Abstract
Background
Benralizumab reduces exacerbations and long-term oral glucocorticosteroid (OCS) exposure in patients with severe eosinophilic asthma. In patients with eosinophilic granulomatosis with polyangiitis (EGPA), uncontrolled symptoms and exacerbations of asthma and chronic rhinosinusitis (CRS) are important reasons for continued OCS therapies. We aimed to describe outcomes of patients with severe asthma and EGPA treated with benralizumab in real-life.
Methods
We retrospectively analyzed adult patients from the Severe Asthma Unit at LMU Munich diagnosed with severe asthma and EGPA treated with benralizumab, differentiating two groups: Group A, patients with a stable daily OCS dose and diagnosis of EGPA >6 months ago; and Group B, patients treated with high-dose daily OCS due to recent diagnosis of EGPA <6 months ago. We compared outcome parameters at baseline and 12 months after initiation of benralizumab, including respiratory exacerbations, daily OCS dose, and lung function.
Results
Group A included 17 patients, all receiving OCS therapy and additional immunosuppressants; 15 patients (88%) continued benralizumab for more than 12 months, demonstrating a significant reduction in daily OCS dose and exacerbations while FEV1 increased. Group B included 9 patients, all with high-dose daily OCS and some receiving cyclophosphamide pulse therapy for life-threatening disease. Benralizumab addition during induction was well tolerated. A total of 7/9 (78%) continued benralizumab for more than 12 months and preserved EGPA remission at the 12-month timepoint.
Conclusion
In this real-life cohort of patients with severe asthma and EGPA, benralizumab initiation during remission maintenance reduced respiratory exacerbations and daily OCS dose. Benralizumab initiation during remission induction was associated with a high rate of clinical EGPA remission.
Introduction
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg–Strauss syndrome, is a rare systemic vasculitis manifesting with a specific combination of clinical features. It occurs in patients with asthma and eosinophilia after a prodromal phase of varying length, with general symptoms like fever and weight loss marking the onset of systemic inflammatory disease.Citation1 EGPA mostly affects the respiratory tract with asthma and pulmonary infiltrates as well as upper airway disease, mostly chronic rhinosinusitis with nasal polyps (CRSwNP). Further, it can involve almost every organ, with cutaneous, cardiovascular, neurological, renal, or gastrointestinal manifestations being most frequent.Citation1,Citation2 Antineutrophil cytoplasmic autoantibodies (ANCA) have been reported in approximately 40% of patients with EGPA in larger, rheumatologic cohorts, usually with specificity against myeloperoxidase (MPO-ANCA) or in rare cases cANCA,Citation3,Citation4 but in recent trials lower proportions of ANCA-positivity have been observed.Citation5 EGPA is classified among the ANCA-associated vasculitis syndromes even though (MPO-) ANCA are often negative and vasculitis is not always detectable.Citation6 Histological characteristics are eosinophilic infiltration and inflammation, necrotizing vasculitis, and extravascular granuloma.Citation1 Oral glucocorticosteroids (OCS) are the mainstay of treatment in both the remission induction as well as the maintenance phase.Citation1,Citation7 For remission induction, high-dose OCS and in case of organ- or life-threatening disease add-on cyclophosphamide or sometimes rituximab are used.Citation1,Citation7
After remission induction, maintenance therapy with steroid-sparing immunosuppressant such as methotrexate (MTX), azathioprine, or rituximab has been available and recommended for many years.Citation1,Citation7,Citation8 However, many patients remain OCS-dependent with subsequent side-effects. Flares requiring increase of OCS doses and pulsed therapies often occur, with exacerbations of asthma and CRSwNP being frequent underlying reasons. In recent years it has become clear that these respiratory exacerbations, also called respiratory relapses, need to be distinguished from systemic relapses.Citation1
Introduction of eosinophil-targeted therapies like antibodies directed against interleukin-5 (IL5) (mepolizumab, reslizumab) or the interleukin-5 receptor (IL5R) (benralizumab) has opened a new chapter in the therapy of severe eosinophilic asthma, reducing exacerbations and OCS use, while improving lung function, airway hyperresponsiveness,Citation9 and clinical symptoms in RCTsCitation10–14 as well as in real life.Citation15,Citation16 Additionally, some agents have also shown efficacy on CRSwNP.Citation17–20 Mepolizumab has proven to be safe and effective in decreasing OCS dose in patients with EGPA, reducing frequent flares and leading to a significantly higher proportion of patients in remission than on placebo in a randomized controlled trial (MIRRA).Citation5 Thus, mepolizumab was recently licensed for EGPA and is now also recommended for remission induction after a relapse and for remission maintenance.Citation7 However, in the MIRRA trial of mepolizumab in EGPA up to half of the included patients did not reach protocol-defined remission, which was defined as the absence of active disease manifestations and a stable dose of OCS with a prednisolone equivalent of ≤7.5 mg/day.Citation5
Benralizumab, an anti-IL5R antibody that rapidly depletes eosinophils via antibody-dependent cell-mediated cytotoxicity (ADCC), is approved for the therapy of severe, eosinophilic asthma. Here, we present experiences and outcome with benralizumab in a cohort of patients with severe asthma and EGPA, stratified by maintenance therapy and induction therapy phase.
Methods
Patient Cohort and Inclusion Criteria
We retrospectively analyzed all adult patients from the Department of Medicine V, LMU Munich, Germany, included in the German Asthma Net (GAN) severe asthma registry who had a diagnosis of severe asthma and EGPA. All patients fulfilled EGPA criteria similar to the MIRRA trial.Citation5 All patients received benralizumab treatment during clinical routine, had an ongoing OCS therapy, and with baseline data available before start of benralizumab and reaching 12-month timepoint prior to this analysis (). All patients gave written informed consent to inclusion in the GAN Severe Asthma Registry, which was approved by the IRB (LMU Munich, 21–0436). The accessed data complied with relevant data protection and privacy regulations. The registry prospectively collects routine clinical parameters of patients with severe asthma at baseline and annual follow-ups as described elsewhere.Citation21,Citation22 Patients were treated in clinical routine with benralizumab given in the indication of severe eosinophilic asthma, with a dose of 30 mg s.c. every 4 weeks three times, then every 8 weeks. Also, prior mepolizumab treatment in some patients was prescribed in an asthma indication dosage of 100 mg s.c. every 4 weeks.
Patients were stratified into two groups: Group A, “maintenance therapy phase”, diagnosis of EGPA longer than 6 months before the initiation of benralizumab and stable daily OCS dose; and Group B, “induction therapy phase”, diagnosis of EGPA within 6 months of start of benralizumab and ongoing high-dose daily OCS therapy for an acute and/or life-threatening organ manifestation.
Baseline Characteristics and Follow-Up
Baseline characteristics, including EGPA manifestations, laboratory results, previous therapy, therapy at start of benralizumab, as well as annualized respiratory exacerbations, asthma control test (ACT), and pulmonary function tests (PFT) were collected. Lung function and blood characteristics during a timeframe of up to three months before benralizumab initiation were acceptable as baseline. Exacerbations were defined as worsening respiratory symptoms necessitating OCS burst therapy of at least 3 consecutive days of prednisolone equivalent dose of at least 20 mg per day. Reasons for starting benralizumab were recorded. At 12 months after treatment initiation, laboratory results, exacerbations, ACT, current therapy, and PFT were collected.
Definition of Remission
As suggested in current guidelinesCitation1 and used in the MIRRA trial,Citation5 we defined clinical remission of EGPA as the absence of active disease manifestations with a stable dose of OCS with a prednisolone equivalent of ≤7.5 mg/day.
Statistical Analysis
Data are presented as numbers (n) and percentages. Mean and standard deviation (SD) are shown in case of normal distribution, otherwise median and interquartile range (IQR) are reported. Paired t-test or Wilcoxon signed-rank test were used as appropriate and specified in the relevant figure legend. P-values <0.05 were considered statistically significant. Statistical analysis was performed with GraphPad Prism 8 (GraphPad Software, San Diego, CA) and SPSS Statistics 25 (IBM SPSS, Armonk, NY).
Results
Maintenance Therapy Phase
We identified 17 patients who started benralizumab during maintenance therapy phase. Reasons for adding benralizumab in this group were side-effects of continuous OCS therapy (65%), persisting respiratory symptoms (53%), persistent eosinophilia (53%), recurrent exacerbations (35%), and failure to taper OCS dose below 7.5 mg/day (35%) (). In this group, 12 patients were female, 5 patients were male, and the mean age was 58 years. The diagnosis of asthma preceded the diagnosis of EGPA by around 20 years (). Median age at EGPA diagnosis was 49 years. Nine patients (53%) had a history of allergies to aeroallergens, with 3 patients (18%) polysensitized (≥3 different allergens). The most frequent clinical manifestations were asthma (17/17, 100%), CRS (16/17, 94%), and pulmonary infiltrates (14/17, 82%); less frequent organ manifestations were neuropathy (6/17, 35%), myocarditis (6/17, 35%), and cutaneous involvement (3/17, 18%).
Table 1 Reasons for Starting Benralizumab in Group A (Maintenance Therapy Phase)
Table 2 Baseline Characteristics of Patients Starting Benralizumab in in Group A and B (Maintenance and Induction Therapy Phase)
Mean blood eosinophil count (BEC) at diagnosis was 3.2 G/L and 29% of peripheral blood leukocytes. pANCA- and MPO-antibodies were evident in 2 patients (12%) and atypical ANCA without MPO-specificity in 2 patients (12%), while no ANCA were detected in 13 (76%). Historical histologic examinations were available for 11/17 patients and demonstrated eosinophilic inflammation and infiltration in all (11/11, 100%), with definitive vasculitis proof in 2 of the biopsies (2/11, 20%).
The spectrum of previous immunosuppressive therapy included cyclophosphamide (4/17, 24%), rituximab (2/17, 12%), azathioprine (6/17, 35%), and MTX (2/17, 12%). Eight patients (47%) had a history of anti-IL5-antibody treatment with mepolizumab (as this was prior to the EMA approval of mepolizumab for the indication of EGPA, mepolizumab was also used in the dosage and indication of severe eosinophilic asthma with 100 mg s.c. every 4 weeks). Median time between the first diagnosis of EGPA and the initiation of benralizumab was 6 years. Current therapy at the start of benralizumab comprised medium-to-high dose ICS/LABA and continuous OCS therapy for all patients, and the mean prednisolone dosage was 8 mg/day ().
Of the initial 17 patients, 15 (88%) continued benralizumab treatment for at least 12 months. One patient discontinued benralizumab and switched to omalizumab because of increasing allergic symptoms after 4 months. One patient with a history of mepolizumab treatment switched to dupilumab after 11 months of benralizumab treatment due to uncontrolled CRSwNP, but this led to severe hypereosinophilia, and consequently successful dual treatment with dupilumab and benralizumab has been applied since.Citation23
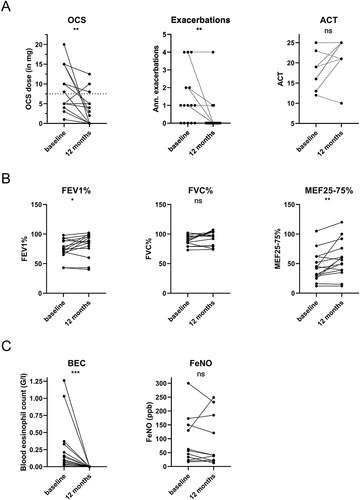
In these 15 patients, respiratory exacerbations decreased significantly (annualized exacerbations baseline vs 12-months: 1 vs 0, p<0.01) during therapy with benralizumab. Asthma control as measured by ACT at both timepoints was available for 8/15 patients and did not significantly differ after 12 months of therapy (ACT baseline vs 12-months: 18 vs 23, p=0.13) (). However, the proportion of patients with well-controlled asthma increased from 2/8 (25%) to 7/8 (88%). Daily OCS dose significantly decreased after 12 months of benralizumab therapy (daily OCS dose baseline vs 12-months: 8 vs 2 mg/day, p<0.01), and 7/15 patients (46%) completely discontinued OCS therapy (, ). Lung function testing showed a significant increase in percent predicted FEV1 (FEV1%) (FEV1% baseline vs 12-months: 75% vs 82%, p=0.04) and percent predicted MEF25-75 (MEF25-75%) after 12 months of therapy (MEF25-75% baseline vs 12-months: 44% vs 58%, p<0.01). Percent predicted FVC (FVC%) (FVC% baseline vs 12-months: 90% vs 94%, p=0.07) and percent predicted RV (RV%) (RV% baseline vs 12-months: 141% vs 131%, p=0.07) were not significantly changed after 12 months (). Further, blood eosinophils were significantly reduced (BEC baseline vs 12-months: 0.12 vs 0.0 G/l, p<0.001), while FeNO was not significantly altered (FeNO baseline vs 12-months: 57 vs 41 ppb, p=0.31) (, ).
Table 3 Comparison of Outcome Parameters at Baseline (Start) and After 12 Months of Benralizumab Therapy in Group A (Maintenance Therapy Phase)
Figure 2 Comparison of parameters of OCS use and symptoms (A), lung function (B), and biomarkers (C) before and after 12 months of benralizumab of patients in maintenance cohort. In this analysis we included all 15 patients who continued benralizumab for 12 months. Statistics: (A), (C): Wilcoxon matched-pairs signed-rank test; (B): paired t-test, *p<0.05, **p< 0.01, ***p<0.001.
Induction Therapy Phase
Group B included 9 patients who started benralizumab within 6 months of diagnosis of EGPA, during acute disease manifestation and ongoing treatment with high-dose corticosteroid therapy. Of these, 4 patients were female and 5 patients were male, with a mean age at EGPA diagnosis of 55 years. A total of 56% of patients had allergies. Asthma and CRS manifestations were most common (9/9, 100% and 8/9, 89%, respectively). Further clinical manifestations included cardiac (6/9, 67%) and neurological involvement (2/9, 22%) ().
The highest BEC measured at diagnosis was at mean 6.2 G/L and 39% of peripheral blood leukocytes. No patient had detectable pANCA or MPO antibodies, while 2 patients presented with atypical ANCA without MPO specificity (2/9, 22%). Biopsies were available for 8/9 (89%), demonstrating eosinophilic inflammation and infiltration in 6/9 (75%), whereas proven vasculitis was seen in none of the biopsies. Three patients (3/9, 33%) received concomitant cyclophosphamide pulse therapy due to life-threatening disease. One patient had a history of a previous mepolizumab therapy. At start of benralizumab, all patients had ongoing high-dose ICS/LABA and continuous OCS therapy with a median dosage of 40 mg/day ().
A total of 7/9 patients (78%) continued benralizumab for more than 12 months, and outcome parameters were analyzed at this timepoint. In these 7 patients benralizumab was well tolerated when added to the standard immunosuppressive treatment of the induction phase, and no adverse events attributed to benralizumab treatment were reported. Two patients discontinued benralizumab: a 41-year-old male patient stopped benralizumab at around 10 months after initiation due to worsening of dyspnea and need for increase of oral steroid therapy occurring 3 days after the last benralizumab application (clinically this was not regarded as an adverse event causally related to the drug; however, the patient requested a switch to mepolizumab). A 72-year-old male patient with initially life-threatening cardiac involvement, who had received 9 cycles of cyclophosphamide and had ongoing therapy with low-dose OCS and MMF, discontinued regular follow-up at our center for unknown reasons and therefore did not receive further benralizumab after 10 months of treatment. He presented to the emergency department 14 months after the last benralizumab application and died shortly after due to severe infection and heart failure.
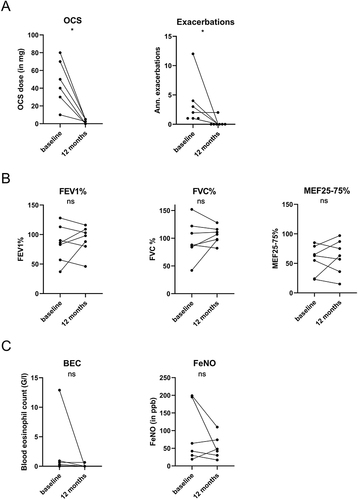
In all 7 patients who continued benralizumab for at least 12 months, clinical remission of EGPA, defined as stable disease without active manifestation and a stable OCS dose with ≤7.5 mg/day was achieved and sustained at the 12-month timepoint. Respiratory exacerbations significantly decreased (baseline vs 12-months: 2 vs 0, p= 0.03). Daily OCS dose was significantly decreased after 12 months of therapy (baseline vs 12-months: 40 vs 2.5 mg/day, p=0.02), while 2 patients (22%) were able to discontinue OCS therapy (). Asthma control as measured by ACT score was only available for 3 patients. Pulmonary function testing parameters were normal at mean during ongoing high-dose OCS treatment at baseline and did not show significant differences in FEV1%, FVC%, RV%, and MEF25-75% at 12-months after initiation of benralizumab (, ). BEC was not significantly altered but was below the lower detection limit in 5 patients. FeNO was not significantly changed (, ).
Table 4 Comparison of Outcome Parameters at Baseline (Start of Benralizumab) and After 12 Months of Benralizumab Therapy in Group B (Induction Therapy Phase)
Figure 3 Comparison of parameters of OCS use and exacerbations (A), lung function (B), and biomarkers (C) before and after 12 months of benralizumab in induction cohort. In this analysis we included all 7 patients who continued benralizumab for 12 months. Statistics: Wilcoxon matched-pairs signed-rank test, *p<0.05.
Long-term follow-up at time of preparation of the manuscript showed that most patients who were treated with benralizumab at the 12-month timepoint are still continuing this treatment to date (14/15 patients in maintenance cohort, 6/7 patients in induction cohort), with a median follow-up of 51 and 46 months, respectively (). A 62-year-old female patient died after 30 months of benralizumab treatment due to long-standing metastatic breast cancer. A 35-year-old male patient stopped benralizumab after 31 months due to repeated increases of BEC at the end of the 8-week application interval and was switched to mepolizumab 300 mg every 4 weeks. After the 12-month timepoint, 6 exacerbations or flares requiring an increase in OCS dose or pulse therapy were documented in the total cohort during follow-up (). One additional patient started dupilumab add-on to benralizumab due to uncontrolled CRSwNP, resulting in improved nasal symptoms and further reduction of daily OCS dose; 1 patient started rituximab add-on to benralizumab due to progressive reticulo-nodular lung abnormalities on CT ().
Table 5 Long-Term Follow-Up in Patients Who Continued Benralizumab Beyond 12-Month Timepoint
Discussion
We demonstrated in this real-life cohort that benralizumab has a steroid-sparing and respiratory exacerbation-reducing effect in EGPA patients in both treatment phases – during maintenance as well as induction therapy. Use of benralizumab in the induction therapy phase was associated with a high rate of EGPA remission, possibly facilitating OCS-downtapering by better controlling asthma and CRSwNP. Of note, in our study we describe the use of benralizumab with a dosage of 30 mg given every 4 weeks three times and then extending the interval to 8 weeks as used in the treatment of asthma, as neither mepolizumab nor benralizumab was approved in Germany at the time of treatment initiation. Our study adds to previously reported studiesCitation25–28 a real-life cohort of EGPA patients treated with benralizumab with a long-term follow-up and additional differentiation between use of treatment in induction versus maintenance therapy phase.
EGPA is a rare multi-organ disease that is characterized by small-vessel vasculitis, tissue eosinophilia, and eosinophilic inflammation and most frequently affects the upper and lower airways.Citation1 The main constituent of therapy are OCS and additional immunosuppressive therapies for remission induction or maintenance, eg cyclophosphamide, rituximab, azathioprine, or methotrexate.Citation1,Citation7 In relapsing or refractory EGPA, addition of the anti-IL5 antibody mepolizumab led to a higher proportion of remission and reduced daily OCS dose.Citation5 Thus, mepolizumab has recently been licensed in Europe for the treatment of EGPA and is also recommended by current guidelines.Citation1,Citation7 However, half of the patients did not achieve remission, and the annual relapse rate was still 1.1 in the randomized controlled trial (MIRRA) despite OCS and mepolizumab therapy.Citation5 Several case reports and series have implied a role for the anti-IL5R antibody benralizumab in the treatment of EGPA.Citation25,Citation26,Citation29–31 Two retrospective multicenter studies demonstrated reduction in OCS use and disease activity.Citation27,Citation28 In the MANDARA trial, a recently published non-inferiority RCT of benralizumab 30 mg/4 weeks compared to mepolizumab 300 mg/4 weeks with the primary endpoint of remission induction in relapsing or refractory EGPA, non-inferiority of benralizumab was shown.Citation32 Interestingly, the reduction of blood eosinophils as well as the percentage of patients that were able to completely stop OCS therapy was numerically greater in the benralizumab group. It remains unclear whether this translates into clinically meaningful differences in practice and which of the two antibodies to choose for which patient.
Diagnosis of EGPA is challenging as there are no diagnostic criteria for EGPA, and biopsies can be inconclusive regarding vasculitis.Citation1 Thus in some cases unambiguous differentiation from hypereosinophilic syndrome may not be possible.Citation33 ANCA-associated vasculitis classification criteria are only applicable when small-vessel vasculitis has already been demonstrated.Citation34 Further, different phenotypes of EGPA have been described, differentiating ANCA-positive patients with more vasculitic manifestations from ANCA-negative patients: glomerulonephritis, peripheral neuropathy, and skin purpura are more frequent in ANCA-positive patients, while lung infiltrates and cardiomyopathy are more frequent in ANCA-negative patients.Citation35–37 In our cohort of patients treated in a severe asthma center, frequent organ manifestations aside from asthma, CRS, and hypereosinophilia were pulmonary infiltrates and cardiomyopathy, and most patients were ANCA-negative. Historical biopsies were not always available or conclusive. We relied on similar EGPA diagnostic criteria as used in the MIRRA trial for inclusion in this analysis. Our real-life cohort resembles the MIRRA cohort, in which <20% of patients were ANCA-positive.Citation5 Therefore, our cohort might differ from cohorts observed in rheumatologic departments that might include a greater proportion of ANCA-positive patients, more renal and neurological involvement, and definitive proof of vasculitis.
We divided our analysis into two cohorts: patients in “maintenance therapy phase” and those in “induction therapy phase”. Patients in “maintenance therapy phase” already had EGPA diagnosis for a median of 6 years, and some had a history of treatment with the anti-IL5 antibody mepolizumab and immunosuppressive therapies but remained OCS-dependent with a median dose above 7.5 mg/d. Importantly, as mepolizumab was licensed for EGPA with a dosage of 300 mg/4 weeks in Germany only in November 2021, the patients presented here had previously been treated with an asthma indication and a dosage of 100 mg of mepolizumab every 4 weeks. Higher dosages are associated with stronger reductions in BEC and might therefore result in better outcome in patients with EGPA, and thus our experiences cannot be transferred to the differing EGPA dosage.
In our analysis of patients in maintenance phase, a significant OCS reduction occurred after start of benralizumab, and almost half of the patients were able to taper off OCS completely. Of note, many of these patients had been on continuous OCS for years or even decades. We further found a significant reduction in respiratory exacerbations, in line with real-life patient cohorts that demonstrated similar OCS-sparing and EGPA-reducing effects.Citation26–29 Lung function of our patients was only mildly impaired at baseline under ongoing OCS therapy and subsequently only showed minor changes, which is in agreement with data from case series.Citation29 Further, parameters of small-airway function like MEF25-75 are highly variable, and two other studies reported no differences in small-airway function after 8 months of IL5 therapy.Citation38,Citation39 Complete depletion of BEC occurred in all patients in the “maintenance therapy phase” group.
A first diagnosis of EGPA is often established when acute manifestations occur, eg respiratory failure due to asthma and pulmonary infiltrates, heart failure due myocarditis, peripheral neuropathy, or renal failure due to glomerulonephritis. To date, the cornerstone of therapy in these patients is a high-dose glucocorticosteroid therapy, in case of organ- or life-threatening disease accompanied by cyclophosphamide pulse or rituximab.Citation1,Citation7 We hypothesize that early initiation of benralizumab in addition to OCS and other indicated immunosuppressive therapies might help to control the disease faster, to prevent further eosinophil-induced damage and facilitate down-tapering OCS thus preventing side-effects. In the induction therapy phase cohort, there was a high number of patients with cardiac manifestations of EGPA. Cardiac manifestations as well as older age at diagnosis are risk factors for death.Citation3,Citation4,Citation36 In our cohort, one patient presenting with both risk factors died: a 74-year-old patient with EGPA-associated eosinophilic necrotizing myocarditis, who had received high-dose OCS and cyclophosphamide pulse therapy alongside benralizumab, was lost to follow-up for 14 months and then presented to our emergency department with severe heart failure and infection leading to death shortly thereafter.
In the induction cohort, addition of benralizumab to high-dose OCS and – if indicated –cyclophosphamide resulted in OCS reduction from an initial median dose of 42 mg/day to 2.5 mg/day after 12 months. During the first 12 months of benralizumab therapy only one patient experienced further flares, the other six remained free of flares and need for increase of OCS therapy. These results must be regarded with caution as this was a real-life study without a control group. However, our data suggest that addition of benralizumab therapy is safe and potentially beneficial in the early remission induction after diagnosis of EGPA.
Benralizumab is well tolerated, side-effects are minimal compared to glucocorticosteroids or classical immunosuppressants, and it might offer the potential to spare some of these therapies. As failure to achieve remission or occurrence of relapses are frequent in patients with EGPA, and active asthma or CRS are frequent reasons for flares, addition of anti-IL5 directed therapies may be helpful in all stages of the disease. Of note, current definitions of remission in asthma require the patient to be completely free of OCS and exacerbations along with no/minimal asthma symptoms for at least one year,Citation40–43 while the currently proposed definition of remission in EGPA requires the absence of clinical signs or symptoms of active disease on a maximum prednisolone dosage of 7.5 mg/day.Citation1 In EGPA, even though eosinophils are crucial for systemic disease manifestation and tissue destruction, other cell types (eg B-cells and T-cells) are also important. Thus, targeting eosinophils alone likely is not sufficient for controlling all aspects of the inflammatory process, and cases of new-onset vasculitis during ongoing anti-eosinophilic therapies have been described.Citation44,Citation45 This might provide a rationale for continuing low-dose OCS and/or other steroid-sparing immunosuppressants after remission induction even with ongoing anti-eosinophilic treatments. However, it is unknown whether this is actually necessary in all patients, eg those with ANCA-negative phenotype, and for how long. In our maintenance therapy cohort, ongoing flares were mainly due to asthma and CRS, and after addition of benralizumab complete withdrawal of OCS was possible in half of the patients without reoccurrence of systemic manifestations. A monotherapy with dupilumab has been associated with unmasking of EGPA in several case reportsCitation46,Citation47 and is therefore not recommended in cases of hypereosinophilia, eg by the German asthma guidelines.Citation40 In two patients in this analysis, persistingly uncontrolled CRSwNP under benralizumab prompted the addition of dupilumab, and dual benralizumab and dupilumab treatment was safe in our experience as previously described.Citation23,Citation48 Our long-term follow-up for a median of more than 4 years found that the majority of patients continued benralizumab treatment long-term, preserving good tolerability and outcome.
We acknowledge the limitations of our study with a retrospective single-center design, lack of a control group, and small sample size. Moreover, even though our patients were treated by an interdisciplinary team, patients were identified from a severe asthma center, thus potentially affecting baseline characteristics, including a low number of ANCA-positive patients, and also affecting the type of pretreatment. Our study should therefore serve as experience for clinicians, corroborating earlier studies as well as generating hypotheses for future controlled studies that need to address early EGPA as well as remission induction therapy of EGPA.
In conclusion, in our retrospective real-life study, benralizumab use in EGPA reduced respiratory exacerbations, enabled reduction of daily OCS doses in maintenance therapy phase, and was associated with high rates of remission when added during induction therapy phase.
Abbreviations
ACT, asthma control test; ANCA, antineutrophil cytoplasmic antibodies; anti-IL5, anti-interleukin 5; anti-IL5R, anti-interleukin 5 receptor; BEC, blood eosinophil count; BMI, body mass index; CNS, central nervous system; CRS, chronic rhinosinusitis; CRSwNP, chronic rhinosinusitis with nasal polyps; EGPA, eosinophilic granulomatosis with polyangiitis; ENT, ear nose and throat; FEV1, forced expiratory volume in 1 second; FEV1%, % predicted value of MEF25-75; FVC, forced vital capacity; FVC%, % predicted value of FVC; ICS, inhaled corticosteroid; LABA, long-acting beta agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; MEF25-75, mean expiratory flow at 25 to 75% of FVC; MEF25-75%, % predicted value of MEF25-75; MPO, myeloperoxidase; MTX, methotrexate; OCS, oral glucocorticosteroid; PFT, pulmonary function testing; RCT, randomized controlled trial; RV, residual volume; RV%, % predicted value of RV.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
CM reports speaker and/or consultancy fees from Sanofi, outside of the submitted work. PM reports speaker and/or consultancy fees from AstraZeneca, ResMed, Insmed, Vertex, all outside of the submitted work. US has received consulting fees from Ablynx, Alexion, and Vifor Pharma and reports research support from Vifor Pharma, Ablynx/Sanofi, and Alexion, all outside the submitted work. UG received speaker honoraria from AstraZeneca outside of the submitted work. HSK served as consultant and advisor or review panel member for AbbVie, Amgen, AstraZeneca, Biogen, BMS, Celgene, Gilead, Galapagos, Hexal Sandoz, Janssen-Cilag, Elli Lilly, Medac, MSD, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, and UCB, all outside the submitted work. JB reports speaker and/or consultancy fees from AstraZeneca, GSK, Novartis, Sanofi, all outside of the submitted work. NK reports speaker and/or consultancy fees from AstraZeneca and GSK, all outside of the submitted work. KM reports speaker and/or consultancy fees from AstraZeneca, Chiesi, GSK, Novartis, Sanofi, all outside of the submitted work. The author report no other conflicts of interest in this work.
Acknowledgment
We thank Christina Rullo, Nicole Seeling and Patricia Sklarek for excellent technical assistance, and all patients for their participation in the registry.
Additional information
Funding
References
- Emmi G, Bettiol A, Gelain E. et al. Evidence-Based Guideline for the diagnosis and management of eosinophilic granulomatosis with polyangiitis. Nat Rev Rheumatol. 2023;19(6):378–393. doi:10.1038/s41584-023-00958-w
- Nguyen Y, Guillevin L. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Semin Respir Crit Care Med. 2018;39(4):471–481. doi:10.1055/s-0038-1669454
- Samson M, Puéchal X, Devilliers H, et al. Long-term outcomes of 118 patients with eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) enrolled in two prospective trials. J Autoimmun. 2013;43:60–69. doi:10.1016/j.jaut.2013.03.003
- Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French vasculitis study group cohort. Arthritis Rheum. 2013;65(1):270–281. doi:10.1002/art.37721
- Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. doi:10.1056/NEJMoa1702079
- Jennette JC, Falk RJ, Bacon PA, et al. revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi:10.1002/art.37715
- Hellmich B, Sanchez-Alamo B, Schirmer JH, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. 2024;83(1):30–47. doi:10.1136/ard-2022-223764
- Yates M, Watts RA, Bajema IM, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75(9):1583–1594. doi:10.1136/annrheumdis-2016-209133
- Chan R, RuiWen Kuo C, Jabbal S, Lipworth BJ. Eosinophil depletion with benralizumab is associated with attenuated mannitol airway hyperresponsiveness in severe uncontrolled eosinophilic asthma. J Allergy Clin Immunol. 2023;151(3):700–705.e10. doi:10.1016/j.jaci.2022.10.028
- Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–1207. doi:10.1056/NEJMoa1403290
- Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371(13):1189–1197. doi:10.1056/NEJMoa1403291
- Castro M, Zangrilli J, Wechsler ME, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, Phase 3 trials. Lancet Respir Med. 2015;3(5):355–366. doi:10.1016/S2213-2600(15)00042-9
- FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388(10056):2128–2141. doi:10.1016/S0140-6736(16)31322-8
- Nair P, Wenzel S, Rabe KF, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376(25):2448–2458. doi:10.1056/NEJMoa1703501
- Kayser MZ, Drick N, Milger K, et al. Real-world multicenter experience with mepolizumab and benralizumab in the treatment of uncontrolled severe eosinophilic asthma over 12 months. J Asthma Allergy. 2021;14:863–871. doi:10.2147/JAA.S319572
- Eger K, Kroes JA, ten BA, Bel EH. Long-term therapy response to anti-IL-5 biologics in severe asthma-a real-life evaluation. J Allergy Clin Immunol Pract. 2021;9(3):1194–1200. doi:10.1016/j.jaip.2020.10.010
- Han JK, Bachert C, Fokkens W, et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9(10):1141–1153. doi:10.1016/S2213-2600(21)00097-7
- Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2022;149(4):1309–1317.e12. doi:10.1016/j.jaci.2021.08.030
- Förster-Ruhrmann U, Stergioudi D, Szczepek AJ, et al. A real-life comparison of pulmonary and nasal outcomes in patients with severe asthma and nasal polyposis treated with T2-biologics. World Allergy Organ J. 2023;16(2):100746. doi:10.1016/j.waojou.2023.100746
- Mümmler C, Dünzelmann K, Kneidinger N, et al. Real-life effectiveness of biological therapies on symptoms in severe asthma with comorbid CRSwNP. Clin Transl Allergy. 2021;11(5):e12049. doi:10.1002/clt2.12049
- Korn S, Milger K, Skowasch D, et al. The German severe asthma patient: baseline characteristics of patients in the German severe asthma registry, and relationship with exacerbations and control. Respir Med. 2022;195:106793. doi:10.1016/j.rmed.2022.106793
- Milger K, Korn S, Buhl R, et al. Age- and sex-dependent differences in patients with severe asthma included in the German Asthma Net cohort. Respir Med. 2020;162:105858. doi:10.1016/j.rmed.2019.105858
- Briegel I, Felicio-Briegel A, Mertsch P, Kneidinger N, Haubner F, Milger K. Hypereosinophilia with systemic manifestations under dupilumab and possibility of dual benralizumab and dupilumab therapy in patients with asthma and CRSwNP. J Allergy Clin Immunol Pract. 2021;9(12):4477–4479. doi:10.1016/j.jaip.2021.07.049
- Barnikel M, Grabmaier U, Mertsch P, et al. Domestic parasitic infections in patients with asthma and eosinophilia in Germany - three cases with learnings in the era of anti- IL5 treatments. J Asthma Allergy. 2023;16:1229–1232. doi:10.2147/JAA.S428607
- Guntur VP, Manka LA, Denson JL, et al. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract. 2021;9(3):1186–1193.e1. doi:10.1016/j.jaip.2020.09.054
- Nanzer AM, Dhariwal J, Kavanagh J, et al. Steroid-sparing effects of benralizumab in patients with eosinophilic granulomatosis with polyangiitis. ERJ Open Res. 2020;6(4):00451–2020. doi:10.1183/23120541.00451-2020
- Bettiol A, Urban ML, Padoan R, et al. Benralizumab for eosinophilic granulomatosis with polyangiitis: a retrospective, multicentre, cohort study. Lancet Rheumatol. 2023;5(12):e707–e715. doi:10.1016/S2665-9913(23)00243-6
- Cottu A, Groh M, Desaintjean C, et al. Benralizumab for eosinophilic granulomatosis with polyangiitis. Ann Rheum Dis. 2023;82(12):1580–1586. doi:10.1136/ard-2023-224624
- Padoan R, Chieco Bianchi F, Marchi MR, et al. Benralizumab as a glucocorticoid-sparing treatment option for severe asthma in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract. 2020;8(9):3225–3227.e2. doi:10.1016/j.jaip.2020.05.033
- Bormioli S, Vultaggio A, Nencini F, et al. Benralizumab: resolution of eosinophilic pulmonary vasculitis in a patient with EGPA. J Investig Allergol Clin Immunol. 2021;31(6):519–521. doi:10.18176/jiaci.0689
- Belfeki N, Abroug S, Ghriss N, et al. Successful benralizumab for eosinophilic myocarditis in eosinophilic granulomatosis with polyangiitis. Clin Exp Rheumatol. 2021;40(4):834–837. doi:10.55563/clinexprheumatol/ahyqld
- Wechsler ME, Nair P, Terrier B, et al. Benralizumab versus mepolizumab for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2024;390(10):911–921. doi:10.1056/NEJMoa2311155
- Wechsler ME, Hellmich B, Cid MC, et al. Unmet needs and evidence gaps in hypereosinophilic syndrome and eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol. 2023;151(6):1415–1428. doi:10.1016/j.jaci.2023.03.011
- Suppiah R, Robson JC, Grayson PC, et al. American college of rheumatology/European alliance of associations for rheumatology classification criteria for microscopic polyangiitis. Ann Rheum Dis. 2022;81(3):321–326. doi:10.1136/annrheumdis-2021-221796
- Cottin V, Bel E, Bottero P, et al. Respiratory manifestations of eosinophilic granulomatosis with polyangiitis (Churg-Strauss). Eur Respir J. 2016;48(5):1429–1441. doi:10.1183/13993003.00097-2016
- Moosig F, Bremer JP, Hellmich B, et al. A vasculitis centre based management strategy leads to improved outcome in eosinophilic granulomatosis and polyangiitis (Churg-Strauss, EGPA): monocentric experiences in 150 patients. Ann Rheum Dis. 2013;72(6):1011–1017. doi:10.1136/annrheumdis-2012-201531
- Sinico RA, Di Toma L, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in churg-strauss syndrome. Arthritis Rheum. 2005;52(9):2926–2935. doi:10.1002/art.21250
- Chan R, Lipworth BJ. Real-life effects of benralizumab on airway oscillometry in severe eosinophilic asthma. BMJ Open Respir Res. 2023;10(1). doi:10.1136/bmjresp-2022-001472
- Chan R, Lipworth BJ. Impact of biologic therapy on the small airways asthma phenotype. Lung. 2022;200(6):691–696. doi:10.1007/s00408-022-00579-2
- Lommatzsch M, Criee C, De Jong C, et al. S2k-Leitlinie zur fachärztlichen Diagnostik und Therapie von Asthma. 2023; Available From: https://register.awmf.org/de/leitlinien/detail/020-009. Accessed February 12, 2024.
- Lommatzsch M, Brusselle GG, Canonica GW, et al. Disease-modifying anti-asthmatic drugs. Lancet. 2022;399(10335):1664–1668. doi:10.1016/S0140-6736(22)00331-2
- Milger K, Suhling H, Skowasch D, et al. Response to biologics and clinical remission in the adult German asthma net severe asthma registry cohort. J All Clini Immun Prac. 2023;11(9):2701–2712.e2. doi:10.1016/j.jaip.2023.05.047
- Thomas D, McDonald VM, Pavord ID, Gibson PG. Asthma remission: what is it and how can it be achieved? Eur Respir J. 2022;60(5):2102583. doi:10.1183/13993003.02583-2021
- Caminati M, Fassio A, Alberici F, et al. Eosinophilic granulomatosis with polyangiitis onset in severe asthma patients on monoclonal antibodies targeting type 2 inflammation: report from the European EGPA study group. Allergy. 2023. doi:10.1111/all.15934
- Umezawa N, Sasaki H, Furusawa H, Kawata D, Hata C, Yasuda S. Development of vasculitis in a case with severe asthma treated with benralizumab and low-dose corticosteroid. Allergol Int. 2023;72(1):179–181. doi:10.1016/j.alit.2022.08.004
- Yamazaki K, Nomizo T, Hatanaka K, Hayama N, Oguma T, Asano K. Eosinophilic granulomatosis with polyangiitis after treatment with dupilumab. J Allergy Clin Immunol Glob. 2022;1(3):180–182. doi:10.1016/j.jacig.2022.03.006
- Suzaki I, Tanaka A, Yanai R, et al. Eosinophilic granulomatosis with polyangiitis developed after dupilumab administration in patients with eosinophilic chronic rhinosinusitis and asthma: a case report. BMC Pulm Med. 2023;23(1):130. doi:10.1186/s12890-023-02415-6
- Lommatzsch M, Suhling H, Korn S, et al. Safety of combining biologics in severe asthma: asthma-related and unrelated combinations. Allergy. 2022;77(9):2839–2843. doi:10.1111/all.15379