Abstract
Allergic rhinitis is a chronic non-infectious inflammation of the nasal mucosa mediated by specific IgE. Recently, the human microbiome has drawn broad interest as a potential new target for treating this condition. This paper succinctly summarizes the main findings of 17 eligible studies published by February 2024, involving 1044 allergic rhinitis patients and 954 healthy controls from 5 countries. These studies examine differences in the human microbiome across important mucosal interfaces, including the nasal and intestinal areas, between patients and controls. Overall, findings suggest variations in the gut microbiota between allergic rhinitis patients and healthy individuals, although the specific bacterial taxa that significantly changed were not always consistent across studies. Due to the limited scope of existing research and patient coverage, the relationship between the nasal microbiome and allergic rhinitis remains inconclusive. The article discusses the potential immune-regulating role of the gut microbiome in allergic rhinitis. Further well-designed clinical trials with large-scale recruitment of allergic rhinitis patients are encouraged.
Introduction
Allergic rhinitis (AR), a chronic and non-infectious IgE-mediated inflammation of the nasal mucosa, manifests through symptoms such as sneezing, congestion, pruritus, and rhinorrhea.Citation1,Citation2 The global incidence of AR has progressively escalated alongside industrialization and urban development. Afflicting 10–20% of the global population, AR has transcended into a pervasive health dilemma.Citation3 Consequences of this condition are multifaceted, ranging from a decline in labor productivity to a deterioration in the quality of life for those affected. Notably, AR impairs sleep and cognitive functions, giving rise to increased levels of irritability and fatigue. It has been documented that AR is intricately linked with diminished academic and professional output, a concern that is accentuated during periods of high pollen exposure.Citation1 The socioeconomic burden of AR is substantial, engendering annual economic losses estimated between 30 to 50 billion euros within European Union nations alone, thereby emphasizing the significant impact of this condition on both individual well-being and economic stability.Citation3
Tari Haahtela’s biodiversity hypothesis posits that a rich human microbiota fosters an equilibrium in immunity, shielding individuals from allergies and other inflammatory conditions.Citation4 In recent scientific discourse, researchers have identified a critical role for imbalances in microbiota populations across multiple key mucosal interfaces, such as the intestinal and nasal areas, in the onset and progression of allergic diseases. Such dysbiosis may detrimentally influence immune system functionality, precipitating conditions including asthma and allergic rhinitis.Citation5–7 Alterations in the gut microbiota are closely intertwined with human physiological and pathological processes. The successful colonization and maintenance of a diverse gut microbiome serve as a formidable barrier against pathogenic bacterial invasion, curtailing nutritional competition between potentially harmful and commensal bacteria.Citation8 Perturbations to the diversity or balance of gut microbiota can lead to gastrointestinal dysbiosis, precipitating alterations in microbial metabolism and immune responses, with consequential impacts on host health.Citation9 Additionally, the gut microbiota is instrumental in stimulating the development of both innate and adaptive immunity through its involvement in the metabolism of short-chain fatty acids (SCFAs), tryptophan (Trp), amino acids, vitamins, and bile acids, making it a potential target for modulating immune responses.Citation10–12
John Bienenstock’s “common mucosal immune system” hypothesis envisages the mucosal immune apparatus scattered throughout the body as a comprehensive, interconnected network. Within this system, immune cells and structures across diverse mucosal tissues form a unified functional whole. This paradigm conceives the mucosal immune system as a cohesive “organ system” wherein internal immune cells facilitate effective communication and collaboration across various mucosal sites to collectively address external stimuli and uphold the host’s immunological stability.Citation13,Citation14 Mucosal tissues, including those in the gastrointestinal, respiratory, and urogenital systems, are pivotal in the host’s defense against the incursion of foreign pathogens. Despite the wealth of knowledge regarding these individual systems, a holistic appraisal of the mucosal immune system as a functional organ system is still markedly lacking.Citation15 Addressing this issue, the current article consolidates recent literature on the variances in the human microbiome at several key mucosal interfaces, including the nasal and gut regions, between individuals with AR and controls, with the aim of enhancing insights into the systemic mucosal microbiota’s association with AR.
Summary of Included Studies
Upon a systematic search of PubMed, Embase, CNKI, and Web of Science databases, this review includes 17 qualifying studies published as of February 2024 (). These studies were conducted in five nations, comprising 12 studies from China, two from the United States, and one each from Australia, Italy, and Japan. Sample sizes ranged from 6 to 572 across case and control cohorts. Within these reports, 8 studies focus on the gut microbiota, while 9 investigate the nasal microbiota.
Table 1 Changes in the gut and nasal microbiota of patients with allergic rhinitis
The Gut Microbiome and AR
Gut Microbiota Presence Patterns in AR Patients
A total of eight studies involving 872 patients with AR focused on the gut microbiota (). Among the research on microbial diversity, six studies reported a marked dysbiosis and a significant reduction in microbial diversity in AR patients compared to healthy controls, with distinct abundance differences.
Research shows that the generation of allergic diseases such as AR has a close connection with the composition and its changes of intestinal microbial diversity.Citation33 More than one thousand diverse species inhabit the gastrointestinal tract,Citation34 and the quantity of bacterial cells is approximately ten times greater than that of eukaryotic cells within the human body.Citation35 The bacteria have evolved along with humans, and it transforms into a mutualistic relationship. Hence, the gut microbiota might be implicated in the progression of specific diseases, and the significance of the gut microbiota is worthy of investigation in the development of AR. demonstrates the alterations of intestinal microbes in AR patients. Compared with healthy individuals, the intestinal microbial diversity of AR patients is decreased, the relative abundance of Firmicutes is significantly reduced, while the proportion of Bacteroidetes is increased. In particular, the colonization and alteration of intestinal microbes have been verified before any clinical manifestations occur in the early stages of life, indicating that the intestinal microbial dysbiosis is one of the causes of AR. Infants with less intestinal microbial diversity seem prone to developing AR. A clinical study of 411 children by Bisgaard et al showed that reduced gut microbiota diversity in infancy was associated with increased risk of school-age AR.Citation36 The research by Johansson et al shows that the colonization of early Lactobacillus (Firmicutes) seems to be associated with the reduction of the risk of allergic diseases at the age of 5.Citation37 It is reported that compared with Russia, the dust samples in Finnish families are found to have a more abundant content of Oxalobacteraceae, and therefore the incidence rate of atopic diseases in this region is 4 times lower than that in Russia.Citation38 Although these studies have not conducted intestinal microbial-related research on atopic disease patients in this region, they provide evidence for the potential connection between intestinal microbiota and the prevalence of atopic diseases.
Zhou et al observed dysbiosis in the gut microbiota of AR patients compared to healthy individuals, with decreased α and β diversity. The AR patients had a reduced abundance of Firmicute. At the genus level the abundance of Blautia, Eubacterium, Romboutsia, Collinsella, Dorea, Subdoligranulum and Fusicatenibacter was significantly lower in the AR group. Notably, fecal SCFAs levels were lower in AR patients, correlating with the presence of Eubacterium hallii and Blautia.Citation16 Watts et al compared the gut microbiome of 57 AR patients with 23 healthy controls using 16S rRNA sequencing. The study showed a significantly lower α-diversity in the AR group, based on Shannon index, OTU (operational taxonomic units counts), inverse Simpson index, and Chao1, compared to HCs. They found a greater presence of Bacteroidetes and a lower abundance of Firmicutes in AR patients. Moreover, an increased abundance of Prevotella and reduced levels of Oscillospira and Fusobacterium were noted in the AR cohort.Citation17 In 2022, Yamaguchi assessed the gut microbiota diversity and composition in 1092 Japanese subjects, focusing on varying ages, allergen sensitivity, and symptom presence. They discovered significant disparities in gut microbiota between allergic and non-allergic individuals, with a marked reduction in α-diversity (including observed species, Chao1, and Shannon index) in allergen-sensitive subjects. However, no meaningful statistical difference was found between symptomatic and asymptomatic groups, suggesting that gut microbiota may not play a role in symptomatic allergic rhinitis episodes. At the phylum level, the allergen-sensitive subjects showed a higher relative abundance of Bacteroidetes and a lower abundance of Firmicutes. At the order level, the percentage of Bacteroidales was significantly higher in the sensitized group than in the unsensitized group in all cases, while the relative abundance of Clostridiales and Lactobacillus tended to be lower.Citation18 Liu et al found significant differences in gut microbiota between 93 AR patients and 72 HCs, with the AR group displaying notably lower α-diversity (including Chao1 index, observed species index, Shannon index, and Simpson index). At the phylum level, Bacteroidetes were more abundant while Actinobacteria and Proteobacteria were less abundant in AR patients compared to HCs. At the genus level, the AR group showed significantly higher relative abundances of Escherichia, Shigella, Prevotella, and Paraprevotella.Citation19 Candela et al included 19 atopic children (17 with AR) and 12 healthy controls aged 10–14, and discovered that the atopic children’s gut microbiota were significantly depleted in species, such as those belonging to Clostridium cluster IV, Faecalibacterium, and Akkermansia muciniphila, while showing an increased relative abundance of Enterobacteriaceae. Unfortunately, the study did not investigate the diversity of gut microbiota between the two groups.Citation20 Chiu et al compared gut microbiota in 28 AR children with 26 HCs, observing lower, but not significantly different, Chao1 and Shannon indices in AR children, with similar microbial clustering to the HCs. The AR group also had notably less abundance of Firmicutes. Correlation analysis revealed that Escherichia positively correlated with house mite IgE, Clostridium with total fecal IgE, and a negative correlation between Dorea spp. and fecal IgE levels.Citation21 Zhang et al conducted a study encompassing a cohort of 24 pediatric patients with AR and compared them to 25 HCs. The findings elucidate a reduction in microbial diversity among AR children in Mainland China when juxtaposed with the HCs group, highlighting distinct microbial signatures. Notably, a significant decrement in the Shannon diversity index and a concurrent marked escalation in the Simpson index were observed at both the phylum and genus taxonomic levels within the AR pediatric cohort.Citation22
Contrary to previous findings, Zhu et al’s 2020 study on 33 AR patients revealed higher bacterial diversity in AR patients compared to HCs, potentially due to an expansion of Firmicutes. They observed a more complex network topology in AR patients’ Firmicutes, suggesting a stubborn dominance in microbial crosstalk. The study noted a lower average relative abundance of Proteobacteria and higher Firmicutes and Clostridiales in AR patients, while the non-AR group possessed unique Lentisphaerae phyla.Citation23
Modulation of AR Immune Responses by Gut Microbiota and Their Metabolites
Regulation of AR Immune Responses by Gut Microbiota
Contemporary immunological frameworks suggest that AR is characterized by a predominant Th1/Th2 disequilibrium with a pronounced shift towards Th2 responsiveness.Citation39 Th1 cytokines are produced by interleukin 12 (IL-12) to stimulate initial T cell differentiation and reduce AR symptoms by producing anti-inflammatory factors such as interferon-γ (IFN-γ) and IL10 to suppress Th2 immune responses. Th2 cells are generated by IL-4 stimulated initial T cell differentiation and produce Th2-like cytokines such as IL-5 and IL-13, which act on inflammatory cells such as B lymphocytes, mast cells, eosinophils, and dendritic cells, thus causing a cascade response of inflammation.Citation40 At the same time, type 2 innate lymphocytes (ILC2) and dendritic cells (DC), generated under the stimulation of epithelial-derived cytokines such as IL25, IL33 and thymic stromal lymphopoietin (TSLP), initiate the differentiation of native T cells into Th2 cells and coordinate the production of Th2 cytokines to assist in the generation of a series of inflammatory responses.Citation41 Accumulating empirical data underscore the intestinal microbiota as a crucial regulator, intricately influencing the dynamic Th1/Th2 balance, thus impacting the immunological pathways implicated in AR.Citation42 Chua et al found that infants with allergies had higher fecal Lachnospiraceae frequencies, linked to overgrowth of R. gnavus, compared to non-allergic infants. Further studies in sensitized mice revealed that R. gnavus induces Th2 and dendritic cell expansion and eosinophilic infiltration in the colon by stimulating cytokine secretion (IL-25, IL-33, TSLP) from colonic epithelial cells. This infiltration could lead to systemic allergic inflammation via eosinophils mobilizing through blood and lymphatic routes.Citation43 Additionally, they observed that ovalbumin-sensitized mice orally administered with R. gnavus had increased levels of IL-25, IL-33, and TSLP in colonic tissue compared to controls, promoting enhanced Th2 differentiation and cytokine secretion. Mazmanian et al study demonstrates that in contrast to mice colonized with Bacteroides fragilis, germ-free mice exhibit a Th1/Th2 dysregulation with a propensity towards Th2 polarization. The underlying mechanism is hypothesized to be the ability of Bacteroides fragilis to modulate this dysregulation via polysaccharide A (PSA), thereby restoring immunological equilibrium.Citation44 The aforementioned research provides robust evidence supporting the role of intestinal bacteria in the immunological mechanisms of AR. These studies offer insight into the complex interactions between gut microbiota and host immunity, reaffirming the potential influence of microbial populations in the modulation of immune responses characteristic of AR.
In the immune response of AR, Toll-like receptors (TLRs) act as the initial element for recognizing and responding to pathogen-associated molecular patterns (PAMPs). TLRs are important pattern recognition receptors composed of cytoplasmic, transmembrane, and extracellular regions that induce the body’s anti-microbial defense system and produce various inflammatory mediators and chemokines. There are 10 TLRs identified in the human body, TLR1 to TLR10, of which TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10 are distributed on the cell membraneCitation45 and can recognize PAMPs such as lipopolysaccharide, peptidoglycan, and yeast glycan, and when TLRs bind to these PAPMs, they initiate a signal transduction cascade to activate an innate immune response to eliminate pathogens that breach mucosal barrier.Citation46 Other TLRs, TLR3, TLR7, TLR8, and TLR9, detect nucleic acids intracellularly.Citation47 TLRs can regulate DCs, lymphocytes, mast cells and other cells closely related to AR, thus indirectly regulating the balance of the Th1/Th2 cytokine network, and playing an important role in the occurrence and development of AR.Citation48 He et al have demonstrated that TLRs in the gut microbiota can activate intestinal epithelial cells (IECs), thereby inducing the production of A Proliferation-Inducing Ligand (APRIL) and TSLP, which assist in the generation of Th2 cytokines and facilitate local inflammatory responses.Citation49 Sjögren et al demonstrated in a prospective study of 64 Swedish infants that the colonization intensity of Bacteroides fragilis in the first week and month after birth is negatively associated with TLR4 mRNA expression. It is speculated that Bacteroides fragilis may regulate the Th1/Th2 balance by suppressing the expression of TLR4, potentially inhibiting the incidence of AR.Citation50 The pediatric cohort study by Amenyogbe et al indicates a significant correlation between the response to TLR2 and the relative abundance of the Bacteroides and Prevotella genera in children from Canada and Ecuador. A higher abundance of Prevotella and a lower abundance of Bacteroides are associated with an increased cytokine response to TLR2.Citation51 The capsular PSA of the gut commensal Bacteroides fragilis has been proven to be a typical immunomodulatory molecule in the gut, capable of limiting pathological inflammation in the intestinal and distal tissues.Citation52–54 PSA requires both innate and adaptive immune responses to exert a protective influence, according to Dasgupta et al. They revealed that plasmacytoid dendritic cells (PDCs) exposed to PSA do not produce pro-inflammatory cytokines because PSA preferentially binds to TLR2 on PDCs, which prompts the secretion of the anti-inflammatory cytokine IL10 by regulatory T cells (Treg).Citation55
Recent scholarly research suggests that an imbalance between T helper cell 17 (Th17) and Treg is closely associated with the pathogenesis of AR.Citation56 Treg and Th17 cells are both derived from CD4+ T cells and are mediated by a common signaling pathway by transforming growth factor-β (TGF-β). In vitro and in vivo studies have shown that TGF-β promotes the development of Th17 and Treg cells by inducing the expression of retinoic acid receptor-related orphan receptor gt (RORγt) and Forkhead box protein P3 (Foxp3), respectively.Citation57 Th17 and Treg cells maintain a dynamic balance, producing an immune response of appropriate intensity and contributing to the maintenance of a stable immune state in vivo. When CD4+ T cells differentiate towards pro-inflammatory Th17, the body then develops a series of inflammatory manifestations. Studies have shown that Th17 activates Th2 cells, resulting in increased serum IgE specificity and aggregation of eosinophils and neutrophils in the nasal mucosa.Citation58,Citation59 At the same time, Th17 secretes pro-inflammatory factors such as IL-17A, IL-17F, IL-6 and tumor necrosis factor (TNF)-α, which cause a specific inflammatory response in the nasal cavity. Among them, IL-17 is the most important pro-inflammatory factor. Clinical studies have shown that serum levels of Th17, IL-17, and TGF-β are elevated in AR patients compared to controls, and that IL-17 correlates with the degree of clinical symptoms inflammation.Citation60 In addition et al found that knockdown of IL-17A in mice significantly reduced allergic symptoms.Citation61 Treg cells are a small but critical subpopulation of CD4+ T with a role in maintaining immune homeostasis. Treg cells, a small but critical subpopulation of CD4+T, develop from TGF-β-induced Foxp3 and play a role in maintaining immune homeostasis in AR. Treg cells are a small but critical subpopulation of CD4+ T that play a role in maintaining immune homeostasis in AR. TGF-β promotes Treg cell development by inducing Foxp3 expression, and under Treg-induced conditions, cells fail to differentiate into Th17 cells, which eventually develop into Foxp3+ Treg cells.Citation62 Nguyen et al found that alpha-lipoic acid (LA) significantly alleviated nasal symptoms such as rubbing and sneezing in AR mice through upregulation of the Treg cytokine IL-10 and the transcription factor Foxp3.Citation56 Jiao et al showed that Notch2 suppresses the development of allergic rhinitis by promoting Foxp3 expression and Treg cell differentiation.Citation63 The intestinal microbiome and its metabolic byproducts are increasingly recognized as critical players in the regulation of the Th17/Treg balance. Atarashi et al demonstrated that regulatory Treg are most abundant in the colonic mucosa of mice, with clusters IV and XIVa of the genus Clostridium enhancing the accumulation of Treg cells.Citation64 Additionally, they found that the colonization of spore-forming Clostridia in the mouse colon creates a rich TGF-β milieu, influencing the immune status both within the gut and systemically through an increase in Foxp3+ Treg numbers.Citation65 Dasgupta et al discovered that PSA, as a typical immunomodulatory molecule from the gut symbiont Bacteroides fragilis, can induce regulatory T cells to secrete the anti-inflammatory cytokine IL10, thereby limiting pathological inflammation in the intestine and distal organs.Citation66 ()
Figure 1 Impact of gut and nasal microbial dysbiosis on AR. Disturbance of the gut microbiota and a decrease in intestinal metabolites (eg, short-chain fatty acids and tryptophan,) induces the production of TSLP, IL-25, and IL-33, which contributes to Th2 cytokine production and promotes localized inflammatory responses. It also creates an environment rich in transforming growth factor-β, which promotes cellular differentiation toward pro-inflammatory Th17 by increasing the amount of RORγ-t. Vibrio traumaticus in the nasal cavity induces cytokines such as IL-1β, IL-6, and TNF-α, participates in NF-κβ signaling, and elicits allergic reactions by releasing IFN-β, IL-27, and IL-1β. A. baumannii activates the Nod-like receptor NLRP3 via caspase-1, which promotes the release of IL-1β and TNF-α from macrophages, thereby triggering AR. In addition, A. baumannii induces the production of reactive oxygen species from dendritic cells, which activates NLRP3 and promotes the differentiation of cells towards pro-inflammatory Th2, triggering the immune response typical of AR.
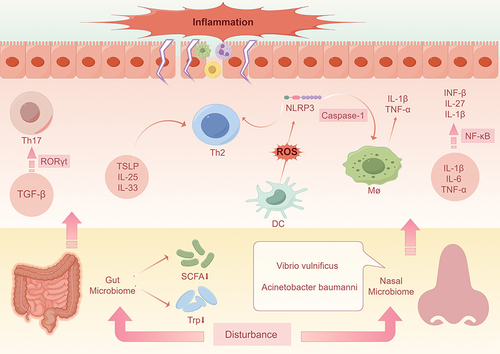
Regulation of Immune Responses in Allergic Rhinitis by Metabolic Products of Gut Microbiota
The gut microbial community is a complex micro-ecosystem, its biodiversity supported by a spectrum of distinct microbial species.Citation66 The dynamic equilibrium of this community is vital for the homeostasis of the host’s immune system.Citation67,Citation68 In the interaction between the host and gut microbiota, SCFAs as key metabolic by-products of microbial communities, play a significant role.Citation69–72 SCFAs are metabolic products generated by gut microbiota via the fermentation of indigestible dietary fibers, primarily including acetate, propionate, and butyrate. Recent studies indicate that SCFAs have important roles in modulating immune responses and exerting anti-inflammatory effects, particularly in the onset and development of allergic diseases.Citation73,Citation74 Studies have identified SCFAs as key metabolites facilitating the differentiation of naïve T lymphocytes into Treg cells in the intestinal tract. Interaction with initial T cells by butyrates and propionates enhances the acetylation of the Foxp3 transcription factor promoter, which is essential for Treg differentiation.Citation75 Remarkably, Rooks et al have demonstrated that a high-fiber diet or supplementation with SCFAs not only mitigates colonic inflammation but also suppresses allergic airway disease by augmenting the suppressive activity of Foxp3+ Treg cells.Citation76 Consequently, by modulating the gut microbiome and its metabolic byproducts, we can effectively influence the host’s immune response, providing new avenues for the treatment of allergic diseases.
Roduit’s research has revealed that infants with reduced levels of SCFAs are more prone to develop allergic conditions. The study shows that in toddlers, those with higher butyrate levels tend to have significantly lower rates of food allergies and allergic rhinitis. In addition, oral administration of SCFAs in mice significantly mitigated symptoms of allergic airway inflammation.Citation77 Cheng et al have corroborated this conclusion, delineating an association between lower levels of gut short-chain fatty acids in early life and an elevated susceptibility to atopic outcomes in childhood.Citation58 Moreover, Chen et al developed an AR mouse model with dysbiosis of the gut microbiome induced by vancomycin and ovalbumin (OVA) sensitization.Citation78 They discovered that such dysbiosis could increase the susceptibility and clinical severity of allergic airway diseases by reducing the bacteria that produce SCFAs and the levels of SCFAs themselves. The supplementation of SCFA-producing bacteria or SCFAs was found to alleviate allergic airway diseases.Citation79–81 Synthesizing current research findings, it becomes apparent that an imbalance in the gut microbiome and reduced SCFAs levels are key drivers in the progression of allergic airway inflammatory diseases. Strategic enhancement of SCFA-producing microbes or direct supplementation of SCFAs may offer a novel interventional route for the prevention and treatment of such conditions. This approach has the potential to modulate the host’s immune response and alleviate inflammation, carving out new therapeutic directions for the clinical management of allergic airway diseases.
Another key product in microbial metabolic processes, Trp, has been demonstrated to safeguard the intestinal barrier’s integrity and ensure the stability of immune cells through the activation of the aryl hydrocarbon receptor (AhR).Citation82,Citation83 Consequently, they are recognized as vital active biomarkers.Citation84 Tryptophan, as an important intestinal metabolic product, undergoes metabolism through three distinct routes under homeostatic conditions: Firstly, the Kynurenine Pathway (KP), which is facilitated by the action of the enzyme indoleamine 2.3-dioxygenase 1 (IDO1); secondly, the production of 5-Hydroxytryptamine within enterochromaffin cells, a process catalyzed by Tryptophan hydroxylase 1 (TpH1); and thirdly, the direct conversion of tryptophan into indole, its derivatives, and tryptamine by intestinal microbiota using the enzyme tryptophanase, leading to the synthesis of a diverse array of metabolites such as Indole-3-acetic acid (IAA), Indole-3-acetaldehyde (IAAld), Indole-3-aldehyde (IAld), Indolepyruvic acid (IPA), and Indoleacetaldehyde (IA).Citation85 Studies have shown that tryptophan metabolites have a major impact on the immune system, especially at the mucosal barrier. Their ability to reduce the viability of T cell responses and stimulate the production of Treg is critical for immune regulation.Citation86,Citation87 Reduced levels of these potentially anti-inflammatory tryptophan metabolites may bias the immune system toward a Th2-dominant response, which may increase susceptibility to Th2-mediated diseases such as allergic rhinitis.
Research indicates that Kyn is capable of elevating the expression levels of the Foxp3 transcription factor, which plays a crucial role in enhancing the differentiation of Treg cells. Simultaneously, it inhibits the proliferation of Th17 type cells.Citation88–91 Hossain et al demonstrated that in the absence of IDO, mice respond to viral infections with pronounced Th17 and Th1-type airway inflammation. This pathological state is marked by intense neutrophil infiltration, increased levels of IL-17 and IFN-γ, and significant airway hyperresponsiveness.Citation92 Within the murine model of asthma, stimulation of TLR-9 via bacterial DNA sequences has been shown to trigger the upregulation of IDO and the aromatic receptor,Citation93 leading to a reduction in inflammatory airway hyperresponsiveness. Based on the findings presented by Kepert, the pre-administration of D-tryptophan prior to the experimental induction of asthma in murine models resulted in an augmented presence of regulatory T cells in the pulmonary system, a diminished Th2 type immune reaction, and a notable amelioration in both allergic airway inflammation and hyperresponsiveness.Citation94 A different metabolite of tryptophan, 3-Hydroxy-Anthranilic acid (3-HAA), has been identified to specifically inhibit the phosphorylation of 3-phosphoinositide-dependent protein kinase 1 (PDK1), thereby obstructing the activation of NF-κB induced by T cell antigen receptors. This action results in the impairment and apoptosis of activated Th2 cells within the organism, effectively mitigating experimental asthma prompted by Th2 immune reactions in murine models.Citation95 Consequently, despite the precise function of tryptophan metabolism in the etiology of atopic disorders including asthma and allergic rhinitis remaining somewhat elusive, the significance of tryptophan metabolism and its intermediary metabolites as critical regulators of immune responses is undeniable.Citation96
The Nasal Microbiome and AR
Patterns of Nasal Microbiota in Patients with AR
Nine studies examining the nasal microbiome of 447AR patients () yielded divergent conclusions. Most studies observed no significant change or difference in microbial diversity between AR patients and healthy controls. However, two studies reported a greater species diversity in the AR group compared to controls, while another study indicated a notably lower microbial diversity in AR patients. All nine studies found significant variances in microbial abundance between the AR group and healthy controls.
Wu et al incorporated 28 Chinese AR patients and 28 HCs in their study and found no statistically significant differences in the alpha diversity indices (including Ace, Chao1, Shannon, Simpson, and Coverage) of nasal microbiota between the two groups. In the allergic rhinitis cohort, the relative abundance of Streptococcus, Staphylococcus aureus, Haemophilus, Clostridium, Veillonella, and Proteobacteria was significantly higher compared to controls, while the relative abundance of Propionibacterium acnes, Actinobacteria, Propionibacterium, Corynebacterium, Rodentibacter, and Propionibacterium was notably lower. Furthermore, Pearson correlation analysis demonstrated a significant positive correlation between Proteobacteria and IgE levels, and a significant negative correlation between Propionibacterium acnes and IgE levels, confirming a certain relationship between nasal microbiota and IgE in AR patients.Citation24 In a comparative study by Li et al, involving nasal microbiome markers and metabolites of 40 individuals with AR and 20 HCs, no discernible differences were found in the alpha and beta diversities of nasal microbes. At a higher taxonomic level, the AR group had elevated levels of Alphaproteobacteria, Clostridia, and Actinobacteria, while Bacilli and Firmicutes were reduced compared to the healthy subjects.Citation25 Hyun and their team investigated the relationship between mucosal microbiota and AR, the number of sensitizing allergens, and specific and total IgE levels by comparing AR subjects with healthy controls. Participants, 42 in total, were categorized into three groups: non-sensitized, mono-sensitized to a single allergen, and polysensitized to multiple allergens, with no observed differences in microbial diversity or community composition among the groups. Further analyzing nasal microbiota in relation to serum IgE sensitization, they divided subjects into high AR-specific/total serum IgE, low AR-specific/total serum IgE, and non-AR/low total IgE levels. They reported lower microbial biodiversity and higher relative abundance of Firmicutes (notably Staphylococcus aureus) and lower Actinobacteria (specifically Propionibacterium acnes) in individuals with high total IgE compared to those with low IgE levels.Citation26 In a study conducted by Wang et al, a comparative analysis of the microbiota of the nasal vestibule between AR patients and HC was performed. A total of 30 subjects were included, with 15 in each group, AR and HC. Through PCA, the research identified significant differences in the structure of the microbial communities between the two groups. However, when examining the OTUs, no statistically significant differences were observed in the richness (such as the Ace index and Chao1 index) and evenness (such as the Simpsoneven index) of the microbial communities between the two groups. Further analysis revealed that at the phylum level, there was a significant reduction in the relative abundance of Actinobacteria in the AR group, while there was a significant increase in the relative abundance of Fusobacteria, with these differences being statistically significant. At the genus level, nine genera were identified to have significant differences in abundance between the AR and HC groups. Specifically, the abundance of Corynebacterium, Peptoniphilus, Anaerococcus, Finegoldia, Propionibacterium, and Brachybacterium was reduced in the AR group compared to the HC group. Conversely, the abundance of Shewanella, Halomonas, and Paracoccus was increased in the AR group, and these differences were also statistically significant.Citation27 In a small-scale study by Zhang et al, even though an uptrend in species diversity indices was noted among six AR patients, these changes were not statistically significant when compared to six HCs. Notably, there was a significant decrease in Bacillales and an increase in Clostridiales within the AR group. At the genus level, Propionibacterium was more abundant in AR patients, whereas various other genera, including Lactobacillus and Staphylococcus, were less prevalent. At higher taxonomic hierarchies, a shift toward lower Clostridiales and higher Bacillales was observed, with altered abundances in certain families, signifying variable microbiota profiles between AR sufferers and healthy individuals.Citation28 Choi et al have observed a significant proliferation of microbial species within the middle meatus of the nasal cavity among subjects with Seasonal Allergic Rhinitis (SAR) during the allergic season, as opposed to non-allergic subjects. This observation was accompanied by an increase in bacterial diversity, quantified by the Shannon diversity index. Furthermore, a significant positive correlation was detected between the bacterial diversity within the middle meatus during the allergic season and the eosinophil count in nasal lavage fluid from SAR subjects.Citation29 Yuan et al found no difference in the alpha diversity (Chao1 and Simpson indices) of nasal microbiota between 28 AR patients and HCs. Nevertheless, notable shifts in microbial composition were observed, the AR group showed a significant increase in Actinobacteria, and genera such as Klebsiella, Prevotella, and Staphylococcus, with a marked decrease in the abundance of Pelomonas.Citation30 Wang et al analyzed the nasal microbiota of 8 AR patients and 7 HCs in China, finding a statistically significant increase in species diversity (including Chao1, ACE, Shannon, npShannon, and Simpson indices) in the AR group. The study also highlighted a significant rise in the relative abundance of Proteobacteria and a decrease in Actinobacteria and Firmicutes among AR patients compared to healthy controls. At the genus level, a noteworthy deficit in Bacillus was detected in the AR patients’ nasal microenvironment, indicating clear microbial distinctions between the two groups.Citation31 Miao and co-researchers, contrasting the nasal microbiota of 55 AR patients with 105 HCs, reached a divergent conclusion, noting a significant discrepancy in microbial composition, with reduced diversity in AR individuals. At the phylum level, an enhanced presence of Firmicutes was recorded. Moreover, they identified the most prevalent OTU in AR patients as a specific strain of Streptococcus salivarius. Compared to HCs, this particular OTU displayed a marked and significant increase in AR patients, suggesting a unique and strong association with AR within the nasal microbiome.Citation32
The Impact of Microbial Dysbiosis on AR
The nasal microbiota serves as a microbial shield against external irritants and an immune regulatory interface, predominantly nested in the mucus layer atop epithelial cells. Encompassing a diverse array of microorganisms—bacteria, fungi, viruses, protozoa, and archaea—this ecosystem is both populous and compositionally varied.Citation97 In the context of AR, individuals exhibit distinct patterns of nasal microbiome diversity and abundance compared to healthy individuals. Dysbiosis may prompt the proliferation of specific pathogens, leading to nasal mucosal inflammation. For instance, Pseudomonas possesses the capacity to survive and replicate within phagocytes, triggering the activation of immunocompetent cells in hypersensitivity reactions, particularly in immunocompromised hosts.Citation98 Burkholderia cepacia complex, a conditionally pathogenic organism, colonizes the nasal cavity and compromises the integrity of the nasal epithelial cells, precipitating a cascade of inflammatory responses.Citation99 In a study by Che et al, an investigation into the nasal microbiome characteristics of patients with AR versus Non-Allergic Rhinitis (NAR) revealed a significantly higher mean relative abundance of Vibrio vulnificus and Acinetobacter baumannii in the AR group.Citation97 Vibrio vulnificus, commonly known as marine vibrio, is a Gram-negative bacterium capable of inducing cytokine production, namely IL-1β, IL-6, and TNF-α.Citation100 These cytokines instigate inflammation by recruiting and activating immune cells such as neutrophils, monocytes, and macrophages. They activate the mTOR pathway, engage NF-κβ signaling via TLRs or NLRs, and incite allergic responses through the release of GM-CSF, IFN-β, IL-27, and IL-1β. Moreover, Vibrio vulnificus has been shown to prompt proliferation and inflammatory reactions in Kupffer cells, aligning with the pathogenic mechanisms of AR.Citation101–103 Acinetobacter baumannii, on the other hand, activates the Nod-like receptor NLRP3 via caspase-1, promoting the release of IL-1β and TNFα from macrophages, hence triggering asthma.Citation104 Furthermore, Acinetobacter comes equipped with various virulence factors, including pore-forming toxins, and its outer membrane protein A induces reactive oxygen species (ROS) production in dendritic cells, activating NLRP3 and fostering immune reactions typical of asthma and AR.Citation105 ()
Discussion
In the current academic landscape, the correlation between the diversity of the human microbiota at essential mucosal interfaces, such as the nasal and gastrointestinal tracts, and the development of allergic rhinitis has captivated increasing interest. The homeostasis of the microbiota is a cornerstone of human well-being, with imbalances potentially triggering disease onset. Contemporary studies suggest that the gastrointestinal tract’s resistance to extrinsic disturbances may positively correlate with the diversity and equilibrium of its resident microbial populations.Citation106 Furthermore, metrics of microbial richness could serve as pivotal indicators in evaluating the stability or “adaptive capacity” of the gut microbiome.Citation106 Current research consistently supports the observation that significant differences exist between the gut microbiota of patients with AR and those of healthy cohorts. A majority of these studies document a trend towards diminished diversity and dysbiosis within the gut microbiome of individuals suffering from AR when compared to healthy counterparts. Notably, numerous investigations have highlighted an increased abundance of the phyla Bacteroidetes and Proteobacteria, and a conspicuously reduced presence of Firmicutes in the AR population. Within the realms of gastrointestinal microbiology, the phyla Bacteroidetes and Firmicutes are heralded as the most abundant bacterial taxonomies populating the gut environment. Their roles are quintessential in gastrointestinal integrity and immunomodulation.Citation107 Bacteroidetes are particularly associated with the production of SCFAs such as acetate and propionate, while Firmicutes are more intimately linked with butyrate generation.Citation108 Integrity disruption in the intestinal barrier can precipitate an increased permeability to pro-inflammatory molecules and antigens, thereby facilitating their translocation into the submucosal layers and systemic circulation, provoking both localized and systemic inflammatory responses. In certain scenarios, a relative preponderance of Bacteroidetes over Firmicutes may result in a reduced synthesis of butyrate, subsequently impacting the fortitude of the intestinal barrier. It is noteworthy that clinical and experimental investigations have unearthed heightened gut permeability in individuals with allergic diseases as compared to their healthy counterparts.Citation109–113
Nine distinct studies have elicited variations in the observed changes to the nasal microbiota of AR patients, rendering a consensus elusive and posing a conundrum. The studies in question exhibit substantial variability in sample sizes, with the smallest incorporating only 15 participants and the largest encompassing 160, which could contribute to the disparate outcomes. It is also worth noting the methodological heterogeneity in specimen types collected, encompassing nasal secretions, inferior turbinate mucosa, and aseptic swabs of both the vestibules and the lower nasal passages, potentially influencing the results. Despite these variations in methodology, sample size, and source of specimen, a consistent finding across all nine studies emerges: AR patients exhibit a significantly different microbial abundance compared to the healthy control group. Concomitantly, it has been observed that 3 studies denote a correlation between changes in microbial abundance (within both the gut and the nasal cavity) in patients with AR and levels of IgE. Specifically, the Actinobacteria phylum has been reported in three separate studies as being inversely related to IgE concentrations.Citation17,Citation78,Citation80 Further investigations have illuminated that Propionibacterium acnes, predominant in the bacterial community of the inferior turbinate in healthy individuals,Citation8 can potentially ameliorate atopic symptoms by inducing Th1 and Treg responses,Citation114 suggesting that a diminished abundance of P. acnes may be implicated in exacerbating AR. Building upon these research findings, it may be postulated that as constituents of the nasal microbiota, members of the Actinobacteria phylum could play a pivotal role in safeguarding the host against allergic incursions by participating in the maintenance of microbial equilibrium. Future studies are expected to shed light on the direct or indirect involvement of Actinobacteria in the pathogenesis of Allergic Rhinitis and determine their viability as potential therapeutic targets. In summary, the Actinobacterial members of the human microbiota might exert an influence on the pathophysiology of Allergic Rhinitis to a certain degree, necessitating further research to elucidate their specific mechanisms and clinical relevance.
Mucosal tissues, encompassing the gastrointestinal, respiratory, constitute the host’s primary line of defense against external pathogens. While substantial knowledge has been gleaned from studying these components in isolation, a comprehensive examination of the mucosal immune system as an interconnected systemic organ remains to be conducted. The common mucosal immune system hypothesis posits that immunological activities within the respiratory tract may be interconnected with immunoregulatory functions occurring in the gastrointestinal milieu.Citation115 This hypothesis suggests that antigens within the gastrointestinal system provoke the local lymphocytes, which, once activated, may migrate to other submucosal regions of the upper and lower respiratory tracts.Citation115 During this immunological cascade, antigens are captured by Peyer’s patches and transported to antigen-presenting cells.Citation116 Naive T cells and B cells demonstrate heightened sensitivity to antigens situated within Peyer’s patches and possess the capability to enter the bloodstream, consequently exerting their functions across various mucosal tissues such as those found in the intestines and respiratory system.Citation116 Emerging evidence indicates that the gut microbiota can modulate the immune status of the nasal mucosa via the production of metabolic byproducts, such as SCFAs. Butyrate, in particular, has been shown to regulate the function of immune cells, including dendritic cells and T cells, thereby modulating inflammatory responses and immunological reactions. Furthermore, the metabolic byproducts of the gut microbiota can disseminate to the nasal cavity through the bloodstream, influencing the immunological barrier function of the nasal mucosa, and could potentially affect the incidence and progression of allergic diseases, such as AR. Additionally, dysbiosis of the gut microbiota may lead to systemic inflammation, which can impact distant organs, including the nasal passages, via the circulatory system, thereby affecting the immune status and barrier function of the nasal mucosa.Citation117
Although current research has yet to confirm a direct correlation between gut and nasal microbiota, it is hypothesized that these two microbiomes, as significant components of the human microbiome, play a critical role in maintaining host health, and there is likely an interconnected relationship between them. Current evidence, albeit limited due to study heterogeneity and variable outcome measures, suggests that oral administration of probiotics may confer benefits in alleviating symptoms and enhancing the quality of life for patients with Allergic Rhinitis.Citation118–123 Research posits that the gut microbiota can influence systemic immune responses through the generation of metabolic byproducts, such as SCFAs, which can be disseminated to distal organs, including the nasal cavity, via the bloodstream.Citation124 The metabolites produced by the gut microbiota may exert an effect on the immune status of the nasal mucosa, thereby influencing the composition and function of the nasal microbiome.Citation124 Future research should delve into the mechanisms through which the gut microbiota impacts the nasal microbiota, including interactions via the immune system, metabolites, or the nervous system. Investigations into the direct and indirect pathways between the gut and the respiratory tract are pertinent, which might encompass exploring how the gut microbiota asserts influence on nasal health through the gut-brain axis or the gut-lung axis.
Disclosure
The authors report no conflicts of interest in this work.
Acknowledgments
This work was supported by the Sichuan Provincial Department of Science and Technology (2021YJ0175).
References
- Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(86):8–160.
- Maurer M, Zuberbier T. Undertreatment of rhinitis symptoms in Europe: findings from a cross-sectional questionnaire survey. Allergy. 2007;62(9):1057–1063. doi:10.1111/j.1398-9995.2007.01367.x
- Bousquet J, Schünemann HJ, Togias A, et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J Allergy Clin Immunol. 2020;145(1):70–80.e3. doi:10.1016/j.jaci.2019.06.049
- Haahtela T, Holgate S, Pawankar R, et al. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. 2013;6:3. doi:10.1186/1939-4551-6-3
- Zhou Y, Jackson D, Bacharier LB, et al. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat Commun. 2019;10(1):5714. doi:10.1038/s41467-019-13698-x
- Renz H, Skevaki C. Early life microbial exposures and allergy risks: opportunities for prevention. Nat Rev Immunol. 2021;21(3):177–191.
- Garn H, Neves JF, Blumberg RS, Renz H. Effect of barrier microbes on organ-based inflammation. J Allergy Clin Immunol. 2013;131(6):1465–1478. doi:10.1016/j.jaci.2013.04.031
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–214. doi:10.1038/nature11234
- Larsson E, Tremaroli V, Lee YS, et al. Analysis of gut microbial regulation of host gene expression along the length of the gut and regulation of gut microbial ecology through MyD88. Gut. 2012;61(8):1124–1131. doi:10.1136/gutjnl-2011-301104
- Agustí A, García-Pardo MP, López-Almela I, et al. Interplay Between the gut-brain axis, obesity and cognitive function. Front Neurosci. 2018;12:155. doi:10.3389/fnins.2018.00155
- Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–273. doi:10.1038/s41574-019-0156-z
- Lin Q, Kuypers M, Liu Z, et al. Invariant natural killer T cells minimally influence gut microbiota composition in mice. Gut Microbes. 2022;14(1):2104087. doi:10.1080/19490976.2022.2104087
- McDermott MR, Bienenstock J. Evidence for a common mucosal immunologic system. I. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122(5):1892–1898. doi:10.4049/jimmunol.122.5.1892
- McDermott MR, Clark DA, Bienenstock J. Evidence for a common mucosal immunologic system. II. Influence of the estrous cycle on B immunoblast migration into genital and intestinal tissues. J Immunol. 1980;124(6):2536–2539. doi:10.4049/jimmunol.124.6.2536
- Gill N, Wlodarska M, Finlay BB. The future of mucosal immunology: studying an integrated system-wide organ. Nat Immunol. 2010;11(7):558–560. doi:10.1038/ni0710-558
- Zhou M, Zhang B, Gao Z, et al. Altered diversity and composition of gut microbiota in patients with allergic rhinitis. Microb Pathogenesis. 2021;161:105272. doi:10.1016/j.micpath.2021.105272
- Watts AM, West NP, Zhang P, Smith PK, Cripps AW, Cox AJ. The gut microbiome of adults with allergic rhinitis is characterised by reduced diversity and an altered abundance of key microbial taxa compared to controls. Int Arch Allergy Immunol. 2021;182(2):94–105. doi:10.1159/000510536
- Yamaguchi T, Nomura A, Matsubara A, et al. Effect of gut microbial composition and diversity on major inhaled allergen sensitization and onset of allergic rhinitis. Allergol Int. 2023;72(1):135–142. doi:10.1016/j.alit.2022.06.005
- Liu X, Tao J, Li J, et al. Dysbiosis of fecal microbiota in allergic rhinitis patients. Am J Rhinol Allergy. 2020;34(5):650–660. doi:10.1177/1945892420920477
- Candela M, Rampelli S, Turroni S, et al. Unbalance of intestinal microbiota in atopic children. BMC Microbiol. 2012;12(1):95. doi:10.1186/1471-2180-12-95
- Chiu CY, Chan YL, Tsai MH, Wang CJ, Chiang MH, Chiu CC. Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Organ J. 2019;12(3):100021. doi:10.1016/j.waojou.2019.100021
- Zhang P, Zhou X, Tan H, et al. Microbial signature of intestine in children with allergic rhinitis. Front Microbiol. 2023;14:1208816. doi:10.3389/fmicb.2023.1208816
- Zhu L, Xu F, Wan W, et al. Gut microbial characteristics of adult patients with allergy rhinitis. Microb Cell Fact. 2020;19(1):171. doi:10.1186/s12934-020-01430-0
- wu B, Youyong C, Jinhong D. Characteristics of nasal flora in allergic rhinitis patients and its relationship with serum IgE and mucosal eosinophils. Chin J Microecol. 2019;31(9):1072–1075.
- Yan L, Xiao Y, zhou L, Xie L. Analysis of the difference of nasal bacteria between patients with allergic rhinitis and healthy people. J Liaoning Univ Tradit Chin Med. 2023;25(11):6–13.
- Hyun DW, Min HJ, Kim MS, et al. Dysbiosis of inferior turbinate microbiota is associated with high total IgE levels in patients with allergic rhinitis. Infect Immun. 2018;86(4):e00934–17. doi:10.1128/IAI.00934-17
- Di W, Hao C, Mengya X, Zihao L, Shan X, Zheng L. Microbial community composition of nasal vestibule in allergic rhinitis patients: an analysis by 16S rRNA sequencing. Chin J Microecol. 2018;30(4):383–391.
- Zhang R. Mechanism of modified yu-ping-feng nasal spray improves the epithelial barrier in allergic rhinitis based on epithelial-derived cytokines [dissertation]. Chengdu: Chengdu University of TCM; 2023.
- Ch C, P V, W S, et al. Seasonal allergic rhinitis affects sinonasal microbiota. Am Jj Rhinol Allergy. 2014;28(4):1.
- Yuan Y, Wang C, Wang G, et al. Airway microbiome and serum metabolomics analysis identify differential candidate biomarkers in allergic rhinitis. Front Immunol. 2021;12:771136. doi:10.3389/fimmu.2021.771136
- wang M. Research on the role of the microecological environment in the pathogenesis and mechanism of allergic rhinitis [dissertation], Xian: Air Force Medical University; 2016.
- Miao P, Jiang Y, Jian Y, et al. Exacerbation of allergic rhinitis by the commensal bacterium Streptococcus salivarius. Nat Microbiol. 2023;8(2):218–230. doi:10.1038/s41564-022-01301-x
- Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi:10.1371/journal.pbio.1002533
- Albenberg L, Kelsen J. Advances in gut microbiome research and relevance to pediatric diseases. J Pediatr. 2016;178:16–23. doi:10.1016/j.jpeds.2016.08.044
- Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361(9356):512–519. doi:10.1016/S0140-6736(03)12489-0
- Bisgaard H, Li N, Bonnelykke K, et al. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol. 2011;128(3):646–52.e525. doi:10.1016/j.jaci.2011.04.060
- Johansson MA, Sjögren YM, Persson JO, Nilsson C, Sverremark-Ekström E. Early colonization with a group of Lactobacilli decreases the risk for allergy at five years of age despite allergic heredity. PLoS One. 2011;6(8):e23031. doi:10.1371/journal.pone.0023031
- Pakarinen J, Hyvärinen A, Salkinoja-Salonen M, et al. Predominance of Gram-positive bacteria in house dust in the low-allergy risk Russian Karelia. Environ Microbiol. 2008;10(12):3317–3325. doi:10.1111/j.1462-2920.2008.01723.x
- Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. 2017;66(4):515–522. doi:10.1016/j.alit.2017.07.010
- Pawankar R, Mori S, Ozu C, Kimura S. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1(3):157–167. doi:10.5415/apallergy.2011.1.3.157
- Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56. doi:10.1038/ni.3049
- Lee MJ, Park YM, Kim B, et al. Disordered development of gut microbiome interferes with the establishment of the gut ecosystem during early childhood with atopic dermatitis. Gut Microbes. 2022;14(1):2068366. doi:10.1080/19490976.2022.2068366
- Chua HH, Chou HC, Tung YL, et al. Intestinal dysbiosis featuring abundance of ruminococcus gnavus associates with allergic diseases in infants. Gastroenterology. 2018;154(1):154–167. doi:10.1053/j.gastro.2017.09.006
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi:10.1016/j.cell.2005.05.007
- Dowling JK, Mansell A. Toll-like receptors: the Swiss army knife of immunity and vaccine development. Clin Transl Immunology. 2016;5(5):e85. doi:10.1038/cti.2016.22
- McGirt LY, Beck LA. Innate immune defects in atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):202–208. doi:10.1016/j.jaci.2006.04.033
- Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32(3):305–315. doi:10.1016/j.immuni.2010.03.012
- Dębińska A, Boznański A. Rola receptorów Toll-podobnych (TLR) w patogenezie schorzeń alergicznych – gdzie leży [The role of Toll-like receptors in the pathogenesis of allergic diseases - where is the truth?]. Postepy Hig Med Dosw (Online). 2014;68:230–237. doi:10.5604/17322693.1093202
- He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A2 class switching by inducing epithelial-cell secretion of the cytokine April. Immunity. 2007;26(6):812–826. doi:10.1016/j.immuni.2007.04.014
- Sjögren YM, Tomicic S, Lundberg A, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy. 2009;39(12):1842–1851. doi:10.1111/j.1365-2222.2009.03326.x
- Amenyogbe N, Dimitriu P, Smolen KK, et al. Biogeography of the relationship between the child gut microbiome and innate immune system. mBio. 2021;12(1):e03079–20. doi:10.1128/mBio.03079-20
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi:10.1038/nature07008
- Ochoa-Repáraz J, Mielcarz DW, Wang Y, et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3(5):487–495. doi:10.1038/mi.2010.29
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107(27):12204–12209. doi:10.1073/pnas.0909122107
- Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, Kasper DL. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe. 2014;15(4):413–423. doi:10.1016/j.chom.2014.03.006
- Van Nguyen T, Piao CH, Fan YJ, et al. Anti-allergic rhinitis activity of α-lipoic acid via balancing Th17/Treg expression and enhancing Nrf2/HO-1 pathway signaling. Sci Rep. 2020;10(1):12528. doi:10.1038/s41598-020-69234-1
- Lochner M, Wang Z, Sparwasser T. The special relationship in the development and function of T Helper 17 and Regulatory T Cells. Prog Mol Biol Transl Sci. 2015;136:99–129.
- Yu S, Han B, Liu S, et al. Derp1-modified dendritic cells attenuate allergic inflammation by regulating the development of T helper type1(Th1)/Th2 cells and regulatory T cells in a murine model of allergic rhinitis. Mol Immunol. 2017;90:172–181. doi:10.1016/j.molimm.2017.07.015
- Borowczyk J, Shutova M, Brembilla NC, Boehncke WH. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148(1):40–52. doi:10.1016/j.jaci.2020.12.628
- Bayrak Degirmenci P, Aksun S, Altin Z, et al. Allergic Rhinitis and Its Relationship with IL-10, IL-17, TGF-β, IFN-γ, IL 22, and IL-35. Dis Markers. 2018;2018:9131432. doi:10.1155/2018/9131432
- Quan SH, Zhang YL, Han DH, Iwakura Y, Rhee CS. Contribution of interleukin 17A to the development and regulation of allergic inflammation in a murine allergic rhinitis model. Ann Allergy Asthma Immunol. 2012;108(5):342–350. doi:10.1016/j.anai.2012.02.014
- Palomares O, Martín-Fontecha M, Lauener R, et al. Regulatory T cells and immune regulation of allergic diseases: roles of IL-10 and TGF-β. Genes Immun. 2014;15(8):511–520. doi:10.1038/gene.2014.45
- Jiao WE, Sun L, Xu S, et al. Notch2 suppresses the development of allergic rhinitis by promoting FOXP3 expression and Treg cell differentiation. Life Sci. 2021;284:119922. doi:10.1016/j.lfs.2021.119922
- Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331(6015):337–341. doi:10.1126/science.1198469
- Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi:10.1038/nature12331
- Moszak M, Szulińska M, Bogdański P. You are what you eat-the relationship between diet, microbiota, and metabolic disorders-A review. Nutrients. 2020;12(4):1096. doi:10.3390/nu12041096
- Murdaca G, Gerosa A, Paladin F, Petrocchi L, Banchero S, Gangemi S. Vitamin D and Microbiota: is There a Link with Allergies? Int J Mol Sci. 2021;22(8):4288. doi:10.3390/ijms22084288
- Yang W, Cong Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell Mol Immunol. 2021;18(4):866–877.
- Paparo L, Nocerino R, Ciaglia E, et al. Butyrate as a bioactive human milk protective component against food allergy. Allergy. 2021;76(5):1398–1415. doi:10.1111/all.14625
- Fusco W, Lorenzo MB, Cintoni M, et al. Short-Chain fatty-acid-producing bacteria: key components of the human gut microbiota. Nutrients. 2023;15(9):2211. doi:10.3390/nu15092211
- Luu M, Monning H, Visekruna A. Exploring the molecular mechanisms underlying the protective effects of microbial SCFAs on intestinal tolerance and food allergy. Front Immunol. 2020;11:1225. doi:10.3389/fimmu.2020.01225
- Blanco-Pérez F, Steigerwald H, Schülke S, Vieths S, Toda M, Scheurer S. The dietary fiber pectin: health benefits and potential for the treatment of allergies by modulation of gut microbiota. Curr Allergy Asthma Rep. 2021;21(10):43. doi:10.1007/s11882-021-01020-z
- Kaisar MMM, Pelgrom LR, van der Ham AJ, Yazdanbakhsh M, Everts B. Butyrate conditions human dendritic cells to prime type 1 regulatory T cells via both histone deacetylase inhibition and G protein-coupled receptor 109A signaling. Front Immunol. 2017;8:1429. doi:10.3389/fimmu.2017.01429
- Shi Y, Xu M, Pan S, et al. Induction of the apoptosis, degranulation and IL‐13 production of human basophils by butyrate and propionate via suppression of histone deacetylation. Immunology. 2021;164(2):292–304. doi:10.1111/imm.13370
- Kehrmann J, Effenberg L, Wilk C, et al. Depletion of Foxp3+ regulatory T cells is accompanied by an increase in the relative abundance of Firmicutes in the murine gut microbiome. Immunology. 2020;159(3):344–353. doi:10.1111/imm.13158
- Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341–352. doi:10.1038/nri.2016.42
- Roduit C, Frei R, Ferstl R, et al. High levels of butyrate and propionate in early life are associated with protection against atopy. Allergy. 2019;74(4):799–809. doi:10.1111/all.13660
- Cheng HY, Chan JCY, Yap GC, et al. Evaluation of stool short chain fatty acids profiles in the first year of life with childhood atopy-related outcomes. Front Allergy. 2022;3:873168. doi:10.3389/falgy.2022.873168
- Chen Z, Xu Q, Liu Y, et al. Vancomycin-induced gut microbiota dysbiosis aggravates allergic rhinitis in mice by altered short-chain fatty acids. Front Microbiol. 2022;13:1002084. doi:10.3389/fmicb.2022.1002084
- Hu W, Lu W, Li L, et al. Both living and dead Faecalibacterium prausnitzii alleviate house dust mite-induced allergic asthma through the modulation of gut microbiota and short-chain fatty acid production. J Sci Food Agric. 2021;101(13):5563–5573. doi:10.1002/jsfa.11207
- Thorburn AN, McKenzie CI, Shen S, et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun. 2015;6:7320. doi:10.1038/ncomms8320
- Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi:10.1016/j.immuni.2013.08.003
- Lamas B, Richard ML, Leducq V, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. doi:10.1038/nm.4102
- Dong F, Hao F, Murray IA, et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes. 2020;12(1):1–24. doi:10.1080/19490976.2020.1788899
- Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol. 2019;10:2113. doi:10.3389/fimmu.2019.02113
- Crestani E, Harb H, Charbonnier LM, et al. Untargeted metabolomic profiling identifies disease-specific signatures in food allergy and asthma. J Allergy Clin Immunol. 2020;145(3):897–906. doi:10.1016/j.jaci.2019.10.014
- Raitala A, Karjalainen J, Oja SS, Kosunen TU, Hurme M. Indoleamine 2,3-dioxygenase (IDO) activity is lower in atopic than in non-atopic individuals and is enhanced by environmental factors protecting from atopy. Mol Immunol. 2006;43(7):1054–1056. doi:10.1016/j.molimm.2005.06.022
- Gostner JM, Becker K, Kofler H, Strasser B, Fuchs D. Tryptophan metabolism in allergic disorders. Int Arch Allergy Immunol. 2016;169(4):203–215. doi:10.1159/000445500
- Romani L, Zelante T, De Luca A, Fallarino F, Puccetti P. IL-17 and therapeutic Kynurenines in pathogenic inflammation to fungi. J Immunol. 2008;180(8):5157–5162. doi:10.4049/jimmunol.180.8.5157
- de Araújo EF, Feriotti C, Galdino NAL, Preite NW, Calich VLG, Loures FV. The IDO–AhR axis controls Th17/Treg immunity in a pulmonary model of fungal infection. Front Immunol. 2017;24:880. doi:10.3389/fimmu.2017.00880
- Prendergast GC, Malachowski WP, DuHadaway JB, Muller AJ. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res. 2017;77(24):6795–6811. doi:10.1158/0008-5472.CAN-17-2285
- Hossain FMA, Park SO, Kim HJ, et al. Indoleamine 2,3-dioxygenase in hematopoietic stem cell-derived cells suppresses rhinovirus-induced neutrophilic airway inflammation by regulating Th1- and Th17-type responses. Immune Netw. 2021;21(4):e26. doi:10.4110/in.2021.21.e26
- Hayashi T, Beck L, Rossetto C, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Investig. 2004;114:270–279. doi:10.1172/JCI21275
- Kepert I, Fonseca J, Müller C, et al. D-tryptophan from probiotic bacteria influences the gut microbiome and allergic airway disease. J Allergy Clin Immunol. 2017;139:1525–1535. doi:10.1016/j.jaci.2016.09.003
- Hayashi T, Mo JH, Gong X, et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci USA. 2007;104(47):18619–18624. doi:10.1073/pnas.0709261104
- Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23(6):716–724. doi:10.1016/j.chom.2018.05.003
- Che Y, Wang N, Ma Q, et al. Microbial characterization of the nasal cavity in patients with allergic rhinitis and non-allergic rhinitis. Front Cell Infect Microbiol. 2023;13:1166389. doi:10.3389/fcimb.2023.1166389
- Qin S, Xiao W, Zhou C, et al. Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther. 2022;7(1):199. doi:10.1038/s41392-022-01056-1
- Chen J, Rao C, Liu W, et al. Construction of fluorescence-labeled Burkholderia pseudomallei and its interaction with host cells. J Army Med Univ. 2022;44(20):2105–2112.
- Blake PA, Merson MH, Weaver RE, Hollis DG, Heublein PC. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N Engl J Med. 1979;300(1):1–5. doi:10.1056/NEJM197901043000101
- Weichhart T, Costantino G, Poglitsch M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29(4):565–577. doi:10.1016/j.immuni.2008.08.012
- Xie DL, Zheng MM, Zheng Y, et al. Vibrio vulnificus induces mTOR activation and inflammatory responses in macrophages. PLoS One. 2017;12(7):e0181454. doi:10.1371/journal.pone.0181454
- Blériot C, Dupuis T, Jouvion G, Eberl G, Disson O, Lecuit M. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42(1):145–158. doi:10.1016/j.immuni.2014.12.020
- Chai L, Wang Q, Si C, Gao W, Zhang L. Potential association between changes in microbiota level and lung diseases: a meta-analysis. Front Med Lausanne. 2022;8:723635. doi:10.3389/fmed.2021.723635
- Kang MJ, Jo SG, Kim DJ, Park JH. NLRP3 inflammasome mediates interleukin-1β production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice. Immunology. 2017;150(4):495–505. doi:10.1111/imm.12704
- Bäckhed F, Fraser CM, Ringel Y, et al. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12(5):611. doi:10.1016/j.chom.2012.10.012
- Johnson EL, Heaver SL, Walters WA, Ley RE. Microbiome and metabolic disease: revisiting the bacterial phylum Bacteroidetes. J Mol Med (Berl). 2017;95(1):1–8. doi:10.1007/s00109-016-1492-2
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340.
- Ukabam SO, Mann RJ, Cooper BT. Small intestinal permeability to sugars in patients with atopic eczema. Br J Dermatol. 1984;110(6):649–652. doi:10.1111/j.1365-2133.1984.tb04699.x
- Pike MG, Heddle RJ, Boulton P, Turner MW, Atherton DJ. Increased intestinal permeability in atopic eczema. J Invest Dermatol. 1986;86(2):101–104. doi:10.1111/1523-1747.ep12284035
- Rosenfeldt V, Benfeldt E, Valerius NH, Paerregaard A, Michaelsen KF. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr. 2004;145(5):612–616. doi:10.1016/j.jpeds.2004.06.068
- Hijazi Z, Molla AM, Al-Habashi H, Muawad WM, Molla AM, Sharma PN. Intestinal permeability is increased in bronchial asthma. Arch Dis Child. 2004;89(3):227–229. doi:10.1136/adc.2003.027680
- Majamaa H, Isolauri E. Evaluation of the gut mucosal barrier: evidence for increased antigen transfer in children with atopic eczema. J Allergy Clin Immunol. 1996;97(4):985–990. doi:10.1016/S0091-6749(96)80074-1
- Kitagawa H, Yamanaka K, Kakeda M, et al. Propionibacterium acnes vaccination induces regulatory T cells and Th1 immune responses and improves mouse atopic dermatitis. Exp Dermatol. 2011;20(2):157–158. doi:10.1111/j.1600-0625.2010.01180.x
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7(4):265–276. doi:10.1007/BF00915547
- Kudsk KA. Current aspects of mucosal immunology and its influence by nutrition. Am J Surg. 2002;183(4):390–398. doi:10.1016/S0002-9610(02)00821-8
- Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med. 2014;211(12):2397–2410. doi:10.1084/jem.20140625
- Ried K, Travica N, Paye Y, Sali A. Effects of a probiotic formulation on seasonal allergic rhinitis in adults-a randomized double-blind placebo-controlled trial: the probiotics for hay fever trial. Front Nutr. 2022;9:887978. doi:10.3389/fnut.2022.887978
- Yan S, Ai S, Huang L, et al. Systematic review and meta-analysis of probiotics in the treatment of allergic rhinitis. Allergol Immunopathol. 2022;50(3):24–37. doi:10.15586/aei.v50i3.507
- Liu P, Hu T, Kang C, et al. Research advances in the treatment of allergic rhinitis by probiotics. J Asthma Allergy. 2022;15:1413–1428. doi:10.2147/JAA.S382978
- Güvenç IA, Muluk NB, Mutlu FŞ, et al. Do probiotics have a role in the treatment of allergic rhinitis? A comprehensive systematic review and meta-analysis. Am J Rhinol Allergy. 2016;30(5):157–175. doi:10.2500/ajra.2016.30.4354
- Farahmandi K, Mohr AE, McFarland LV. Effects of probiotics on allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. Am J Rhinol Allergy. 2022;36(4):440–450. doi:10.1177/19458924211073550
- Zajac AE, Adams AS, Turner JH. A systematic review and meta-analysis of probiotics for the treatment of allergic rhinitis: probiotics for the treatment of AR. Int Forum Allergy Rhinol. 2015;5(6):524–532. doi:10.1002/alr.21492
- de Vos WM, Tilg H, Van Hul M, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–1032. doi:10.1136/gutjnl-2021-326789
