Abstract
Ragweed (Ambrosia spp.) is an annually flowering plant whose pollen bears high allergenic potential. Ragweed-induced allergic rhinoconjunctivitis has long been seen as a major immunologic condition in Northern America with high exposure and sensitization rates in the general population. The invasive occurrence of ragweed (A. artemisiifolia) poses an increasing challenge to public health in Europe and Asia as well. Possible explanations for its worldwide spread are climate change and urbanization, as well as pollen transport over long distances by globalized traffic and winds. Due to the increasing disease burden worldwide, and to the lack of a current and comprehensive overview, this study aims to review the current and emerging treatment options for ragweed-induced rhinoconjunctivitis. Sound clinical evidence is present for the symptomatic treatment of ragweed-induced allergic rhinoconjunctivitis with oral third-generation H1-antihistamines and leukotriene antagonists. The topical application of glucocorticoids has also been efficient in randomized controlled clinical trials. Combined approaches employing multiple agents are common. The mainstay of causal treatment to date, especially in Northern America, is subcutaneous immunotherapy with the focus on the major allergen, Amb a 1. Beyond this, growing evidence from several geographical regions documents the benefit of sublingual immunotherapy. Future treatment options promise more specific symptomatic treatment and fewer side effects during causal therapy. Novel antihistamines for symptomatic treatment are aimed at the histamine H3-receptor. New adjuvants with toll-like receptor 4 activity or the application of the monoclonal anti-immunoglobulin E antibody, omalizumab, are supposed to enhance conventional immunotherapy. An approach targeting toll-like receptor 9 by synthetic cytosine phosphate–guanosine oligodeoxynucleotides promises a new treatment paradigm that aims to modulate the immune response, but it has yet to be proven in clinical trials.
Specifics of ragweed-induced rhinoconjunctivitis
Botanical characteristics and ecologic factors
Ragweed (genus Ambrosia in the family Asteraceae) is an annual herbaceous flowering plant that is originally native to Northern America. Worldwide, the two most widespread species are common (or short) ragweed (A. artemisiifolia) and great ragweed (A. trifida), which are, in turn, clinically the most relevant for their high potential to cause allergic rhinitis. The distribution of the ragweed species is, however, dependent upon the geographical region. For example, the species causing the most severe symptoms in allergic patients in Israel and parts of the Mediterranean region is A. maritime.Citation1
Ragweed is an invasive species with a growing occurrence in different world regions. Presumably because of climate change, ragweed has reportedly shown an increasing length of pollen season in Northern America,Citation2 implying a potential increasing disease burden for atopic patients in affected areas. A rise in atmospheric carbon dioxide concentrations and air temperature, brought about not only by climate change but also by urbanization, are conditions that stimulate ragweed growth, which is signified by biomass production and flowering date.Citation3 Since ragweed is becoming increasingly common in Europe, pollen load in several regions is also a growing health problem.Citation4 Excluding mountain ranges, almost all of Western, Southern, and Eastern Europe are assumed to be viable to sustain ragweed occurrence.Citation5
Globalization in trade and traffic has been identified as relevant to the distribution of ragweed seeds, contributing to the plant’s invasive characteristics. This is shown, for example, in the contamination of birdseeds with germinable ragweed seeds in France.Citation6 Also, ragweed pollen has been estimated to be transported over long distances, with Eastern Europe being a possible major source.Citation7–Citation10 This has important implications since it makes containment more difficult, while it increases the potential exposure of atopic populations.
Allergens and epidemiology
Presumably, due to high nicotinamide adenine dinucleotide phosphate oxidase,Citation11 as well as serine and cysteine protease activity in its pollen,Citation12 ragweed carries high potential to cause allergic rhinitis. The major allergens of ragweed, Amb a 1 and Amb a 2, belong to the pectate-lyase family and are secreted, acidic, nonglycosylated, single-chain proteins. Minor allergens include Amb a 3 (secreted basic glycoprotein, copper-binding domain), Amb a 5 (secreted basic protein, homologous to Amb t 5 and Amb p 5), Amb a 6 (secreted basic protein, homologous to nonspecific lipid transfer proteins), and Amb a 7 (basic protein, plastocyanin domain).Citation13 As recently as 2010, Amb a 4 has been described for the first time; it is a glycoprotein with a defensin-like domain and is highly homologous to Art v 1, the sunflower protein P22357, and the feverfew allergen Par h 1.Citation14 Further, minor allergens of ragweed include Amb a 11 (cysteine protease)Citation15,Citation16 and Amb a CPI (a cysteine protease inhibitor).Citation17,Citation18 Amb a 8 (profilin), Amb a 9 (polcalcin), and Amb a 10 (polcalcin) are known pan-allergens.Citation13,Citation19 Amb a 6 and Amb a 8 (with potential homologues Cuc m2 and Mus xp 1) are candidates for the cross-reactive structures involved in the clinical manifestation of the supposed ragweed–melon–banana association, which is a pollen–food syndrome.Citation20
With respect to patients allergic to ragweed, in an in vitro study, all patients displayed serum reactivity to Amb a 1, while 30% were monosensitized against Amb a 1 alone.Citation19 A high prevalence of cosensitivity to mugwort (Artemisia vulgaris) has been noted in patients hypersensitive to ragweed; 93% of mugwort-sensitized patients were sensitized to ragweed, whereas 38% of ragweed-hypersensitive patients were sensitized to mugwort.Citation21 This might partially be explained by the homology of Amb a 4 to Art v 1, the major allergen of mugwort.Citation14
In Northern America, ragweed allergy has long been recognized as a major health problem. Sensitization rates are estimated to be as high as 10%–26% in the common population.Citation22,Citation23 Recently, attention has also been drawn to ragweed in Europe, since a rising prevalence of ragweed sensitization has been observed beginning in the 1990s.Citation24 Ragweed sensitization rates in the general population in Europe are given as 10%–30%, as assessed by pollen extracts of ragweed,Citation25,Citation26 and 1.9% for purified Amb a 1.Citation26 In atopic patients, a great variety of sensitization rates has been observed, with values reported from 7.9%–70.0%.Citation27–Citation31 Comparable to the situation in Europe, pollen count and sensitization rates for ragweed in Asia have been increasing for several decades now, as exemplified by the situation in Korea.Citation32 For distinct Asian populations, up to 21%–29% of atopic patients were reported to be sensitized to a specific ragweed species.Citation1 Due to the high cross-reactivity between ragweed and mugwort,Citation21,Citation33,Citation34 however, data on the sensitization rates for ragweed have to be interpreted with care since extracts, and not recombinant allergens, are often used and the values could, at least in part, reflect an effect of allergen homology.
In epidemiologic studies, a high rate of comorbidity of allergic rhinitis, chronic rhinosinusitis, and asthma bronchiale is well established today.Citation35 Specific to ragweed-induced allergic rhinitis, this correlation with concomitant maxillary sinusitis during the allergy season has been confirmed in a clinical study.Citation36 A foundation for this was brought forward in an experimental setting by showing that an intranasal challenge with ragweed allergens leads to an inflammatory reaction of the maxillary sinus.Citation37
Diagnosis
Diagnostic approaches in ragweed-induced allergic rhinitis include obtaining the patient’s precise medical history, especially in terms of potential sources of exposure, seasonal symptoms of nasal inflammation, and relevant comorbidities like asthma. Following this, a skin prick testCitation38 and nasal provocation are the main clinical tests applied today. In vitro testing allows for the subclassification of allergens and cross-reactions.Citation39
Symptomatic treatment options
Several agents in topical and systemic dosage forms are available for the symptomatic treatment of ragweed-induced rhinoconjunctivitis. In clinical trials, first- and second-generation H1-antihistamines and leukotriene receptor antagonists dispensed orally, as well as topical glucocorticoids, were studied alone and in various combinations.
For the symptomatic treatment of conjunctival affection caused by ragweed allergy, an uncontrolled, nonblinded, randomized clinical trial attributed benefit to the topical use of the mast cell stabilizer, nedocromil, with oral application of the third-generation H1-antihistamine, fexofenadine.Citation40 Due to the lack of a proper control group, however, the level of evidence for this finding is considerably low.
H1-antihistamines are the traditional mainstay for the symptomatic treatment of allergies. A longstanding debate has argued whether a reduction in vigilance observed in patients suffering from nasal allergies is caused by the condition itself or by first-generation antihistamines as a possible sedating medication.Citation41 Evidence exists from an experimental study that attributed vigilance reduction to the condition itself in ragweed-induced allergic rhinitis.Citation42 Regarding antihistamines, it was shown that for the symptomatic treatment of ragweed allergy, the first-generation H1-antagonist, diphenhydramine, and the third-generation H1-antagonist, desloratadine, effected a comparable reduction in nasal allergy symptoms compared to placebo.Citation43 However, vigilance and cognitive function were significantly more reduced by diphenhydramine, confirming the value of newer antihistaminic agents.
Olopatadine hydrochloride acts as an antihistamine with anticholinergic and mast cell stabilizing effects. In topical dosage forms, it reduced nasal symptoms, as assessed by a nasal symptom score, when compared to placebo in a 1-day trial.Citation44 During and after exposure in an allergen challenge chamber, a concentration of 0.6% proved to be the most efficient.Citation44 Of note, this was the only study that validated the results obtained in the environmental exposure chamber against previous studies on natural exposure.Citation45,Citation46
The relative benefit of different classes of symptomatic medication is essentially a question of clinical relevance, and therefore the comparison of different agents among each other and against placebo have been the subject of randomized controlled trials. Therefore, the oral application of the third-generation H1-antagonist, levocetirizine, was tested against the leukotriene receptor antagonist, montelukast, and placebo.Citation47 Efficacy was shown in the reduction of nasal symptoms for 5 mg of levocetirizine once daily, while 10 mg of montelukast once daily failed to cause significant mitigation of symptoms in a 2-day trial. The investigation took place in an allergen challenge chamber,Citation47 thereby providing only an approximation of the situation in the natural environment with potentially different allergen characteristics.
Combination treatments with antihistamines are increasingly investigated. No statistically significant differences were found when the second-generation H1-antagonist, fexofenadine, in combination with the sympathomimetic decongestant, pseudoephedrine, was compared to a combination of the second-generation H1-antagonist, loratadine, and the leukotriene receptor antagonist, montelukast.Citation48 Both interventions improved symptoms from baseline. However, without an a priori stated primary endpoint, this study bears a high risk of bias.Citation48 It should be noted that this was the only study available for ragweed-induced rhinoconjunctivitis where the Rhinoconjunctivitis Quality of Life Questionnaire was used as the validated outcome measure for quality of life.Citation49 In another trial, the second-generation H1-antagonist, loratadine, in combination with the leukotriene receptor antagonist, montelukast, as a tablet and solution reduced nasal congestion significantly in patients with ragweed-induced allergic rhinoconjunctivitis in comparison to placebo, while the common over-the-counter decongestant, phenylephrine (an alpha 1-adrenergic receptor agonist), failed to prove efficacy in a parallel arm of the study.Citation50
Increasing interest focuses on topical glucocorticoids for the symptomatic treatment of allergic rhinitis. In patients with ragweed-induced allergic rhinoconjunctivitis, the synthetic glucocorticoid, fluticasone furoate, was shown to be superior with 110 mg applied once daily in a 15-day trial during the allergy season versus placebo, with a symptom score as the primary endpoint.Citation51
Taken together, there is sound evidence to support the clinical application of oral antihistamines and topical glucocorticoids for the symptomatic treatment of ragweed allergy. Leukotriene receptor antagonists were effective in combination with antihistamines, but they have yet to be proven as monotherapy. An overview of the recent prospective, randomized, controlled, double-blind Phase III studies for the symptomatic treatment of ragweed allergy is given in .
Table 1 Recent prospective, randomized, controlled, double-blind Phase III studies for the symptomatic treatment of seasonal allergic rhinitis caused by ragweed pollen
The choice of meaningful outcome measures in clinical trials of the symptomatic application of topical glucocorticoids has recently been evaluated. In a prospective clinical evaluation,Citation52 a nasal challenge was effected by the topical delivery of allergens. Sneeze count, symptom scores, and albumin level in nasal lavage were validated as being reproducibly influenced by the intervention, and they were therefore deemed to be suitable outcome parameters. The further candidate, lysozyme level in nasal lavage, was not influenced by glucocorticoid application, while kinin level was not reproducible.Citation52 For the design of future studies, these results should be honored.
Causal treatment options
Subcutaneous immunotherapy is the traditional mainstay for the causal treatment of allergic disorders. Subcutaneous immunotherapy for ragweed is considerably well documented in Northern America. A prospective, randomized clinical trialCitation53 from Southern Europe also reported some benefit for the efficacy of this treatment option in another geographical region. However, without an a priori stated primary endpoint, this study is at high risk of biased results.
Sublingual immunotherapy is generally gaining interest due to its supposed lower risk of side effects and potentially better compliance.Citation54,Citation55 However, a considerable number of questions has remained open regarding the mechanisms of the desired effect and the optimal dosage.Citation56 As will be detailed, several randomized, controlled clinical trials have therefore been conducted to investigate the efficacy and safety of sublingual immunotherapy for ragweed allergy.
Testing a formulation with a continuous dose applied as a solid tablet, André et alCitation57 found significant benefit of an oral immunotherapy of a standardized ragweed extract in a multicenter study from Western Europe. However, this study did not include an a priori defined primary endpoint and thus also bears considerable risk of bias.
Two prospective clinical trials from Northern America failed to show a significant difference in the primary endpoint for efficacy, which included the improvement of a symptom scoreCitation58 or improvements in combined symptom and medication scores,Citation59 during the respective local ragweed pollen season. However, both formulations of ragweed extract used for sublingual application were deemed to be safe for further clinical investigations. As a reason for not being able to demonstrate efficacy, a possibly polysensitized patient cohortCitation58 and variations in pollen load between the study centersCitation59 were hypothesized.
Recently, two studies with considerably high numbers of randomized patients (565 patientsCitation60 and 784 patientsCitation61) were undertaken to investigate the effect of a sublingual allergy immunotherapy tablet. Both studies showed the efficacy of this treatment in Northern America,Citation60 as well as in Northern America and Eastern Europe.Citation61 In both cases, the primary endpoint was a combined symptom and medication score, while the effective doses were 6 μg and 12 μgCitation60 or 12 μgCitation61 of the major allergen Amb a 1 in each single tablet.
Taken together, growing evidence supports the clinical value of sublingual immunotherapy in ragweed allergies. An overview of the recent prospective, randomized, controlled, double-blind Phase III studies for the causal treatment of ragweed allergy is given in .
Table 2 Recent prospective, randomized, controlled, double-blind Phase III studies for the causal treatment of seasonal allergic rhinitis caused by ragweed pollen
Future perspectives
In the future, an important challenge in Europe will be the reduction of pollen exposure to the population by limitation of ragweed expansion. As a potential basic measure, repetitive mowing during the annual life cycle of the plant has been shown to be effective in the reduction of pollen load.Citation62 Beyond this, combined efforts of manual uprooting, herbicides, and mowing have been proposed according to local conditions.Citation63 Furthermore, advanced knowledge of pollen load might help affected patients avoid allergen exposure. Numerous studies investigated region-specific pollen counts,Citation64–Citation66 helping to develop several forecasting models.Citation67–Citation69
Allergies to different ragweed species might carry different clinical profiles. A recent studyCitation70 compared the symptoms elicited during the natural pollen season in a region where giant ragweed is more prevalent to the symptoms elicited in an allergen challenge chamber by short ragweed pollen. It thereby identified clusters with three distinct response profiles among the patients tested, signifying low and high responses during the natural season and experimental challenge. This implies the possibility of subtypes in ragweed pollinosis that could bear consequences for treatment.Citation70
Several nonpharmacological treatment approaches have been explored to treat ragweed-induced allergic rhinoconjunctivitis. Prospective open clinical studies explored the value of intranasal phototherapy three times a week during the ragweed pollen season. The defined dose of a combination of ultraviolet (UV)-B, UV-A, and visible light led to improvements in a nasal symptom score,Citation71 even in comparison to the second-generation H1-antagonist, fexofenadine.Citation72 Another open study found a beneficial effect of photochemotherapy by irradiation of the nasal cavity with UV-A light after topical sensitization with a psoralen (psoralen with ultraviolet A light [PUVA]) in terms of a symptom score.Citation73 A simple barrier method like nasal filters was also described as being effective in reducing symptoms during exposure to ragweed allergens.Citation74
New agents are nearing clinical application. Proposals for symptomatic treatments include selective histamine-H3 receptor antagonists, with the compounds JNJ-39220675 and PF-03654746 being principally able to relieve nasal allergy symptoms.Citation75,Citation76 In preclinical investigations, new target structures are being evaluated for their value in the treatment of ragweed-induced allergies. While traditional leukotriene receptor antagonists like montelukast block the cysteinyl leukotriene receptor 1, the leukotriene B4 receptor antagonist ONO-4057 was able to inhibit itching after a ragweed challenge in an experimental animal model of conjunctivitis.Citation77 In a comparable model, the selective DP2 receptor antagonist, AM 156, has been shown to reduce the symptoms of an allergen challenge.Citation78
Improvements of subcutaneous immunotherapy include the addition of adjuvants or omalizumab, an anti-immunoglobulin (Ig)E antibody, as well as modified allergens.Citation79 Considerable effort is being put into the development of toll-like receptor agonists, with toll-like receptors 4 and 9 as the most promising target structures.Citation80
The monoclonal antibody, omalizumab, has been approved for clinical use in several countries now. It is able to block IgE binding to the high-affinity IgE receptor, Fc epsilon RI (FCER1), on mast cells and basophils by inactivating soluble and membrane-bound IgECitation81 and reducing the overall serum level of IgE after continuous therapy.Citation82 It acts as a mast cell stabilizing agentCitation81 and has the potential to limit the effects of aeroallergen exposure mediated by basophilCitation83 or dendritic cells.Citation84 Therefore, the expression of FCER1 on immune cells is reduced.Citation82 A randomized controlled clinical trial was able to show that omalizumab pretreatment was able to ameliorate the side effects of subcutaneous rush immunotherapy for ragweed seasonal allergic rhinitis,Citation85 while it has also been shown that this was caused by the inhibition of allergen-specific IgE binding.Citation86 However, an issue that still needs to be resolved for ragweed-induced allergic rhinoconjunctivitis is the cost effectiveness of omalizumab. For allergic asthma, the benefits of omalizumab have been calculated to outweigh the cost, but only in severely affected subgroups of patients.Citation87
An entirely new approach is the toll-like receptor 9-based inhibition of immune responses mediated by type 2 T-helper cells, which modulate dendritic cells and mononuclear cells.Citation88 This has been attempted by an immunostimulatory conjugate of the ragweed major allergen, Amb a 1, and an oligodeoxyribonucleotide DNA sequence containing a CpG motif. Favorable redirection of the immunoreaction has been shown,Citation89–Citation94 in addition to a reduction of symptoms and an improvement in quality of life for up to two consecutive seasons, following a regime containing only six injections in a pilot clinical trial.Citation95 However, further treatments were not able to prove clinical benefit, and there are currently no new data on this approach.Citation79
New treatment regimens and formulations for subcutaneous immunotherapy are also being evaluated. An ultrashort treatment course applying an allergoid with the toll-like receptor 4 agonist, monophosphoryl lipid A, as an adjuvant was demonstrated to be safe,Citation96 and it had effected a significant improvement in the symptom score.Citation97
A field that is currently not employed for the treatment of allergic rhinitis is cytokine signaling. In studies employing an animal model of experimental conjunctivitis, a role for interleukin-10Citation98,Citation99 and interleukin-16Citation100 has been described in the development of the reaction in response to an allergen challenge. Correspondingly, suppressors of cytokine signaling (SOCS)3 and SOCS5 have been reported to bear the potential to regulate allergic responses.Citation101 Taken together, there are several more promising targets for the treatment of allergic rhinitis currently still outside clinical investigation.
Methods
The search strategy on MEDLINE via PubMed applied the medical subject heading (MeSH) terms “Ambrosia” in combination with either “Rhinitis, Allergic, Seasonal”, “Rhinitis, Allergic, Perennial”, “Conjunctivitis, Allergic”, or “Allergic Rhinitis [Supplementary Concept]”.
The search was restricted to English- and German-language publications and yielded 182 single entries as of September 2014. The review was limited to publications published since 2002 (170 entries). Obtained publications were systematically screened by the abstract. For the evaluation of symptomatic and causal treatment options, only publications reporting Phase III randomized, controlled, double-blind clinical trials were included. This resulted in five studies reporting symptomatic and six studies reporting causal treatments.
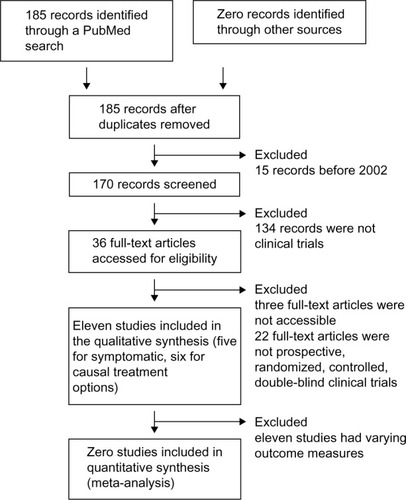
This low number of studies presents a considerable risk for publication bias. The variety in study designs and primary outcome measures found in the literature, as well as the lack of validated outcome measures, did not allow us to pool the study results for the meta-analysis. A flow diagram of the review process according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statementCitation102 is shown in .
Conclusion
Ragweed-induced allergic rhinoconjunctivitis is prevalent in Northern America and Europe. For symptomatic treatment, clinical evidence exists for antihistamines, leukotriene antagonists, and glucocorticoids. Options for causal treatment include subcutaneous and sublingual immunotherapy. Novel antihistamines, refined adjuvants, and combination therapy with the anti-IgE antibody, omalizumab, as well as an immunomodulatory approach involving toll-like receptor 9 are promising for the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- WaiselYEshelAKeynanNLanggutDAmbrosia: a new impending disaster for the Israeli allergic populationIsr Med Assoc J2008101285685719160941
- ZiskaLKnowltonKRogersCRecent warming by latitude associated with increased length of ragweed pollen season in central North AmericaProc Natl Acad Sci U S A2011108104248425121368130
- ZiskaLHGebhardDEFrenzDAFaulknerSSingerBDStrakaJGCities as harbingers of climate change: common ragweed, urbanization, and public healthJ Allergy Clin Immunol2003111229029512589347
- RidoloEAlbertiniRGiordanoDSolianiLUsbertiIDall’AglioPPAirborne pollen concentrations and the incidence of allergic asthma and rhinoconjunctivitis in northern Italy from 1992 to 2003Int Arch Allergy Immunol2007142215115717057413
- ChapmanDSHaynesTBealSEsslFBullockJMPhenology predicts the native and invasive range limits of common ragweedGlob Chang Biol201420119220224038855
- ThibaudonMColonnelloCBesancenotJPTolobaYFrançoisHCaillaudDCan birdseed contribute to the spread of ragweed?J Investig Allergol Clin Immunol2012223234236
- KasprzykINon-native Ambrosia pollen in the atmosphere of Rzeszów (SE Poland); evaluation of the effect of weather conditions on daily concentrations and starting dates of the pollen seasonInt J Biometeorol200852534135118046583
- StachASmithMSkjøthCABrandtJExamining Ambrosia pollen episodes at Poznań (Poland) using back-trajectory analysisInt J Biometeorol200751427528617120063
- CecchiLLorenzoCMorabitoMLong distance transport of ragweed pollen as a potential cause of allergy in central ItalyAnn Allergy Asthma Immunol2006961869116440538
- PucMRagweed pollen in the air of SzczecinAnn Agric Environ Med2004111535715236498
- DharajiyaNBoldoghICardenasVSurSRole of pollen NAD(P) H oxidase in allergic inflammationCurr Opin Allergy Clin Immunol200881576218188019
- GunawanHTakaiTIkedaSOkumuraKOgawaHProtease activity of allergenic pollen of cedar, cypress, juniper, birch and ragweedAllergol Int2008571839118209508
- GadermaierGDedicAObermeyerGFrankSHimlyMFerreiraFBiology of weed pollen allergensCurr Allergy Asthma Rep20044539140015283880
- LéonardRWopfnerNPabstMA new allergen from ragweed (Ambrosia artemisiifolia) with homology to art v 1 from mugwortJ Biol Chem201028535271922720020576600
- RadauerCNandyAFerreiraFUpdate of the WHO/IUIS Allergen Nomenclature Database based on analysis of allergen sequencesAllergy201469441341924738154
- MariARasiCPalazzoPScalaEAllergen databases: current status and perspectivesCurr Allergy Asthma Rep20099537638319671381
- RogersBLPollockJKlapperDGGriffithIJSequence of the proteinase-inhibitor cystatin homologue from the pollen of Ambrosia artemisiifolia (short ragweed)Gene199313322192217916719
- PopovicMMMilovanovicMBurazerLCysteine proteinase inhibitor Act d 4 is a functional allergen contributing to the clinical symptoms of kiwifruit allergyMol Nutr Food Res201054337338019885843
- GadermaierGWopfnerNWallnerMArray-based profiling of ragweed and mugwort pollen allergensAllergy200863111543154918925891
- EggerMMutschlechnerSWopfnerNGadermaierGBrizaPFerreiraFPollen-food syndromes associated with weed pollinosis: an update from the molecular point of viewAllergy200661446147616512809
- AseroRWopfnerNGruberPGadermaierGFerreiraFArtemisia and Ambrosia hypersensitivity: co-sensitization or co-recognition?Clin Exp Allergy200636565866516650052
- GergenPJTurkeltaubPCKovarMGThe prevalence of allergic skin test reactivity to eight common aeroallergens in the US population: results from the second National Health and Nutrition Examination SurveyJ Allergy Clin Immunol19878056696793680811
- ArbesSJJrGergenPJElliottLZeldinDCPrevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination SurveyJ Allergy Clin Immunol2005116237738316083793
- BurbachGJHeinzerlingLMRöhneltCBergmannKCBehrendtHZuberbierTGA(2)LEN studyRagweed sensitization in Europe – GA(2)LEN study suggests increasing prevalenceAllergy200964466466519210367
- BoehmeMWGabrioTDierkesmannRSensitization to airborne ragweed pollen – a cause of allergic respiratory diseases in Germany?Dtsch Med Wochenschr200913428–2914571463 German19572244
- BoehmeMWKompauerIWeidnerUPiechotowskiIGabrioTBehrendtHRespiratory symptoms and sensitization to airborne pollen of ragweed and mugwort of adults in Southwest GermanyDtsch Med Wochenschr20131383316511658 German23913351
- RuëffFPrzybillaBWalkerASensitization to common ragweed in southern Bavaria: clinical and geographical risk factors in atopic patientsInt Arch Allergy Immunol20121591657422572962
- CanisMBeckerSGrögerMKramerMFIgE reactivity patterns in patients with allergic rhinoconjunctivitis to ragweed and mugwort pollensAm J Rhinol Allergy2012261313522391077
- TosiAWüthrichBBoniniMPietragalla-KöhlerBTime lag between Ambrosia sensitisation and Ambrosia allergy: a 20-year study (1989–2008) in Legnano, northern ItalySwiss Med Wkly2011141w1325321984071
- TestiSCarabelliACecchiLMulticenter investigation to assess the prevalence of ambrosia pollen allergy in TuscanyJ Investig Allergol Clin Immunol2009193251252
- Ackermann-LiebrichUSchindlerCFreiPSensitisation to Ambrosia in Switzerland: a public health threat on the waitSwiss Med Wkly20091395–6707519204839
- KimJHOhJWLeeHBChanges in sensitization rate to weed allergens in children with increased weeds pollen counts in Seoul metropolitan areaJ Korean Med Sci201227435035522468096
- OberhuberCMaYWopfnerNPrevalence of IgE-binding to Art v 1, Art v 4 and Amb a 1 in mugwort-allergic patientsInt Arch Allergy Immunol200814529410117823540
- HanDLaiXGjesingBZhongNZhangLSpangfortMDThe specific IgE reactivity pattern of weed pollen-induced allergic rhinitis patientsActa Otolaryngol2011131553353821189055
- JarvisDNewsonRLotvallJAsthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in EuropeAllergy2012671919822050239
- BaroodyFMMuchaSMdeTineoMNaclerioRMEvidence of maxillary sinus inflammation in seasonal allergic rhinitisOtolaryngol Head Neck Surg2012146688088622301108
- BaroodyFMMuchaSMDetineoMNaclerioRMNasal challenge with allergen leads to maxillary sinus inflammationJ Allergy Clin Immunol2008121511261132 e718367240
- GungorAHouserSMAquinoBFA comparison of skin endpoint titration and skin-prick testing in the diagnosis of allergic rhinitisEar Nose Throat J2004831546014986760
- JiangXDLiGYDongZZhuDDCorrelation analysis of two serum-specific immunoglobulin E test systems and skin-prick test in allergic rhinitis patients from northeast ChinaAm J Rhinol Allergy201125211611921294972
- AlexanderMPatelPAllegroSHicksASupplementation of fexofenadine therapy with nedocromil sodium 2% ophthalmic solution to treat ocular symptoms of seasonal allergic conjunctivitisClin Experiment Ophthalmol200331320621212786770
- DruceHMPerformance impairment revisitedAnn Allergy Asthma Immunol200289434434512392375
- WilkenJABerkowitzRKaneRDecrements in vigilance and cognitive functioning associated with ragweed-induced allergic rhinitisAnn Allergy Asthma Immunol200289437238012392381
- WilkenJAKaneRLEllisAKA comparison of the effect of diphenhydramine and desloratadine on vigilance and cognitive function during treatment of ragweed-induced allergic rhinitisAnn Allergy Asthma Immunol200391437538514582817
- PatelPRolandPSMarpleBFAn assessment of the onset and duration of action of olopatadine nasal sprayOtolaryngol Head Neck Surg2007137691892418036421
- RatnerPHHampelFCAmarNJSafety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis to mountain cedarAnn Allergy Asthma Immunol200595547447916312171
- MeltzerEOHampelFCRatnerPHSafety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitisAnn Allergy Asthma Immunol200595660060616400902
- PatelPPatelDEfficacy comparison of levocetirizine vs montelukast in ragweed sensitized patientsAnn Allergy Asthma Immunol2008101328729418814452
- MoinuddinRdeTineoMMaleckarBNaclerioRMBaroodyFMComparison of the combinations of fexofenadine-pseudoephedrine and loratadine-montelukast in the treatment of seasonal allergic rhinitisAnn Allergy Asthma Immunol2004921737914756468
- JuniperEFGuyattGHDevelopment and testing of a new measure of health status for clinical trials in rhinoconjunctivitisClin Exp Allergy199121177832021881
- DayJHBriscoeMPRatzJDDanzigMYaoREfficacy of loratadine-montelukast on nasal congestion in patients with seasonal allergic rhinitis in an environmental exposure unitAnn Allergy Asthma Immunol2009102432833819441605
- KaiserHBNaclerioRMGivenJTolerTNEllsworthAPhilpotEEFluticasone furoate nasal spray: a single treatment option for the symptoms of seasonal allergic rhinitisJ Allergy Clin Immunol200711961430143717418384
- ProudDRikerDKTogiasAReproducibility of nasal allergen challenge in evaluating the efficacy of intranasal corticosteroid treatmentClin Exp Allergy201040573874420337650
- MironeCAlbertFTosiAEfficacy and safety of subcutaneous immunotherapy with a biologically standardized extract of Ambrosia artemisiifolia pollen: a double-blind, placebo-controlled studyClin Exp Allergy20043491408141415347374
- CanonicaGWPassalacquaGNoninjection routes for immunotherapyJ Allergy Clin Immunol20031113437448 ; quiz 44912642818
- LeathermanBDOwenSParkerMSublingual Immunotherapy: Past, present, paradigm for the future? A review of the literatureOtolaryngol Head Neck Surg20071363 SupplS1S2017321336
- CoxLSLarenas LinnemannDNolteHWeldonDFinegoldINelsonHSSublingual immunotherapy: a comprehensive reviewJ Allergy Clin Immunol200611751021103516675328
- AndréCPerrin-FayolleMGrosclaudeMA double-blind placebo-controlled evaluation of sublingual immunotherapy with a standardized ragweed extract in patients with seasonal rhinitis. Evidence for a dose-response relationshipInt Arch Allergy Immunol2003131211111812811019
- SkonerDGentileDBushRFasanoMBMcLaughlinAEschRESublingual immunotherapy in patients with allergic rhinoconjunctivitis caused by ragweed pollenJ Allergy Clin Immunol20101253660666, 666. e1–e666. e420153030
- BowenTGreenbaumJCharbonneauYCanadian trial of sub-lingual swallow immunotherapy for ragweed rhinoconjunctivitisAnn Allergy Asthma Immunol200493542543015562880
- NolteHHébertJBermanGRandomized controlled trial of ragweed allergy immunotherapy tablet efficacy and safety in North American adultsAnn Allergy Asthma Immunol20131106450456 e423706715
- CreticosPSMaloneyJBernsteinDIRandomized controlled trial of a ragweed allergy immunotherapy tablet in North American and European adultsJ Allergy Clin Immunol2013131513421349 e623622121
- SimardMJBenoitDLEffect of repetitive mowing on common ragweed (Ambrosia artemisiifolia L.) pollen and seed productionAnn Agric Environ Med2011181556221736270
- GajnikDPeternelRMethods of intervention in the control of ragweed spread (Ambrosia artemisiifolia L.) in the area of Zagreb County and the city of ZagrebColl Antropol20093341289129420102083
- LaaidiMLaaidiKBesancenotJPThibaudonMRagweed in France: an invasive plant and its allergenic pollenAnn Allergy Asthma Immunol200391219520112952115
- PeternelRMusic MilanovicSSrnecLAirborne ragweed (Ambrosia artemisiifolia L.) pollen content in the city of Zagreb and implications on pollen allergyAnn Agric Environ Med200815112513018581990
- SikoparijaBRadisicPPejakTSimicSAirborne grass and ragweed pollen in the southern Panonnian Valley – consideration of rural and urban environmentAnn Agric Environ Med200613226326617195999
- CsépeZMakraLVoukantsisDPredicting daily ragweed pollen concentrations using Computational Intelligence techniques over two heavily polluted areas in EuropeSci Total Environ2014476–477542552
- HowardLELevetinEAmbrosia pollen in Tulsa, Oklahoma: aerobiology, trends, and forecasting model developmentAnn Allergy Asthma Immunol2014113664164625240331
- LaaidiMThibaudonMBesancenotJPTwo statistical approaches to forecasting the start and duration of the pollen season of Ambrosia in the area of Lyon (France)Int J Biometeorol2003482657312783292
- JacobsRLHarperNHeWResponses to ragweed pollen in a pollen challenge chamber versus seasonal exposure identify allergic rhinoconjunctivitis endotypesJ Allergy Clin Immunol20121301122127 e822554707
- KoreckAICsomaZBodaiLRhinophototherapy: a new therapeutic tool for the management of allergic rhinitisJ Allergy Clin Immunol2005115354154715753902
- GaracziEBoros-GyeviMBellaZCsomaZKeményLKoreckAIntranasal phototherapy is more effective than fexofenadine hydrochloride in the treatment of seasonal allergic rhinitis: results of a pilot studyPhotochem Photobiol201187247447721366599
- CsomaZKoreckAIgnaczFPUVA treatment of the nasal cavity improves the clinical symptoms of allergic rhinitis and inhibits the immediate-type hypersensitivity reaction in the skinJ Photochem Photobiol B2006831212616406552
- O’MearaTJSercombeJKMorganGReddelHKXuanWToveyERThe reduction of rhinitis symptoms by nasal filters during natural exposure to ragweed and grass pollenAllergy200560452953215727589
- BarchukWTSalapatekAMGeTD’AngeloPLiuXA proof-of-concept study of the effect of a novel H3-receptor antagonist in allergen-induced nasal congestionJ Allergy Clin Immunol20131324838846 e1–e623791513
- StokesJRRomeroFAJrAllanRJThe effects of an H3 receptor antagonist (PF-03654746) with fexofenadine on reducing allergic rhinitis symptomsJ Allergy Clin Immunol20121292409412, 412. e1–e222196768
- AndohTSakaiKUrashimaMKitazawaKHonmaAKuraishiYInvolvement of leukotriene B4 in itching in a mouse model of ocular allergyExp Eye Res2012989710322504036
- StebbinsKJBroadheadARMusiyenkoADP2 (CRTh2) antagonism reduces ocular inflammation induced by allergen challenge and respiratory syncytial virusInt Arch Allergy Immunol2012157325926822042170
- CasaleTBStokesJRImmunotherapy: what lies beyondJ Allergy Clin Immunol20141333612619 : quiz 62024581428
- AryanZHolgateSTRadziochDRezaeiNA new era of targeting the ancient gatekeepers of the immune system: toll-like agonists in the treatment of allergic rhinitis and asthmaInt Arch Allergy Immunol20141641466324853609
- ChangTWShiungYYAnti-IgE as a mast cell-stabilizing therapeutic agentJ Allergy Clin Immunol2006117612031212 ; quiz 121316750976
- LinHBoeselKMGriffithDTOmalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophilsJ Allergy Clin Immunol2004113229730214767445
- SainiSBloomDCBienemanAVasagarKTogiasASchroederJSystemic effects of allergen exposure on blood basophil IL-13 secretion and FcepsilonRIbetaJ Allergy Clin Immunol2004114476877415480314
- PrussinCGriffithDTBoeselKMLinHFosterBCasaleTBOmalizumab treatment downregulates dendritic cell FcepsilonRI expressionJ Allergy Clin Immunol200311261147115414657874
- CasaleTBBusseWWKlineJNImmune Tolerance Network GroupOmalizumab pretreatment decreases acute reactions after rush immunotherapy for ragweed-induced seasonal allergic rhinitisJ Allergy Clin Immunol2006117113414016387596
- KlunkerSSaggarLRSeyfert-MargolisVImmune Tolerance Network GroupCombination treatment with omalizumab and rush immunotherapy for ragweed-induced allergic rhinitis: Inhibition of IgE-facilitated allergen bindingJ Allergy Clin Immunol2007120368869517631952
- FariaRMcKennaCPalmerSOptimizing the position and use of omalizumab for severe persistent allergic asthma using cost- effectiveness analysisValue Health201417877278225498772
- HayashiTRazETLR9-based immunotherapy for allergic diseaseAm J Med200611910897 e1–e617000223
- NayakASTripathyILevittDNovel Amb a 1 CpG oligodeoxy-ribonucleotide conjugate ragweed vaccine administered to childrenJ Allergy Clin Immunol20061172 SupplS159
- SimonsFEShikishimaYVan NestGEidenJJHayGlassKTSelective immune redirection in humans with ragweed allergy by injecting Amb a 1 linked to immunostimulatory DNAJ Allergy Clin Immunol200411361144115115208597
- SimonsFEHayGlassKTImmunotherapy with a ragweed vaccineN Engl J Med200735618687 ; author reply 8717205611
- AsaiKFoleySCSumiYAmb a 1-immunostimulatory oligodeoxynucleotide conjugate immunotherapy increases CD4+CD25+ T cells in the nasal mucosa of subjects with allergic rhinitisAllergol Int200857437738118797179
- TulicMKChristodoulopoulosPFisetPOLocal induction of a specific Th1 immune response by allergen linked immunostimulatory DNA in the nasal explants of ragweed-allergic subjectsAllergol Int200958456557219776676
- TulicMKFisetPOChristodoulopoulosPAmb a 1- immunostimulatory oligodeoxynucleotide conjugate immunotherapy decreases the nasal inflammatory responseJ Allergy Clin Immunol2004113223524114767435
- CreticosPSSchroederJTHamiltonRGImmune Tolerance Network GroupImmunotherapy with a ragweed-toll-like receptor 9 agonist vaccine for allergic rhinitisN Engl J Med2006355141445145517021320
- BaldrickPRichardsonDWoronieckiSRLeesBPollinex Quattro Ragweed: safety evaluation of a new allergy vaccine adjuvanted with monophosphoryl lipid A (MPL) for the treatment of ragweed pollen allergyJ Appl Toxicol200727439940917299813
- PatelPHoldichTFischer von Weikersthal-DrachenbergKJHuberBEfficacy of a short course of specific immunotherapy in patients with allergic rhinoconjunctivitis to ragweed pollenJ Allergy Clin Immunol20141331121129 e1–e223870670
- FukushimaASumiTFukudaKKumagaiNNishidaTUenoHEndogenous interleukin-10 produced by antigen-irrelevant cells promotes the development of experimental murine allergic conjunctivitisInt Arch Allergy Immunol20071441798417505142
- FukushimaASumiTFukudaKInterleukin 10 and transforming growth factor beta contribute to the development of experimentally induced allergic conjunctivitis in mice during the effector phaseBr J Ophthalmol200690121535154116914468
- El BassamSPinsonneaultSKornfeldHRenFMenezesJLabergeSInterleukin-16 inhibits interleukin-13 production by allergen-stimulated blood mononuclear cellsImmunology20061171899616423044
- OzakiASekiYFukushimaAKuboMThe control of allergic conjunctivitis by suppressor of cytokine signaling (SOCS)3 and SOCS5 in a murine modelJ Immunol200517585489549716210657
- MoherDLiberatiATetzlaffJAltmanDGPRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statementPLoS Med200967e100009719621072