusion of the risk–benefit analysis marked the beginning of the technology development phase, during which an optimal set of devices was selected, and user experience and system integration were tested.
Across a 4-week period, 13 devices were tested by 17 healthy volunteers aged 25–65 years who expressed an interest in health and technology. Three volunteers tested each device, and ≥2 devices were tested for each parameter. The devices included a wearable biometric patch (HealthPatch MD, VitalConnect, San Jose, California, USA), a vital sign monitor (Zephyr BioPatch, Zephyr, Maryland, USA), a chest-based wearable activity monitor (Zephyr BioHarness 3, Zephyr, Maryland, USA), four smart band activity trackers (Microsoft Band 2, Microsoft, Seattle, USA; Healbe GoBe, Healbe, Seattle, USA; Mio FUSE, Mio Global, Physical Enterprises, British Columbia, Canada; and Garmin Vivosmart HR, Garmin Ltd, Canton of Schaffhausen, Switzerland), a heart rate monitor (BodyGuardian Heart, Preventice Solutions, Minnesota, USA), a sleep monitor (MySleep S+, ResMed, California, USA), an electronic peak expiratory flow meter (Smart Peak Flow, Smart Respiratory Products, London, UK), two smart spirometers (Spirobank II, Medical International Research, Italy; Smart Spirometry, NuvoAir Air, Pond Health Care Innovation, Sweden), and a fractional exhaled nitric oxide (FeNO) monitor (NiOx Vero, Circassia, North Carolina, USA).
The devices varied in their capacity to measure the types and numbers of parameters, multiple variables across parameters, and the frequency at which data were collected. At Weeks 2 and 4, volunteers responded to a set of survey questions derived from the System Usability Scale (SUS)Citation23 and an internally developed questionnaire to quantify the usability of the respective devices based on their first impressions, ease of start-up, ease of use, comfort, connectivity and recording, data quality, and overall likelihood of recommendation, all of which could influence patient compliance in the study setting (Supplementary Table 1). In addition, the volunteers maintained a diary to record any thoughts and technical issues that they were experiencing with the device(s).
Based on an analysis of the measured parameters, user feedback, data type and quality, and device connectivity, a selected number of optimal biosensors and wearable devices for measuring parameters and delivering prospective data to a mobile application were identified.
Results
Measurable Parameter Analysis in Patients with Asthma
Of the patients who completed the survey, most were female (84%; n = 186) and self-identified as having well-controlled asthma (89%; n = 197). However, based on the 2016 Global Initiative for Asthma (GINA) criteriaCitation24 (Supplementary Figure 1), 171 (77%) patients were classified as having poorly controlled disease, showing a lack of agreement between clinically defined and patients’ view of disease control.
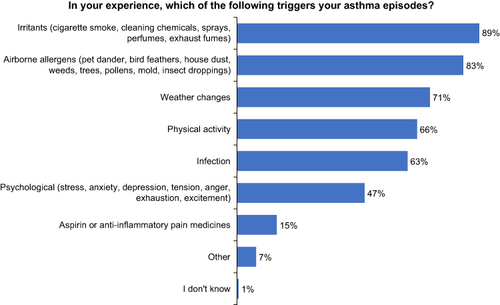
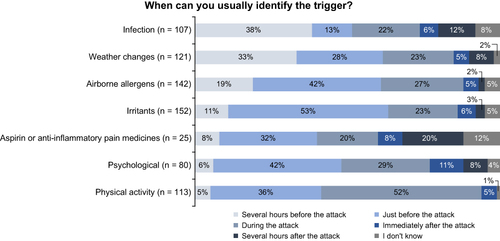
Survey responses from patients with poorly controlled asthma revealed that nearly all believed that they could identify their triggers, but the timing of the trigger identification varied according to the triggering factor. The most common self-reported triggers were irritants, such as smoke, chemicals, and perfumes (89%); aeroallergens, including animal dander, house dust, and molds (83%); weather changes (71%); and physical activity (66%) (). Some triggers (infections, allergens, irritants, and weather changes) could be identified prior to the onset of an asthma episode, whereas others (psychological, drug, and physical activity) were identified during or after an event ().
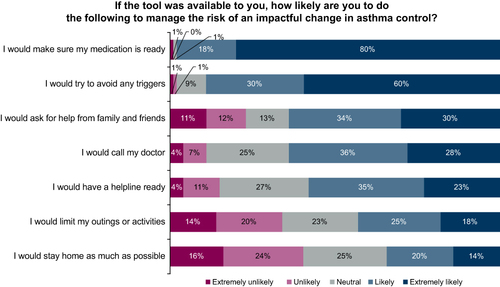
Participants reported that they would likely modify their behaviors after being notified of a change in asthma control, including ensuring access to asthma medication (98%); avoiding triggers (90%); and seeking assistance from family, friends (64%), or their physician (64%) ().
Parameter Selection
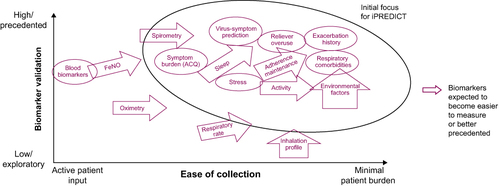
Qualitative risk–benefit analysis of parameters deemed potentially predictive of changes in asthma control, as validated in the literature, identified those that could be recorded with minimal patient burden (). These parameters varied based on whether they could be digitally detected or had to be patient-reported and the timing of trigger identification (). The final seven categories of parameters were chosen for the iPREDICT system based on those identified as important triggers by patients with poorly controlled asthma and the results of the risk–benefit analysis (). These parameters included asthma symptoms, such as dyspnea, coughing, and wheezing; lung function parameters; physiological data, such as heart rate, respiratory rate, and activity; daily asthma medication use; sleep patterns and nocturnal awakenings; specific exposure to common triggers, such as animal dander, house dust, and smoke; and environmental data, including the air-quality index score and pollen count.
Table 1 Prioritization of Triggers Based on Qualitative and Quantitative Assessments
Device Usability Testing in Healthy Volunteers
All volunteers reported being very-to-extremely interested in their health and technology. Of the 17 healthy volunteers, only six were using a health- or wellness-related tool at the time of the study, and four others had previously used such tools. The devices tested varied by the type and number of parameters measured, with some, including the wearable biometric patch, vital sign monitor, and chest-based activity monitor, capable of measuring several variables across parameters, whereas others, such as sleep and FeNO monitors, focused on a single output (Supplementary Table 2).
The usability survey revealed a number of between-device differences that could influence user compliance in the study setting (). The FeNO monitor and one of the two spirometers evaluated achieved the highest usability scores at Week 2, whereas at Week 4, the sleep monitor attained the highest usability score.
Table 2 Usability Scores of Devices Tested
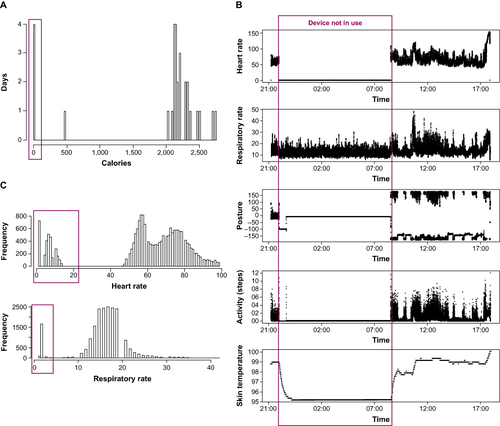
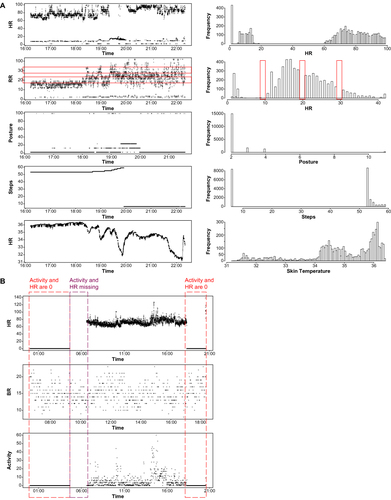
With respect to evaluation of the quality of the data captured, several disparities were discovered among the biosensors and connected devices (). These included the presence of background noise, typically encountered with the spirometers, even when volunteers were not testing the devices, and data capture out of physiologic range such that unrealistic outputs were observed for data streams (). In addition, missing data were common and appeared as no values or empty values, given as not available (NA) or NaN (not a number) by data export convention. Although the absence of values was the easiest to identify, a numeric entry of “0” could potentially be misinterpreted as an actual measurement.
Prioritized Devices
Based on the usability testing and validity of data capture, three devices, that is, a spirometer (Spirobank II, Medical International Research, Italy), a vital sign monitor (Zephyr BioPatch, Zephyr, Maryland, USA), and a sleep monitor (MySleep S+, ResMed, California, USA), were prioritized and selected for testing in the iPREDICT program. Together with an iPREDICT mobile application, which was developed to unobtrusively integrate data collected from most of the biosensors and connected devices, these components comprised the iPREDICT integrated system and were applied in the 24-week iPREDICT proof-of-concept study, which included patients with severe uncontrolled asthma to develop predictive models using identifiable, patient-specific disease parameters to anticipate changes in asthma control. The results of the pilot study are published separately.Citation23
Discussion
The iPREDICT program aims to transform disease management by applying digital health technologies to improve patient outcomes. To accomplish this overriding objective, we initially surveyed patients with asthma to understand their perceptions of asthma control, triggers, and management. We observed that most patients with asthma overestimated their degree of disease control. However, they reported an ability to identify environmental and physiological triggers and reacted positively to an accurate predictive tool that could signal the deterioration of asthma control. The most commonly cited asthma triggers were irritants, air pollution, airborne allergens, weather changes, and physical activity.
We then systematically characterized these sensory and environmental triggers to establish a set of measurable, validated, and non-invasive parameters that could be collected passively, imposing minimal patient burden and exhibiting the potential to predict changes in asthma control. These parameters included asthma symptoms, pulmonary function tests, physiological data, daily asthma medication use, sleep quality, environmental data, and exposure to common triggers. Subsequently, during the usability test portion of this study, healthy volunteers ranked devices and biosensors that could passively capture related data based on the type and number of parameters, as well as any variables across the parameters that could measure usability, and data quality.
Based on comparative results from usability testing, an optimal set of three devices, that is, a spirometer, vital sign monitor, and sleep monitor, could be incorporated into the iPREDICT system while maintaining the necessary data quality. In the iPREDICT pilot study of patients with physician-diagnosed, severe, uncontrolled asthma, this integrated system of devices, together with up to two connected medication inhalers and two integrated mobile applications, were evaluated for their ability to generate multifaceted data, which could be leveraged to inform the development of an optimal prognostic algorithm for disease control from streaming data based on the detected changes from individual baseline variables. The results of this pilot study will be published separately.Citation23
The findings from the patient perception and asthma trigger survey are consistent with published literature.Citation14,Citation15,Citation24,Citation25 Patients commonly overestimate their asthma control, and reports indicate that as many as 80% of patients with uncontrolled disease perceive their asthma to be well controlled.Citation24,Citation25 Additionally, the most common triggers for asthma episodes reside in the environment and may not be recognized or monitored accurately by the patient alone.Citation15 These observations suggest that an objective digital tool capable of tracking the disease status in real time may benefit patients with severe asthma and potentially those with other chronic diseases.
Technological advancements in wearable biosensors and connected devices capable of monitoring physiological and environmental parameters have enabled a vast amount of data capture. In parallel, machine learning has facilitated the analysis of large datasets and predictions of data variations in real time through statistical modeling.Citation26,Citation27 However, to ensure high-quality data, the utility of rapidly evolving wearable sensor technology requires an iterative process of systematic, comparative assessments. The data quality evaluation in the device testing phase of the iPREDICT program unearthed several unexpected issues. Medical-grade devices, such as the wireless biometric patch, did not necessarily generate the most accurate data, and successfully integrating sensor data with other data streams will require consideration of both the frequency of data collection and the variability in their respective formats and units. Intrusive background noise would necessitate appropriate data smoothing to improve the signal-to-noise ratio. Thus, the development of a digitized intervention program for asthma management demands a proper framework for device selection, which accounts for the essential parameters of scaled usability, connectivity, and data quality.
Several other integrated digital health systems exist, distinguished by a variety of devices, sensors, and functionalities.Citation11,Citation13,Citation15,Citation28–32 For instance, the Asthma Tuner, an electronic clinical decision support system, consists of a patient application (Android or iOS), healthcare interface, and spirometer connected via Bluetooth.Citation19 Patients register symptoms in the application and measure lung function using the spirometer. In turn, they receive automated feedback on asthma control and the recommended medication, including the required dose. In another digital health system, the kHealth asthma framework, the health status of the patient and environmental data are monitored continuously to convey information to physicians about personalized asthma triggers with the goal of fostering more individualized asthma management.Citation15 Similar to iPREDICT, the myAirCoach mobile health system features a portable spirometer, an inhaler adapter, an indoor air-quality monitor, a FeNO monitor, an exhaled breath temperature device, a FitBit HR Charge activity monitor, and smart inhalers that record medication adherence and the need for a rescue inhaler.Citation13 The mHealth system collects and analyzes data to determine parameters that may predict periods of uncontrolled asthma. Self-management, aided by the essential elements of myAirCoach, has proven effective in improving asthma control, quality of life, and acceptance of the technology among patients, in addition to reducing severe exacerbation rates.Citation18
Patient compliance to asthma self-management plans is often low and declines over time,Citation33 potentially contributing to poor disease control. Additionally, dropout rates for patients with chronic diseases in mobile health self-management studies tend to be high,Citation34 highlighting the need for improving patient experience and engagement with digital health systems. While a wide range of devices have been developed for monitoring and improving compliance to a prescribed regimen,Citation35 most studies in asthma employing digital devices have focused on monitoring compliance to inhaled medications.Citation20,Citation36–39 In the present study, assessment of device usability in healthy volunteers provided insights into factors that may influence compliance with the digital health system. Although device compliance was not assessed as part of the present study, findings from the iPREDICT proof-of-concept study conducted in patients with severe uncontrolled asthma showed that compliance to digital devices (75–90%)Citation23 was considerably higher than reported in previous studies (40–60%).Citation40–42 With the exception of a few studies that have employed the use of integrated digital health systems in asthma,Citation15,Citation19 most research has explored the use of remote consultations and mobile phone text messaging to improve patient outcomes.Citation43–45 To our knowledge, findings from the present study represent the first step towards thorough identification of perceived asthma triggers and device usability assessment with the aim of developing the multimodal iPREDICT system, thereby paving the way for continuous data streaming, daily disease monitoring, and predicting changes in asthma control to enable early intervention.
Taken together, digital approaches to improve healthcare solutions that target medication adherence, real-time disease monitoring, trigger identification, and patient-physician communication offer opportunities for effective self-management in patients with asthma. The iPREDICT system, which aims to predict the deterioration of asthma control in real time via continuous monitoring of individual physiological and environmental data, represents a step forward in the field of digital healthcare. However, this model will require validation through long-term studies. For instance, the iPREDICT system did not include an FeNO monitor because a suitable device was not available during the earlier study phases. Additional devices may be evaluated for non-invasive data acquisition as future iterations of iPREDICT technology evolve.
The limitations of digital health solutions should also be considered. Gaps in access to technology and an uneven technology infrastructure may restrict their dissemination to patients most in need.Citation22,Citation46 The complexity of the connected devices, biosensors, data collection, systems integration, and access to broadband internet, compounded by privacy concerns, may also hinder broader uptake and application of digitized self-management programs.Citation47 The ingress of integrated digital solutions in routine medical practice will thus mandate privacy and data protection safeguards and greater digital inclusion. Nonetheless, digital solutions such as the iPREDICT system demonstrate the potential to benefit a variety of stakeholders. Patients may use this technology to better understand their personal experiences with asthma and its triggers, self-manage their disease, proactively modify risk factors toward an improved quality of life, and engage in a more informed and productive dialogue with their treating physicians. Physicians may also benefit from digital sensor technology as it provides objective information on individual disease status, thereby assisting in structuring tailored, optimized therapy. In the context of clinical trials, such a system can generate anonymized patient data that may not only expedite the identification of asthma treatment paths but also potential asthma phenotypes. For payers, the iPREDICT system can track medication adherence in real time and potentially generate quality-of-care metrics.
Conclusion
In conclusion, a systematic approach that commenced with a survey of patients with asthma to better understand their perceptions of disease control and unmet self-management needs proceeded to identify measurable and validated parameters with the potential to predict changes in disease control. Finally, a structured, systematic comparison of devices and biosensors based on factors of usability, connectivity, data collection, and quality laid the foundation for the iPREDICT program. The findings reported herein delineate the framework for technology selection and testing that may inform future studies designed to evaluate the contribution of digital sensor technology to the management of chronic diseases such as asthma.
Abbreviations
ACQ, Asthma Control Questionnaire; BR, breathing and relaxation; CO, carbon monoxide; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; GINA, Global Initiative for Asthma; GPP, Good Publication Practice; GSR, galvanic skin response; HR, heart rate; iPREDICT, individualized PREdiction of DIsease Control using digital sensor Technology; NA not available; NaN, not a number; NO2, nitrogen dioxide; PEF, peak expiratory flow; PM, particulate matter; PRO, patient-reported outcome; RR, respiratory rate; SO2, sulfur dioxide; SUS, System Usability Scale; US, United States; UV, ultraviolet.
Ethics Approval and Informed Consent
The Regional Ethics Committee for Gothenburg (formerly known as Ethics Committee for Västra Götaland) exempted the part of the study involving healthy volunteers. IRB approval was not sought for part of the study involving patients with asthma as no intervention steps were involved and surveys were conducted by PatientsLikeMe, a web-based application where members are willing to share detailed computable data about symptoms, treatments, and health in order to learn from the experience of others and improve their outcomes.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Mario Castro reports grants/research support from the NIH, ALA, PCORI, AstraZeneca, Gala Therapeutics, Genentech, GSK, Novartis, Pioneering Medicines, Pulmatrix, Sanofi-Aventis, Shionogi, and Theravance; consulting fees/honoraria from Allakos, Amgen, Arrowhead, AstraZeneca, Genentech, GSK, Merck, Novartis, OM Pharma, Regeneron, Sanofi, and Teva; and royalties from Aer Therapeutics and Elsevier. Bhaskar Dutta was an employee of AstraZeneca when this study was conducted. All other authors are employees of AstraZeneca. Annika Rutgersson and Magnus Jörntén-Karlsson are currently engaged by Evinova, a separate healthtech business within the AstraZeneca group, launched in 2023. The authors report no other conflicts of interest in this work.
Acknowledgments
We thank the patients, volunteers, and the investigators who participated in the iPREDICT program.
Editorial support was provided by Michelle Rebello, PhD, CMPP, and Praveen Kaul, PhD, of Cactus Communications (Mumbai, India) in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). This study was fully funded by AstraZeneca.
Parts of these data were presented at the American Thoracic Society International Conference, 17–22 May 2019, Dallas, TX, USA. The poster’s abstract was published in “American Thoracic Society International Conference Abstracts” in American Journal of Respiratory and Critical Care Medicine [https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A3039.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Additional information
Funding
References
- Asthma and Allergy Foundation of America. Asthma facts and figures; 2022. Available from: https://www.aafa.org/asthma-facts/. Accessed April 28, 2023.
- Centers for Disease Control and Prevention. 2018 National Health Interview Survey (NHIS); 2018. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed April 28, 2023.
- Nurmagambetov T, Kuwahara R, Garbe P. The economic burden of asthma in the United States, 2008–2013. Ann Am Thoracic Soc. 2018;15(3):348–356. doi:10.1513/AnnalsATS.201703-259OC
- Foster JM, McDonald VM, Guo M, Reddel HK. “I have lost in every facet of my life”: the hidden burden of severe asthma. Eur Respir J. 2017;50(3):1700765. doi:10.1183/13993003.00765-2017
- Nelsen LM, Kimel M, Murray LT, et al. Qualitative evaluation of the St George’s Respiratory Questionnaire in patients with severe asthma. Respir Med. 2017;126:32–38. doi:10.1016/j.rmed.2017.02.021
- Gibson PG, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003;(1):CD001117. doi:10.1002/14651858.CD001117
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2022; 2022. Available from: https://ginasthma.org. Accessed April 28, 2023.
- Pinnock H. Supported self-management for asthma. Breathe. 2015;11(2):98–109. doi:10.1183/20734735.015614
- Deloitte Centre for Health Solutions; 2016. Available from: https://www2.deloitte.com/content/dam/Deloitte/uk/Documents/life-sciences-health-care/deloitte-uk-connected-health.pdf. Accessed April 28, 2023.
- Ritz T, Steptoe A, Bobb C, Harris AH, Edwards M. The asthma trigger inventory: validation of a questionnaire for perceived triggers of asthma. Psychosom Med. 2006;68(6):956–965. doi:10.1097/01.psy.0000248898.59557.74
- Ainsworth B, Greenwell K, Stuart B, et al. Feasibility trial of a digital self-management intervention “My Breathing Matters” to improve asthma-related quality of life for UK primary care patients with asthma. BMJ Open. 2019;9(11):e032465. doi:10.1136/bmjopen-2019-032465
- Bousquet J, Bedbrook A, Czarlewski W, et al. Guidance to 2018 good practice: ARIA digitally-enabled, integrated, person-centred care for rhinitis and asthma. Clin Transl Aller. 2019;9(1):16. doi:10.1186/s13601-019-0252-0
- Honkoop PJ, Simpson A, Bonini M, et al. MyAirCoach: the use of home-monitoring and mHealth systems to predict deterioration in asthma control and the occurrence of asthma exacerbations; study protocol of an observational study. BMJ oOpen. 2017;7(1):e013935. doi:10.1136/bmjopen-2016-013935
- Kagen S, Garland A. Asthma and allergy mobile apps in 2018. Curr Aller Asthma Rep. 2019;19(1):6. doi:10.1007/s11882-019-0840-z
- Venkataramanan R, Thirunarayan K, Jaimini U, et al. Determination of personalized asthma triggers from multimodal sensing and a mobile app: observational study. JMIR Pediat Parent. 2019;2(1):e14300. doi:10.2196/14300
- Hagger L, Dutta B, Jörnten-Karlsson M. iPREDICT: transforming severe uncontrolled asthma management and patient outcomes using digital technology [Abstract]. Am J Respir Crit Care Med. 2019;199:A3039.
- Bui AAT, Hosseini A, Rocchio R, et al. Biomedical REAl-Time Health Evaluation (BREATHE): toward an mHealth informatics platform. JAMIA Open. 2020;3(2):190–200. doi:10.1093/jamiaopen/ooaa011
- Khusial RJ, Honkoop PJ, Usmani O, et al. Effectiveness of myAirCoach: a mHealth self-management system in asthma. J Allergy Clin Immunol Pract. 2020;8(6):1972–1979 e1978. doi:10.1016/j.jaip.2020.02.018
- Ljungberg H, Carleborg A, Gerber H, et al. Clinical effect on uncontrolled asthma using a novel digital automated self-management solution: a physician-blinded randomised controlled crossover trial. Eur Respir J. 2019;54(5):1900983. doi:10.1183/13993003.00983-2019
- Merchant R, Inamdar R, Henderson K, et al. Digital health intervention for asthma: patient-reported value and usability. JMIR mHealth and uHealth. 2018;6(6):e133. doi:10.2196/mhealth.7362
- Bol N, Helberger N, Weert JCM. Differences in mobile health app use: a source of new digital inequalities? Inf Soc. 2018;34(3):183–193. doi:10.1080/01972243.2018.1438550
- Yu P, Wu MX, Yu H, Xiao GQ. The challenges for the adoption of m-health. Paper presented at: 2006 IEEE International Conference on Service Operations and Logistics, and Informatics; 2006.
- Castro M, Zavod M, Rutgersson A, Jörntén Karlsson M, Dutta B, Hagger L. iPREDICT: proof-of-concept study to develop a predictive model of changes in asthma control; 2022.
- Magnoni MS, Latorre M, Bettoncelli G, et al. Asthma control in primary care: the results of an observational cross-sectional study in Italy and Spain. World Allergy Organ J. 2017;10(1):13. doi:10.1186/s40413-017-0144-5
- Price D, Fletcher M, Van Der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med. 2014;24(1):14009. doi:10.1038/npjpcrm.2014.9
- Tibble H, Tsanas A, Horne E, et al. Predicting asthma attacks in primary care: protocol for developing a machine learning-based prediction model. BMJ Open. 2019;9(7):e028375. doi:10.1136/bmjopen-2018-028375
- Goto T, Camargo CA, Jr., Faridi MK, Yun BJ, Hasegawa K. Machine learning approaches for predicting disposition of asthma and COPD exacerbations in the ED. Am J Emergency Med. 2018;36(9):1650–1654. doi:10.1016/j.ajem.2018.06.062
- Dieffenderfer J, Goodell H, Mills S, et al. Low-power wearable systems for continuous monitoring of environment and health for chronic respiratory disease. IEEE J Biomed Health Inform. 2016;20(5):1251–1264. doi:10.1109/JBHI.2016.2573286
- Su JG, Barrett MA, Henderson K, et al. Feasibility of deploying inhaler sensors to identify the impacts of environmental triggers and built environment factors on asthma short-acting bronchodilator use. Environ Health Perspect. 2017;125(2):254–261. doi:10.1289/EHP266
- Melvin E, Cushing A, Tam A, Kitada R, Manice M. Assessing the use of BreatheSmart® mobile technology in adult patients with asthma: a remote observational study. BMJ Open Respir Res. 2017;4(1):e000204. doi:10.1136/bmjresp-2017-000204
- Chan Y-FY, Bot BM, Zweig M, et al. The asthma mobile health study, smartphone data collected using ResearchKit. Scientific Data. 2018;5(1):180096. doi:10.1038/sdata.2018.96
- van der Kamp MR, Klaver EC, Thio BJ, et al. WEARCON: wearable home monitoring in children with asthma reveals a strong association with hospital based assessment of asthma control. BMC Med Inf Decis Making. 2020;20(1):192. doi:10.1186/s12911-020-01210-1
- Kaya Z, Erkan F, Ozkan M, et al. Self-management plans for asthma control and predictors of patient compliance. J Asthma. 2009;46(3):270–275. doi:10.1080/02770900802647565
- Meyerowitz-Katz G, Ravi S, Arnolda L, Feng X, Maberly G, Astell-Burt T. Rates of attrition and dropout in app-based interventions for chronic disease: systematic review and meta-analysis. J Med Internet Res. 2020;22(9):e20283. doi:10.2196/20283
- Zijp TR, Mol PGM, Touw DJ, van Boven JFM. Smart medication adherence monitoring in clinical drug trials: a prerequisite for personalised medicine? EClinicalMedicine. 2019;15:3–4. doi:10.1016/j.eclinm.2019.08.013
- Morton RW, Elphick HE, Rigby AS, et al. STAAR: a randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax. 2017;72(4):347–354. doi:10.1136/thoraxjnl-2015-208171
- Vasbinder EC, Goossens LM, Rutten-van Molken MP, et al. e-Monitoring of Asthma Therapy to Improve Compliance in children (e-MATIC): a randomised controlled trial. Eur Respir J. 2016;48(3):758–767. doi:10.1183/13993003.01698-2015
- Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract. 2016;4(3):455–463. doi:10.1016/j.jaip.2015.11.022
- Jaimini U, Thirunarayan K, Kalra M, Venkataraman R, Kadariya D, Sheth A. “How is my child’s asthma?” digital phenotype and actionable insights for pediatric asthma. JMIR Pediat Parent. 2018;1(2):e11988. doi:10.2196/11988
- Beiwinkel T, Kindermann S, Maier A, et al. Using smartphones to monitor bipolar disorder symptoms: a pilot study. JMIR Ment Health. 2016;3(1):e2. doi:10.2196/mental.4560
- Cohen S, Waks Z, Elm JJ, et al. Characterizing patient compliance over six months in remote digital trials of Parkinson’s and Huntington disease. BMC Med Inform Decis Mak. 2018;18(1):138. doi:10.1186/s12911-018-0714-7
- Depp CA, Kim DH, de Dios LV, Wang V, Ceglowski J. A pilot study of mood ratings captured by mobile phone versus paper-and-pencil mood charts in bipolar disorder. J Dual Diagn. 2012;8(4):326–332. doi:10.1080/15504263.2012.723318
- van Gaalen JL, Beerthuizen T, van der Meer V, et al. Long-term outcomes of internet-based self-management support in adults with asthma: randomized controlled trial. J Med Internet Res. 2013;15(9):e188. doi:10.2196/jmir.2640
- Ahmed S, Ernst P, Bartlett SJ, et al. The effectiveness of web-based asthma self-management system, My Asthma Portal (MAP): a pilot randomized controlled trial. J Med Internet Res. 2016;18(12):e313. doi:10.2196/jmir.5866
- Kinley E, Skene I, Steed E, Pinnock H, McClatchey K. Delivery of supported self-management in remote asthma reviews: a systematic rapid realist review. Health Exp. 2022;25(4):1200–1214. doi:10.1111/hex.13441
- Mitchell UA, Chebli PG, Ruggiero L, Muramatsu N. The digital divide in health-related technology use: the significance of race/ethnicity. Gerontologist. 2019;59(1):6–14. doi:10.1093/geront/gny138
- McCallum C, Rooksby J, Gray CM. Evaluating the impact of physical activity apps and wearables: interdisciplinary review. JMIR mHealth and uHealth. 2018;6(3):e58. doi:10.2196/mhealth.9054