Abstract
In the last decade, many epidemiologic studies have investigated the link between vitamin D deficiency and asthma. Most studies have shown that vitamin D deficiency increases the risk of asthma and allergies. Low levels of vitamin D have been associated with asthma severity and loss of control, together with recurrent exacerbations. Remodeling is an early event in asthma described as a consequence of production of mediators and growth factors by inflammatory and resident bronchial cells. Consequently, lung function is altered, with a decrease in forced expiratory volume in one second and exacerbated airway hyperresponsiveness. Subepithelial fibrosis and airway smooth muscle cell hypertrophy are typical features of structural changes in the airways. In animal models, vitamin D deficiency enhances inflammation and bronchial anomalies. In severe asthma of childhood, major remodeling is observed in patients with low vitamin D levels. Conversely, the antifibrotic and antiproliferative effects of vitamin D in smooth muscle cells have been described in several experiments. In this review, we briefly summarize the current knowledge regarding the relationship between vitamin D and asthma, and focus on its effect on airway remodeling and its potential therapeutic impact for asthma.
Introduction
Vitamin D is an essential nutrient with significant pleiotropic effects, and its role in immunomodulation is considered to be of major importance. Moreover, there is growing evidence that vitamin D also contributes positively to pulmonary health.Citation1 Vitamin D is a secosteroid hormone synthesized by the skin following exposure to ultraviolet B light. Solar radiation photolyzes 7-dehydrocholesterol in the skin to previtamin D3, which is then converted to vitamin D3 (cholecalciferol).Citation2 Cholecalciferol from the skin and diet is hydroxylated in the liver to 25-hydroxyvitamin D3 [25(OH)D]. Hydroxylation of 25(OH)D to its biologically active form, ie, calcitriol (1,25(OH)2D3), takes place mainly in the kidney, but also in other sites, such as immune cells. Vitamin D signaling occurs predominantly through binding of 1,25(OH)2D3 to the vitamin D receptor (VDR), formation of a heterodimer with the retinoid X receptor, and subsequent regulation of gene expression after translocation to the nucleus. Alternatively, 1,25(OH)2D3 can bind to the VDR on the plasma membrane to exert rapid responses via production of second messengers.Citation3,Citation4
Epidemiology
Worldwide, there have been many asthma cohorts looking at vitamin D status in association with asthma and allergy, and these have shown contradictory results. Most studies have demonstrated an association between vitamin D deficiency and increased asthma risk.Citation5 Several case-control studies performed in different countries have shown a high prevalence of vitamin D deficiency in asthmatic children compared with controls, even in sunny areas.Citation6–Citation8 Two studies conducted in Italian children showed that hypovitaminosis D was very frequent in asthmatic children, with only 11% of children having adequate vitamin D levels.Citation9,Citation10 Several studies in children and adults concluded that low levels of vitamin D (defined as circulating 25(OH)D levels <30 ng/mL) were correlated with asthma severity, altered lung function, increased airway hyperresponsiveness, increased reactivity to exercise,Citation9–Citation15 and loss of control.Citation4 Several authors have demonstrated the involvement of vitamin D deficiency in severity and control of asthma. Lower concentrations of vitamin D have been associated with an increased risk of exacerbations.Citation16,Citation17 In one study, children with decreased serum 25(OH)D levels had a risk of asthma exacerbation that was eight times higher than that in children with stable or increased serum 25(OH)D levels.Citation18
However, the data are controversial. Some studies have reported conflicting results, ie, no association between vitamin D status and asthma in either children or adults.Citation19,Citation20 More time spent in the sun in winter between the ages of 6 and 15 years has been associated with an increased risk of hay fever.Citation20
The vitamin D pathway has revealed a number of polymorphisms in its components, including the VDR (the major receptor for the bioactive form of 25(OH)D), the microsomal vitamin D hydroxylase enzyme cytochrome P450 (CYP)2R1, and the vitamin D-binding protein GC.Citation21 Genetic variants in the VDR are variably associated with a risk of asthma.Citation22–Citation24 It is worth noting that, in addition to being expressed by immune cells, airway smooth muscle (ASM) cells also express functional VDR.Citation25 Another interesting gene is sphingosine-1-phosphate phosphatase (SGPP2) that had significant differences in expression depending on serum 25(OH)D level. Single nucleotide polymorphisms of SGPP2 have been associated with forced expiratory volume in one second (FEV1) variations in the general population.Citation26
Vitamin D and airway inflammation
Data from observational studies suggest a protective role of vitamin D in severe asthma. An immune-modulating potential of vitamin D has been implicated in asthma, and there is increasing evidence to support the role of the vitamin D pathway in the regulation of immune function. The VDR has been found on almost all immune cells, including macrophages and dendritic cells, as well as T-cells and B-cells;Citation2,Citation27–Citation29 these receptors increase five-fold following activation of quiescent cells.Citation30 In vivo studies in mouse models suggested this association, with Wittke et al demonstrating that VDR knockout mice do not develop experimental asthma, leading these authors to conclude that vitamin D is required for generation of T-helper (Th)2-driven inflammation in the airways.Citation31
Vitamin D promotes immune regulation. Indeed, in vitro studies have demonstrated the capacity of vitamin D to induce a tolerogenic dendritic cell phenotype producing interleukin (IL)-10, which promotes generation of fork-head box P3 (FOXP3) regulatory T-cells (Tregs).Citation32,Citation33 In children with asthma, serum 25(OH)D levels were significantly correlated with CD25+ FOXP3+ Tregs and IL-10+ CD4+ T lymphocytes.Citation34 These cells are associated with steroid sensitivity. Vitamin D deficiency could hinder expression of FOXP3 in CD4+ Tregs, thereby lowering steroid-induced production of IL-10 and decreasing the anti-inflammatory activity of glucocorticoids. In contrast, vitamin D supplementation reverses steroid resistance, probably via upregulation of FOXP3 in Tregs. Moreover, it has been recently shown that vitamin D inhibits proinflammatory Th17 responses.Citation34–Citation36 Glucocorticoids do not inhibit IL-17A cytokine expression in vivo or in vitro, whereas treatment with 1,25(OH)2D3 significantly reduces IL-17A and IL-22 levels.Citation37 Clinical studies show that the improvement in lung function obtained with inhaled steroid treatment is reduced in children with vitamin D deficiency.Citation38
Lastly, adequate vitamin D status may prevent asthma by upregulating the production of antimicrobial proteins, such as cathelicidin and beta defensins, thereby inhibiting the inflammatory reaction arising from viral respiratory tract infection.Citation39 Considering the major role of viruses in exacerbations of asthma, together with the consequences of recurrent exacerbations for airway remodeling, the potential therapeutic involvement of vitamin D to inhibit remodeling is discussed below.
Vitamin D and airway remodeling
The extensive airway remodeling occurring in asthma is characterized by structural changes that include abnormally thickened epithelium with mucous gland hypertrophy, subepithelial membrane thickening, fibrosis with altered composition and deposition of extracellular matrix, angiogenesis, and greatly increased ASM mass.Citation40 Airway remodeling therefore often causes irreversible airflow limitation and increased airway hyperresponsiveness. The thickness of the basement membrane is negatively correlated with FEV1 and the provocative dose of methacholine.Citation41 Traditionally, remodeling was thought to occur as a result of long-term inflammation but it is now clear that remodeling begins in early childhood before the age of 3 years.Citation42 In ovalbumin-sensitized mice, vitamin D deficiency was associated with greater airway hyperresponsiveness and more marked signs of airway remodeling than that observed in vitamin D-replete mice. Vitamin D supplementation attenuated these proinflammatory effects, but did not completely reverse the features of allergic airway inflammation.Citation43 In a murine model of chronic asthma, vitamin D treatment concomitant with ovalbumin challenge reduced chronic ovalbumin-induced inflammation and attenuated structural changes in the airways, including subepithelial fibrosis, goblet cell hyperplasia, and increased ASM mass. Nuclear translocation of nuclear factor-kappa B (NF-κB) p65 was inhibited, suggesting that vitamin D supplementation could attenuate airway remodeling in asthma via inhibition of NF-κB activation.Citation44
Inflammatory cells such as eosinophils produce and secrete matrix metalloproteinase (MMP)-9, which is capable of digesting type IV collagen, a component of the basement membrane. Overproduction of tissue inhibitors of matrix metalloproteinase 1 (TIMP-1) allows deposition of extracellular matrix and subepithelial fibrosis. Fibroblasts play a critical role in this exaggerated deposition of collagen when activated by tumor growth factor-beta-1 (TGF-β1), a profibrotic cytokine.Citation4,Citation41 In asthma, inflammatory and resident cells such as macrophages, lymphocytes, eosinophils, fibroblasts, and airway epithelial cells synthesize TGF-β. Th2-derived cytokines, including IL-4, play an important role in airway remodeling.Citation45 TGF-β induces TIMP-1 expression in a Th2-dependent manner.Citation4,Citation41 Vitamin D decreases CD4+ T-cell production of the signature Th2 cytokines, IL-4, IL-5, and IL-13, and promotes release of IL-10. The concentration of calcitriol in vitro may be critical in determining the effect on Th2 cell differentiation and function.Citation46 In the same way, 1,25(OH)2D3 inhibited the proliferation and activation of murine fibroblasts treated with TGF-β1 and reduced the expression of extracellular matrix proteins ().Citation47 In vitro experiments have demonstrated that TGF-β1 induces epithelial–mesenchymal transition in bronchial epithelial cells, with a decrease in E-cadherin expression and an increase in expression of vimentin and α-smooth muscle actin.Citation48 Bradykinin induces differentiation of lung fibroblasts into myofibroblasts.Citation49
Figure 1 Potential pathways involving vitamin D in airway remodeling. Airway remodeling can be defined as changes in the composition, content, and organization of the cellular and molecular constituents of the airway wall. These structural changes include epithelial detachment, subepithelial fibrosis, increased ASM mass, goblet cell hyperplasia, mucous gland hyperplasia, and proliferation of blood vessels. Asthma exacerbation is triggered by allergens or viruses, inducing T-helper cell-driven inflammation and resulting in production of growth factors (TGF-β, TNF-α, and VEGF), profibrotic mediators (TGF-β, LTs, PG), MMPs, and ECM protein. Vitamin D insufficiency is associated with alteration in lung development, cellular senescence with inflammation and MMP release, and ASM hyperplasia. Potential beneficial effects of vitamin D are increasing Treg activity, improving innate immunity against viruses, decreasing release of induced mediators, and decreasing production of LTs, PG, MMP, TNF-α and TGF-β, resulting in decreased subepithelial fibrosis and ASM hypertrophy.
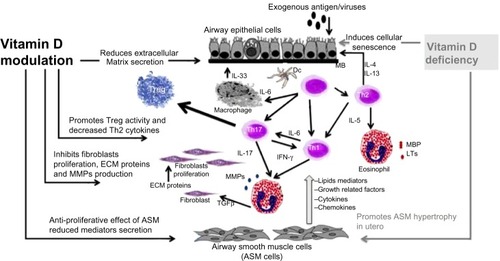
The antifibrotic effects of vitamin D have been described in several disease models. VDR knockout mice showed increased influx of inflammatory cells, phosphoacetylation of NF-κB associated with increased proinflammatory mediators, and upregulation of metalloproteinases in the lung. This was associated with emphysema and a decline in lung function associated with formation of lymphoid aggregates.Citation50 Further, 1,25(OH)2D3 suppresses production of MMPs and enhances TIMP-1 levels in tuberculosis.Citation51 In a human squamous carcinoma cell line, vitamin D3 significantly suppressed production of MMP-9 and MMP-13 mRNA and proteins in a dose-dependent manner.Citation52 In a Crohn’s disease model, a vitamin D analog attenuated the profibrotic response of colonic myofibroblasts to high matrix stiffnesss.Citation53 Calcitriol is known to attenuate epidermal inflammation. Exposure to tumor necrosis factor-alpha (TNF-α) markedly increased protein and mRNA levels of MMP-9, while pretreatment with calcitriol reduced this effect in a dose-dependent manner. This effect was associated with a reduction in activation of NF-κB.Citation54 1,25(OH)2D3 attenuates TNF-α-induced p65 nuclear translocation and NF-κB activity in a VDR-dependent manner because VDR physically interacts with IκB kinase β to block NF-κB activation. This interaction is enhanced by 1,25(OH)2D3.Citation55
Airway remodeling in asthma includes increased ASM mass, reflecting myocyte proliferation.Citation56–Citation58 Hypertrophy of ASM is correlated with a decrease in FEV1 after provocation by histamine.Citation59 Interestingly, vitamin D has antiproliferative effects on ASM, decelerating cell cycling and decreasing hyperplasia.Citation60 Asthmatic ASM cells express higher levels of MMP-9, and ADAM metallopetidase domain 33(ADAM33) suppress disintegrin, and metalloprotease 33 when compared with nonasthmatic ASM cells. Treatment of asthmatic cells with vitamin D reduced the mRNA and protein expression of MMP-9 and ADAM33 genes.Citation4 Further, polymorphisms in the VDR gene have been linked to increased airway resistance in mice.Citation61 A study demonstrated that calcitriol (but not dexamethasone), added to human ASM cultures, inhibited platelet-derived growth factor-induced DNA synthesis. These effects were associated with VDR-mediated expression of CYP24A1, with no effect on apoptosis of ASM cells.Citation62
Gupta et al described major evidence for a role for vitamin D in remodeling in a study of children with severe therapy-resistant asthma. Remodeling was assessed by endobronchial biopsy. Lower vitamin D levels were associated with increased ASM mass and worse asthma control and lung function. ASM mass, but not epithelial shedding or reticular basement membrane thickness, was inversely related to 25(OH)D(3) levels.Citation63
Cysteinyl leukotrienes (LTC4, LTD4) synthesized from arachidonic acid can increase ASM mass, and prostaglandins are even more effective than leukotrienes for inducing ASM remodeling.Citation40 Vitamin D significantly reduced production of prostaglandin E2, regulating its synthesis and degradation by human lung fibroblasts, without any effect on production of TGF-β1, vascular endothelial growth factor (VEGF), or fibronectin. Vitamin D can modulate fibroblast-mediated tissue repair function via this mechanism.Citation64
Human rhinovirus infections are the main trigger for asthma exacerbations.Citation65 Viruses may contribute to airway remodeling by increasing deposition of extracellular matrix, which in turn may contribute to increased ASM mass via increased cell migration.Citation66 Rhinovirus infection induces epithelial production of a number of growth factors and other mediators, eg, activin A and amphiregulin, that are linked to subepithelial membrane thickening, and MMP9 and VEGF, which could contribute to development and progression of the airway remodeling processes in asthma.Citation42 In vitro, rhinovirus induces release of basic fibroblast growth factor by human airway epithelium, and stimulates fibroblast proliferation together with increased metalloproteinase activity.Citation67 Primary human bronchial epithelial cells were treated with vitamin D, and rhinovirus replication and gene expression were evaluated by quantitative polymerase chain reaction.Citation68 Vitamin D did not directly affect replication of rhinovirus in airway epithelial cells, but did influence chemokine synthesis and alter the growth and differentiation of airway epithelial cells. In vitro, treatment of epithelial lung cells with vitamin D significantly decreased H1N1-induced inflammatory cytokine transcription levels.Citation69 Human cathelicidin LL-37 and murine cathelicidin (cathelin-related antimicrobial peptide), expression of which is regulated by VDR activation, showed antiviral activity and modulated the immune response to viral infections.Citation70 Downregulation of expression of monocyte Toll-like receptors by calcitriol leads to reduced production of the proinflammatory cytokine TNF-α in response to stimulation with Toll-like receptor ligands.Citation46 Therefore, vitamin D sufficiency may be important for overall maintenance of pulmonary health via control of innate and adaptive immune inflammatory responses.Citation46
VEGF is a key mediator of angiogenesis and is able to modulate ASM.Citation71 Blood levels of VEGF are significantly increased in asthmatic patients compared with healthy controls. Circulating VEGF levels are inversely correlated with percent predicted FEV1, suggesting a relationship between VEGF production and bronchial remodeling.Citation72 Induction of VEGF may be a potential mechanism via which particulate matter in the urban environment influences lung function in children.Citation73 Isocyanate-induced occupational asthma is associated with upregulated expression of vitamin D-binding protein. In vitro, exposure of epithelial cells to isocyanate induces increased production of VEGF which is reversed by treatment with calcitriol.Citation74 However, in other situations, vitamin D promotes production of VEGF.Citation75
Airway remodeling is considered to be a repair process occurring after injury to the epithelium. Cellular senescence has been examined in lung specimens from patients with asthma, and activation of cellular senescence was detected in epithelial airway samples. Moreover, induction of cellular senescence has been shown to be required for airway remodeling in vitro, and inhibition of cellular senescence blocked airway remodeling.Citation76 Vitamin D deficiency is involved in senescence of different organs by inducing DNA damage, cellular senescence, and production of senescence-associated inflammatory cytokines and MMPs.Citation77
Vitamin D involvement in lung development is important to consider, as remodeling occurs very early in the course of asthma. In fact, vitamin D stimulates synthesis of alveolar type II cell DNA and surfactant production.Citation3 Mouse pups born to mothers with vitamin D deficiency were shown to have decreased lung function, primarily as a result of reduced lung volume. In the same way, lung compliance was decreased in rat pups born to vitamin D-deficient mothers.Citation78 Moreover, the authors of a study on remodeling in severe asthma of childhood hypothesized that ASM hypertrophy resulted from vitamin D deficiency in utero.Citation63 As such, vitamin D deficiency may predispose to asthma or increase the morbidity associated with asthma by altering lung development in early life.Citation3
Potential prevention and reversal of remodeling
Recombinant adeno-associated virus containing antisense against the IL-4 gene (rAAV-asIL4) vector can significantly suppress the expression of IL-4 protein and airway inflammation in the rat. Interestingly, rAAV-asIL4 decreases collagen deposition beneath the basement membrane, suggesting the potential to attenuate the airway remodeling process by inhibition of airway inflammation.Citation45 In the same way, treatment with anti-immunoglobulin E antibody inhibited the development of airway hyperresponsiveness, eosinophilic inflammation, and airway remodeling.Citation79 Vitamin D, by inhibition of Th2 cytokine production, can enhance the effect of asthma drugs on remodeling. Budesonide and formoterol, both alone or in combination, were shown to effectively inhibit bradykinin-induced differentiation of fibroblasts into myofibroblasts and to have the potential to inhibit fibroblast-dependent matrix remodeling in the airways of asthmatic patients. However, there was no consistent evidence of an inhibitory effect of steroids on remodeling.Citation49 Arachidonic acid-derived mediators, ie, cysteinyl leukotrienes and prostaglandins, production of which is decreased by vitamin D, constitute a potential target for modulation of remodeling.Citation40 Monthly vitamin D doses of 60,000 IU significantly reduced the number of exacerbations and improved lung function tests.Citation80 Further, vitamin D supplementation improved symptom scores and decreased steroid requirements, and also increased the Treg subset.Citation81 One plausible mechanism for this reversibility effect on steroid resistance is upregulation of FOXP3 in Tregs by vitamin D.Citation4
Vitamin D supplementation
Data suggesting that vitamin D deficiency results in an increased risk of asthma and allergy continues to accumulate. However, the amount of vitamin D required to decrease the risk for both development and severity of these disorders is still unknown, and the results of ongoing clinical trials on vitamin D supplementation are needed before recommendations can be firmly established.Citation82
With regard to vitamin D supplementation during pregnancy, two epidemiologic studies have shown that higher vitamin D intake is associated with a reduction in the risk of recurrent wheeze in children.Citation83,Citation84 Further, Errkola et al found lower rates of allergic rhinitis and asthma in 5-year-old children whose mothers had a higher vitamin D intake during pregnancy.Citation85 In contrast, a study from the UK reported an increased risk of eczema and asthma in children born to women with higher levels of vitamin D (>75 nmol/L or 30 ng/mL).Citation86 Wjst et al suggested that exposure to higher doses of vitamin D in utero might be a risk factor for development of atopic disorders, and suggested that the current asthma epidemic may be due to food supplementation with vitamin D.Citation87
The large, randomized, multicenter Vitamin D Antenatal Asthma Reduction Trial is testing the hypothesis that vitamin D supplementation in pregnant women can prevent asthma and allergic diseases.Citation88 In the Prevention and Incidence of Asthma and Mite Allergy birth cohort, serum vitamin D concentrations at age 4 years were inversely associated with asthma at the age of 4–8 years.Citation89 However, a trial of supplementation with vitamin D in late pregnancy showed no effect on wheeze frequency or lung function in offspring by the age of 3 years.Citation90 In this study, supplementation improved but did not optimize vitamin D status.Citation90
Even if more evidence is still required to support supplementation of vitamin D to prevent asthma, positive data have been reported with respect to vitamin D supplementation as a common treatment for asthma, with the effect of decreasing the frequency of exacerbation and improving steroid sensitivity.Citation18,Citation91 In a recent prospective study,Citation18 vitamin D supplementation in asthmatic children appeared to prevent exacerbation of asthma triggered by acute respiratory infection. Further, Majak et alCitation18 compared two groups of asthmatic children who were treated with inhaled corticosteroids with or without vitamin D3 supplementation. Children treated with steroids and placebo experienced more asthma exacerbations and had more severe clinical manifestations than children with treated with steroids and vitamin D3. In addition, vitamin D supplementation improved antimicrobial defense against respiratory tract infections.Citation92,Citation93
Conclusion
As shown in this review, airway remodeling involves structural changes in the walls of the airways induced by repeated injury and repair processes, and is characterized by changes in tissue, cellular, and molecular composition affecting the ASM, epithelium, blood vessels, extracellular matrix, and defective lung–blood barriers. Airway remodeling is an early event in asthma and other lung diseases, and is arguably one of the most intractable problems leading to irreversible loss of lung function. Vitamin D may influence airway remodeling by affecting the growth and contractility of ASM and inhibiting TGF-β and MMPs, as well as fibroblast proliferation, promoting antimicrobial pathways, and suppressing Treg activity, as summarized in .
Our knowledge regarding direct mechanistic links between vitamin D and lung disease is limited. In view of the available data, vitamin D insufficiency can be said to be associated with the severity of childhood asthma and airway remodeling. According to experimental evidence, several pathways involve hypovitaminosis D in remodeling, ie, development of alterations in the lung and accelerated cellular senescence associated with an unbalanced process of repair, with excessive Th2 inflammation, fibroblast activation, and smooth muscle cell hypertrophy, suggesting a beneficial therapeutic impact of supplementation on reversal of remodeling. However, major evidence supporting the beneficial effect of vitamin D supplementation on remodeling is needed. Vitamin D supplementation does inhibit several profibrotic mediators and production of growth factors in vitro, but the results of clinical trials are still controversial. The issue lies within the link of causality between vitamin D insufficiency and asthma. Although vitamin D is definitely necessary for pulmonary health, the low levels observed in asthma could be due to the inflammatory process and simply considered as a marker of severity. Recommendations cannot be made regarding vitamin D in asthma until the results of ongoing clinical trials become available.
Disclosure
The authors report no conflicts of interests in this work.
References
- HolickMFVitamin D deficiencyN Engl J Med200735726628117634462
- NagpalSNaSRathnachalamRNoncalcemic actions of vitamin D receptor ligandsEndocr Rev20052666268715798098
- PaulGBrehmJMAlcornJFHolguínFAujlaSJCeledónJCVitamin D and asthmaAm J Respir Crit Care Med201218512413222016447
- PoonAHMahboubBHamidQVitamin D deficiency and severe asthmaPharmacol Ther201314014815523792089
- CamargoCAJrInghamTWickensKCord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthmaNew Zealand Asthma and Allergy Cohort Study Group Pediatrics2011127e180e187
- FreishtatRJIqbalSFPillaiDKHigh prevalence of vitamin D deficiency among inner-city African American youth with asthma in Washington, DCJ Pediatr201015694895220236657
- BenerAEhlayelMSTulicMKHamidQVitamin D deficiency as a strong predictor of asthma in childrenInt Arch Allergy Immunol201215716817521986034
- AlyasinSMomenTKashefSAlipourAAminRThe relationship between serum 25 hydroxyvitamin D levels and asthma in childrenAllergy Asthma Immunol Res2011325125521966605
- ChinellatoIPiazzaMSandriMSerum vitamin D levels and exercise-induced bronchoconstriction in children with asthmaEur Respir J2011371366137021071468
- ChinellatoIPiazzaMSandriMPeroniDPiacentiniGBonerALVitamin D serum levels and markers of asthma control in Italian childrenJ Pediatr201115843744120870246
- BurnsJSDockeryDWNeasLMLow dietary nutrient intakes and respiratory health in adolescentsChest200713223824517475634
- BlackPNScraggRRelationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination surveyChest20051283792379816354847
- SutherlandERGolevaEJacksonLPStevensADLeungDYVitamin D levels, lung function, steroid response in adult asthmaAm J Respir Crit Care Med201018169970420075384
- LiFPengMJiangLVitamin D deficiency is associated with decreased lung function in Chinese adults with asthmaRespiration20118146947521124013
- SearingDAZhangYMurphyJRHaukPJGolevaELeungDYDecreased serum vitamin D levels in children with asthma are associated with increased corticosteroid useJ Allergy Clin Immunol2010125955100020381850
- BrehmJMCeledònJCSoto-QuirosMESerum vitamin D levels and markers of severity of childhood asthma in Costa RicaAm J Respir Crit Care Med200917976577119179486
- BrehmJMSchuemannBFuhlbriggeALSerum vitamin D levels and severe asthma exacerbations in the childhood asthma management program studyJ Allergy Clin Immunol2010126525820538327
- MajakPOlszowiec-ChlebnaMSmejdaKStelmachIVitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infectionJ Allergy Clin Immunol20111271294129621315433
- DevereuxGWilsonAAvenellAMcNeillGFraserWDA case-control study of vitamin D status and asthma in adultsAllergy20106566666719845573
- HughesAMLucasRMPonsonbyALThe role of latitude, ultraviolet radiation exposure and vitamin D in childhood asthma and hayfever: an Australian multicenter studyPediatr Allergy Immunol20112232733320880353
- HuangHPorpodisKZarogoulidisPVitamin D in asthma and future perspectivesDrug Des Devel Ther2013710031013
- RabyBALazarusRSilvermanEKAssociation of vitamin D receptor gene polymorphisms with childhood and adult asthmaAm J Respir Crit Care Med20041701057106515282200
- PoonAHLapriseCLemireMAssociation of vitamin D receptor genetic variants with susceptibility to asthma and atopyAm J Respir Crit Care Med200417096797315282199
- MaalmiHSassiFHBerraiesAAmmarJHamzaouiKHamzaouiAAssociation of vitamin D receptor gene polymorphisms with susceptibility to asthma in Tunisian children: a case control studyHum Immunol20137423424023200756
- BosseYMaghniKHudsonTJ1α,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processesPhysiol Genomics20072916116817213369
- ReardonBJHansenJGCrystalRGVitamin D-responsive SGPP2 variants associated with lung cell expression and lung functionBMC Med Genet20131412224274704
- HewisonMVitamin D and the intracrinology of innate immunityMol Cell Endocrinol201032110311120156523
- ProvvediniDMTsoukasCDDeftosLJManolagasSC1,25 dihydroxyvitamin D3 receptors in human leukocytesScience1983221118111836310748
- WangYZhuJDeLucaHFWhere is the vitamin D receptor?Arch Biochem Biophys201252312313322503810
- BaekeFKorfHOverberghLHuman T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune systemJ Steroid Biochem Mol Biol201012122122720302932
- WittkeAWeaverVMahonBDAugustACantornaMTVitamin D receptor-deficient mice fail to develop experimental allergic asthmaJ Immunol20041733432343615322208
- UrryZChambersESXystrakisEThe role of 1α,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cellsEur J Immunol2012422697270822903229
- GregoriSCasoratiMAmuchasteguiSSmiroldoSDavalliAMAdoriniLRegulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation toleranceJ Immunol20011671945195311489974
- MaalmiHBerraiesATangourEThe impact of vitamin D deficiency on immune T cells in asthmatic children: a case-control studyJ Asthma Allergy20125111922690128
- TangJZhouRLugerDCalcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector responseJ Immunol20091824624463219342637
- DanielCSartoryNAZahnNImmune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profileJ Pharmacol Exp Ther2008324233317911375
- NanzerAMChambersESRyannaKEnhanced production of IL-17A in patients with severe asthma is inhibited by 1α,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashionJ Allergy Clin Immunol201313229730423683514
- WuACTantisiraKLiLFuhlbriggeALWeissSTLitonjuaAChildhood Asthma Management Program Research GroupEffect of vitamin D and inhaled corticosteroid treatment on lung function in childrenAm J Respir Crit Care Med201218650851322798322
- BeardJABeardenAStrikerRVitamin D and the anti-viral stateJ Clin Virol20115019420021242105
- PrakashYSAirway smooth muscle in airway reactivity and remodeling: what have we learned?Am J Physiol Lung Cell Mol Physiol2013305L912L93324142517
- YamauchiKInoueHAirway remodeling in asthma and irreversible airflow limitation-ECM deposition in airway and possible therapy for remodelingAllergol Int20075632132917965575
- ProudDRole of rhinovirus infections in asthmaAsian Pac J Allergy Immunol20112920120822053589
- AgrawalTGuptaGKAgrawalDKVitamin D supplementation reduces airway hyperresponsiveness and allergic airway inflammation in a murine modelClin Exp Allergy20134367268323711130
- LaiGWuCHongJSongY1,25-dihydroxyvitamin D(3) (1,25-(OH) (2)D(3) attenuates airway remodeling in a murine model of chronic asthmaJ Asthma20135013314023157452
- CaoYZengDSongQThe effects of antisense interleukin-4 gene transferred by recombinant adeno-associated virus vector on the airway remodeling in allergic ratsJ Asthma20104795195820831469
- DimeloeSNanzerARyannaKHawrylowiczCRegulatory T cells, inflammation and the allergic response – the role of glucocorticoids and vitamin DJ Steroid Biochem Mol Biol2010120869520227496
- RamirezAMWongtrakoolCWelchTSteinmeyerAZügelURomanJVitamin D inhibition of pro-fibrotic effects of transforming growth factor beta1 in lung fibroblasts and epithelial cellsJ Steroid Biochem Mol Biol201011814215019931390
- YangZCYiMJRanNTransforming growth factor-β1 induces bronchial epithelial cells to mesenchymal transition by activating the Snail pathway and promotes airway remodeling in asthmaMol Med Rep201381663166824126595
- SabatiniFPetecchiaLUsaiCPharmacological modulation of the bradykinin-induced differentiation of human lung fibroblasts: effects of budesonide and formoterolJ Asthma2012491004101123088211
- SundarIKHwangJWWuSSunJRahmanIDeletion of vitamin D receptor leads to premature emphysema/COPD by increased matrix metalloproteinases and lymphoid aggregates formationBiochem Biophys Res Commun201140612713321300024
- AnandSPSelvarajPEffect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosisClin Immunol200913312613119615945
- MeephansanJKomineMTsudaHOhtsukiMSuppressive effect of calcipotriol on the induction of matrix metalloproteinase (MMP)-9 and MMP-13 in a human squamous cell carcinoma cell lineClin Exp Dermatol20123788989622924547
- JohnsonLASauderKLRodanskyESSimpsonRUHigginsPDCARD-024, a vitamin D analog, attenuates the pro-fibrotic response to substrate stiffness in colonic myofibroblastsExp Mol Pathol201293919822542712
- Bahar-ShanyKRavidAKorenRUpregulation of MMP-9 production by TNF alpha in keratinocytes and its attenuation by vitamin DJ Cell Physiol201022272973720020446
- ChenYZhangJGeXVitamin D receptor inhibits nuclear factor κB activation by interacting with IκB kinase β proteinJ Biol Chem2013288194501945823671281
- BentleyJKHershensonMBAirway smooth muscle growth in asthma: proliferation, hypertrophy, and migrationProc Am Thorac Soc20085899618094090
- BergeronCBouletLPStructural changes in airway diseases: characteristics, mechanisms, consequences, and pharmacologic modulationChest20061291068108716608960
- GolevaEHaukPJBoguniewiczJAirway remodeling and lack of bronchodilator response in steroid-resistant asthmaJ Allergy Clin Immunol20071201065107217900681
- TsurikisawaNOshikataCTsuburaiTBronchial reactivity to histamine is correlated with airway remodeling in adults with moderate to severe asthmaJ Asthma20104784184820854030
- IqbalSFFreishtatRJMechanism of action of vitamin D in the asthmatic lungJ Investig Med20115912001202
- BerndtASavageHSStearnsTMPaigenBGenetic analysis of lung functioning inbred mice suggests vitamin D receptor as a candidate geneMol Genet Genomics201128623724621850575
- DameraGFogleHWLimPVitamin D inhibits growth of human airway smooth muscle cells through growth factor-induced phosphorylation of retinoblastoma protein and checkpoint kinase 1Br J Pharmacol20091581429144119814732
- GuptaASjoukesARichardsDRelationship between serum vitamin D, disease severity, and airway remodeling in children with asthmaAm J Respir Crit Care Med20111841342134921908411
- LiuXNelsonAWangXVitamin D modulates PGE2 synthesis and degradation in human lung fibroblastsAm J Respir Cell Mol Biol201450405023941558
- WarkPAToozeMPowellHParsonsKViral and bacterial infection in acute asthma and chronic obstructive pulmonary disease increases the risk of readmissionRespirology201318996100223600594
- KuoCLimSKingNJRhinovirus infection induces extracellular matrix protein deposition in asthmatic and nonasthmatic airway smooth muscle cellsAm J Physiol Lung Cell Mol Physiol2011300L951L95721460120
- SkevakiCLPsarrasSVolonakiERhinovirus-induced basic fibroblast growth factor release mediates airway remodeling featuresClin Transl Allergy201221422908984
- Brockman-SchneiderRAPicklesRJGernJEEffects of vitamin D on airway epithelial cell morphology and rhinovirus replicationPLoS One20149e8675524475177
- KhareDGodboleNMPawarSDCalcitriol [1, 25(OH)2 D3] pre- and post-treatment suppresses inflammatory response to influenza A (H1N1) infection in human lung A549 epithelial cellsEur J Nutr2013521405141523015061
- VandammeDLanduytBLuytenWSchoofsLA comprehensive summary of LL-37, the factotum human cathelicidin peptideCell Immunol2012280223523246832
- MakindeTMurphyRFAgrawalDKImmunomodulatory role of vascular endothelial growth factor and angiopoietin-1 in airway remodelingCurr Mol Med2006683184117168735
- LeeKYLeeKSParkSJClinical significance of plasma and serum vascular endothelial growth factor in asthmaJ Asthma20084573573918972287
- IwanagaKElliottMSVedalSDebleyJSUrban particulate matter induces pro-remodeling factors by airway epithelial cells from healthy and asthmatic childrenInhal Toxicol20132565366024102466
- KimSHChoiGSNamYHRole of vitamin D-binding protein in isocyanate-induced occupational asthmaExp Mol Med20124431932922314196
- GrundmannMHaidarMPlaczkoSVitamin D improves the angiogenic properties of endothelial progenitor cellsAm J Physiol Cell Physiol2012303C954C96222932684
- WuJDongFWangRACentral role of cellular senescence in TSLP-induced airway remodeling in asthmaPLoS One20138e7779524167583
- ShenMLuoYNiuY1,25(OH)2D deficiency induces temporomandibular joint osteoarthritis via secretion of senescence-associated inflammatory cytokinesBone20135540040923624390
- GaultierCHarfABalmainNCuisinier-GleizesPMathieuHLung mechanics in rachitic ratsAm Rev Respir Dis1984130110811106508008
- KangJYKimJWKimJSInhibitory effects of anti-immunoglobulin E antibodies on airway remodeling in a murine model of chronic asthmaJ Asthma20104737438020528589
- YadavMMittalKEffect of vitamin D supplementation on moderate to severe bronchial asthmaIndian J Pediatr1162013 [Epub ahead of print]
- BarisSKiykimAOzenATulunayAKarakoc-AydinerEBarlanIBVitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust miteAllergy20146924625324180595
- LitonjuaAAVitamin D deficiency as a risk factor for childhood allergic disease and asthmaCurr Opin Allergy Clin Immunol20121217918522266772
- CamargoCAJrRifas-ShimanSLLitonjuaAAMaternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of ageAm J Clin Nutr20078578879517344501
- DevereuxGLitonjuaAATurnerSWMaternal vitamin D intake during pregnancy and early childhood wheezingAm J Clin Nutr20078585385917344509
- ErkkolaMKailaMNwaruBIMaternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old childrenClin Exp Allergy20093987588219522996
- GaleCRRobinsonSMHarveyNCMaternal vitamin D status during pregnancy and child outcomesEur J Clin Nutr200862687717311057
- WjstMDoldSGenes, factor X, and allergens: what causes allergic diseases?Allergy19995475775910442534
- ClinicalTrials.govMaternal Vitamin D Supplementation to Prevent Childhood Asthma (VDAART) Available from: http://clinicaltrials.gov/ct2/show/NCT00920621Accessed February 14, 2014
- Van OeffelenAABekkersMBSmitHASerum micronutrient concentrations and childhood asthma: the PIAMA birth cohort studyPediatr Allergy Immunol20112278479321929603
- GoldringSTGriffithsCJMartineauARPrenatal vitamin D supplementation and child respiratory health: a randomised controlled trialPLoS One20138e6662723826104
- XystrakisEKusumakarSBoswellSReversing the defective induction of IL-10–secreting regulatory T cells in glucocorticoid-resistant asthma patientsJ Clin Invest200611614615516341266
- GunvilleCFMouraniPMGindeAAThe role of vitamin D in prevention and treatment of infectionInflamm Allergy Drug Targets20131223924523782205
- LaaksiIRuoholaJPMattilaVAuvinenAYlikomiTPihlajamäkiHVitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish menJ Infect Dis201020280981420632889
