Abstract
Background
Asthma is a heterogeneous disease characterized by different clinical phenotypes and the involvement of multiple inflammatory pathways. During airway inflammation, many cytokines and chemokines are released and some are detectable in the sera.
Objective
Serum chemokines and cytokines, involved in airway inflammation in asthma patients, were investigated.
Methods
A total of 191 asthma patients were classified by hierarchical cluster analysis, including the following parameters: forced expiratory volume in 1 second (FEV1), eosinophil cationic protein (ECP) serum levels, blood eosinophils, Junipers asthma symptom score, and the change in FEV1, ECP serum levels, and blood eosinophils after 3 weeks of asthma therapy. Serum proteins were measured by multiplex analysis. Receiver operating characteristic (ROC) curves were used to evaluate the validity of serum proteins for discriminating between asthma clusters.
Results
Classification of asthma patients identified one cluster with high ECP serum levels, increased blood eosinophils, low FEV1 values, and good FEV1 improvement in response to asthma therapy (n=60) and one cluster with low ECP serum levels, low numbers of blood eosinophils, higher FEV1 values, and no FEV1 improvement in response to asthma therapy (n=131). Serum interleukin (IL)-8, eotaxin, vascular endothelial growth factor (VEGF), cutaneous T-cell-attracting chemokine (CTACK), growth-related oncogene (GRO)-α, and hepatocyte growth factor (HGF) were significantly different between the two clusters of asthma patients. ROC analysis for serum proteins calculated a sensitivity of 55.9% and specificity of 75.8% for discriminating between them.
Conclusion
Serum cytokine and chemokine levels might be predictors for the severity of asthmatic inflammation, asthma control, and response to therapy, and therefore might be useful for treatment optimization.
Keywords:
Introduction
Asthma is a chronic inflammatory disorder of the airways, characterized by reversible airflow obstruction, airway hyper-responsiveness, and typical clinical symptoms such as wheezing, breathlessness, and chest tightness as a result of inflammation in the airways. The heterogeneity of the clinical presentation of asthma patients suggests that different inflammatory pathways play a role in the pathogenesis of asthma.Citation1 Many cell types, including immune cells and tissue cells, are involved in asthmatic inflammation, and several molecular and cellular pathways are activated for the release of chemokines and cytokines.Citation2 In allergic asthma, allergen-specific T-helper (Th)-2 lymphocytes release interleukin (IL)-4, -9, and -13, which are essential for the production of allergen-specific immunoglobulin E (IgE).Citation3 IgE binding on the high-affinity FC ε receptor 1 (FcεR1) activates mast cells and eosinophils, which subsequently secrete inflammatory mediators.Citation4 These mediators cause bronchial smooth muscle contraction and increase airway hyper-reactivity, a cardinal feature of asthma.Citation5 Additionally, other effector T-cell subsets, like Th1 or Th17 cells, can contribute to airway inflammation.Citation6 Th17 cells are thought to be mainly involved in rhinovirus-induced asthmaCitation7 and neutrophil recruitment to the airways,Citation8 whereas Th1 cells are important for induction of apoptosis in tissue cells.Citation9
The identification of inflammatory proteins that are specific for clinical asthma phenotypes is one important approach to facilitate the diagnosis, therapy, and monitoring of asthma. Recently, it was demonstrated that the presence of serum IL-8, vascular endothelial growth factor (VEGF), and metalloproteinase-9 was associated with diisocyanate-induced asthma.Citation10 In addition, increased IL-17 levels in sera of patients with severe asthma were described,Citation11 and an association between serum IL-32 levels and treatment response in asthma patients was demonstrated.Citation12 Moreover, differences in serum cytokine levels between allergic and non-allergic asthma have been demonstrated.Citation13
In this study, we investigated serum and chemokine levels in 191 asthma patients who were classified into two groups according to several clinical and physiological parameters and the response to asthma therapy. Our hypotheses were that these two clinically different groups of asthma patients are characterized by different cytokine and chemokine serum levels and that the upregulation of cytokine and chemokine serum levels might indicate poorly controlled asthma.
Methods
Study design
Adult patients had a physician diagnosis of asthma according to Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) guidelines. To exclude chronic obstructive pulmonary disease, asthma patients had to show a reversibility in forced expiratory volume in 1 second (FEV1) in response to a short-acting β2-agonist of at least 12% predicted. Asthma patients with an acute respiratory infection were excluded. All asthma patients included in the study were admitted to, and treated for at least 3 weeks at, the high-altitude clinic Davos-Wolfgang, which is located 1,600 m above sea level in the Swiss Alps. The patients were admitted for a rehabilitation and asthma treatment optimization program and were treated according to the recent GINA guidelines; there were no acute hospitalizations. All medications related to asthma treatment and changes to it during the stay in the high-altitude clinic are shown in . To classify these patients as atopic or non-atopic, we evaluated their medical history, and skin prick tests were performed with animal dander, food allergens, pollens, fungi, and latex. The NIOX system (Aerocrine, Solna, Sweden) was used to measure fractional exhaled nitric oxide (NO) according to the manufacturer’s instructions. Blood eosinophils and eosinophil cationic protein (ECP) were analyzed in the laboratory of the high-altitude clinic. All clinical features and examinations were evaluated on the day that the patients arrived in the clinic (entry) and after 3 weeks (discharge) and are shown in . The multidisciplinary treatment at high altitude, consisting of personalized treatment plans with physiotherapy and education, aimed to achieve full asthma control with the lowest possible dose of asthma medication. The six-item Asthma Control Questionnaire (Junipers symptom score) was used to assess the level of asthma control.Citation14 Responses to each item were rated on a six-point scale; the mean was subsequently calculated and ranged between 0 (totally controlled) and 6 (severely uncontrolled). Informed consent was obtained from all asthma patients. The study was approved by the local ethical committees of the Cantons of Grissons and Zürich. Data were stored in a database and analyzed using SPSS 17.0 (SPSS Schweiz AG, Zürich, Switzerland) and Graphpad Prism 4 (GraphPad Software, Inc., La Jolla, CA, USA).
Table 1 Characterization of asthma patients
Statistical analysis and cluster formation
Because the total number of patients was limited to 191 in this study, we divided the asthma patients into two groups by hierarchical cluster analysis. Power analysis calculated a total sample size of 59 patients for each group (effect size =0.4, α=0.05, 1 − β =0.95). Pearson’s chi-squared test was used for statistical analyses of categorical variables because the datasets in each cluster were large enough and the values of cluster 1 and 2 are independent. Mann–Whitney U test was used for statistical analyses of cytokine concentrations because they were not normally distributed. Multiple testing correction was performed by the Benjamini and Hochberg false discovery rate test. Unpaired t-test analyses were used for clinical and therapy features, and the calculations are shown as mean ± standard error of the mean. The paired t-test was used to compare parameters before and after therapy. P-values below 0.05 were considered significant. Cytokines, which were significantly different between the asthma clusters, were used for principal component analysis to reduce the variables to a principal component. Subsequently, receiver operating characteristic (ROC) analysis with the identified principal component was performed.
Cytokine and chemokine measurements
Serum probes from asthma patients were taken upon entry of the patients and stored at −80°C until they were analyzed. Serum cytokines were quantified by multiplex measurements (Bioplex; Bio-Rad Laboratories, Hercules, CA, USA). Of 48 serum cytokines or chemokines, 36 were in the detection range in at least 50% of the asthma patients and used for analyses.
Results
Classification of asthma patients according to markers for clinical asthma severity and treatment response
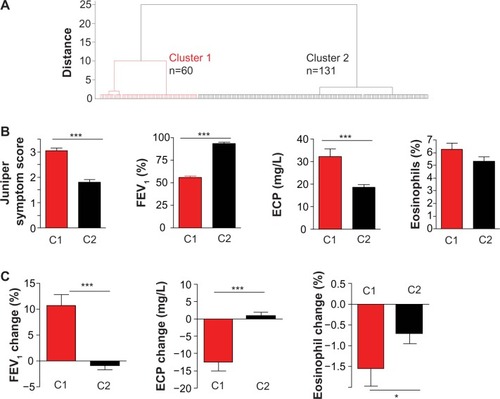
Asthma patients were classified according to Junipers symptom score, FEV1, serum ECP, circulating eosinophils, and the improvement in FEV1, ECP, and circulating eosinophils after 3 weeks of therapy in the high-altitude clinic in Davos-Wolfgang. Using hierarchical cluster analysis, two clusters of asthma patients were obtained (). Asthma patients in cluster 1 (n=60) had significantly higher Junipers symptom scores, lower FEV1 values, higher ECP serum levels, and a tendency towards higher blood eosinophils than asthma patients in cluster 2 (n=131) on the day they arrived in the clinic (). The response to asthma therapy differed between these two groups. Asthma patients in cluster 1 showed a higher FEV1 improvement, blood eosinophil decrease, and serum ECP decrease after 3 weeks of intensive asthma therapy ().
Figure 1 Classification of asthma patients according to airway inflammation.
Abbreviations: ECP, eosinophil cationic protein; FEV1, forced expiratory volume in 1 second; n, number of asthma patients.
Characterization of asthma patients in clusters 1 and 2
Asthma patients in clusters 1 and 2 were further characterized by physical examination results, medications, and questionnaire data. The frequency of asthma patients fulfilling American Thoracic Society criteria (for detailed information see http://www.thoracic.org) for severe asthma was significantly higher in cluster 1 than in cluster 2. The average age of patients in cluster 1 was higher (54.4±12.0 years) than of those in cluster 2 (48.1±15.9 years), the asthma patients in cluster 1 had significantly more asthma exacerbations over the previous 12 months and less well controlled asthma. In addition, asthma patients in cluster 1 received systemic steroids, β2-mimetics (long and short acting), and theophylline as asthma medications more frequently as at the day of entry. There was no difference between these two groups in terms of age of onset, sex, exposure to cigarette smoke, body mass index, serum IgE levels, allergic sensitization, adherence to therapy, and therapy with inhaled steroids (see ).
To further specify the response to asthma therapy, FEV1, serum ECP, blood eosinophils, and exhaled NO were measured on the day of entry and at discharge after 3 weeks. FEV1 increased significantly from 56.0%±13.6% to 66.7%±19.1% in asthma patients belonging to cluster 1, whereas there was no significant change in FEV1 in asthma patients in cluster 2 (entry 93.8%±16.3%; discharge 92.9%±18.2%, ). In addition, ECP serum levels decreased from 32.3±25.8 μg/L to 19.8±16.2 μg/L in asthma patients in cluster 1, whereas they did not change for those in cluster 2 (entry 18.7±12.5 μg/L, discharge 19.4±13.9 μg/L, ). Blood eosinophils and exhaled NO significantly decreased in both groups after 3 weeks of therapy. Concerning asthma medication, there was no significant change in systemic steroids, inhaled steroids, and long-acting β2 agonists between entry and discharge in both clusters, whereas the frequency of short-acting β2 agonist usage decreased significantly in both groups after asthma therapy ().
Figure 2 Asthma patients in cluster 1 respond better to asthma therapy.
Abbreviations: ECP, eosinophil cationic protein; FEV1, forced expiratory volume in 1 second; n, number of asthma patients; NO, nitric oxide.
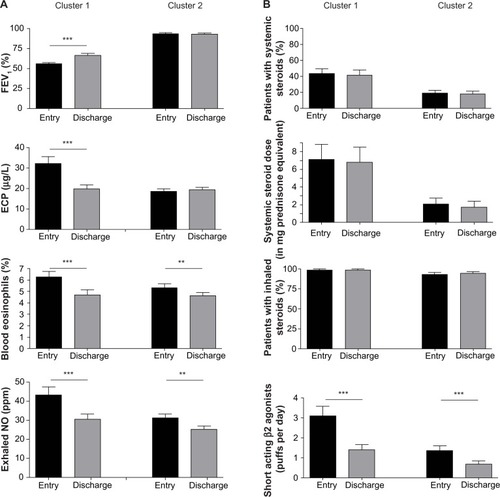
Taken together, asthma patients in cluster 1 are characterized by a less well controlled disease and better response to asthma therapy than asthma patients in cluster 2.
Increased serum inflammatory protein levels in asthma patients between cluster 1 and cluster 2
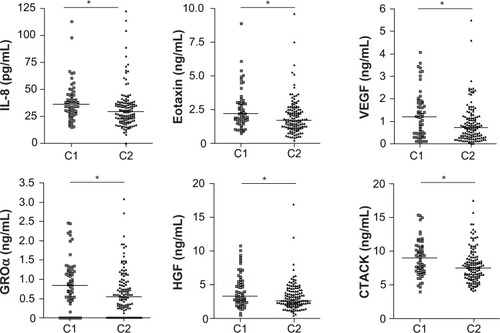
Next, inflammatory serum protein levels in all asthma patients were analyzed. IL-8, eotaxin, VEGF, cutaneous T-cell-attracting chemokine (CTACK), growth-related oncogene (GRO)-α, and hepatocyte growth factor (HGF) in the sera of asthma patients belonging to cluster 1 were significantly higher than in patients in cluster 2 (; for full names of the following cytokines and chemokines, see Table S1). There was no significant difference in IL-1Ra, IL-2, -4, -6, -9, -10, -13, -15, -16, -17, -18, IFN-γ, TNF-α, MIP-1α, MIP-1β, MIF, MIG, SCF, SCGFβ, G-CSF, IFN-α, LIF, MCP-3, MCSF, FGF, SDF-1α, MCP-1, IP-10, and TRAIL levels (see Table S1).
Figure 3 Upregulation of serum cytokines and chemokines in asthma patients belonging to uster 1.
Abbreviations: CTACK, cutaneous T-cell-attracting chemokine; GRO, growth-related oncogene; HGF, hepatocyte growth factor; IL, interleukin; VEGF, vascular endothelial growth factor.
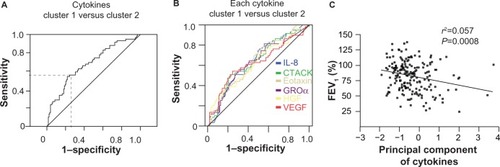
Principal component analysis of the concentrations of all cytokines and chemokines that are upregulated in cluster 1 was performed. An ROC for the principal component revealed a sensitivity of 55.9% and a specificity of 75.8% for distinguishing between asthma patients in clusters 1 and 2 (area under the curve [AUC] 0.683; ). The AUC for single cytokines was lower than the principal component analysis of all upregulated cytokines in cluster 1 (AUC IL-8: 0.645; CTACK: 0.639; eotaxin: 0.640; GROα: 0.632; HGF: 0.625; VEGF: 0.627; ). In addition, there is a significant negative correlation between cytokine serum levels of the upregulated cytokines in cluster 1 and FEV1 ().
Figure 4 Prognostic value of serum cytokines to distinguish between asthma patients belonging to cluster 1 or 2.
Abbreviations: CTACK, cutaneous T-cell-attracting chemokine; FEV1, forced expiratory volume in 1 second; GRO, growth-related oncogene; HGF, hepatocyte growth factor; IL, interleukin; ROC, receiver operating characteristic; VEGF, vascular endothelial growth factor.
Discussion
Using hierarchical cluster analysis with the clinical routine parameters FEV1, ECP serum levels, blood eosinophil levels, or Junipers asthma symptom score, we were able to classify our population of asthma patients into two groups. One group of asthma patients (cluster 1) was characterized by higher ECP serum levels, higher numbers of circulating eosinophils, lower FEV1 values, and better response to asthma therapy. Further characterization of these asthma patients demonstrated that they had less well controlled asthma with more exacerbations over the previous year. Importantly, these asthma patients had higher serum levels of certain pro-inflammatory cytokines and chemokines. In addition, the profile of serum cytokines could predict, with a sensitivity of 55.9% and specificity of 75.8%, the cluster to which asthma patients belonged.
Asthma is a heterogeneous disease, which could be divided into subgroups according to therapy response, fixed airway obstruction, obesity, or trigger factors such as allergens, air pollution, occupational irritants, cigarette smoke, aspirin, and exercise.Citation15 The identification of asthma phenotype-specific inflammatory pathways is one important approach to improve the diagnosis and treatment of asthma. In this context, the term endotypeCitation16 was recently introduced, which suggests that asthma phenotypes are characterized by certain inflammatory mechanisms, which correlate with treatment response.Citation1 Accordingly, we classified the asthma patients into two groups by parameters used routinely in our clinic and that reflected clinical asthma severity and response to asthma treatment. Serum cytokine and chemokine levels were measured to investigate if different inflammatory pathways were activated in asthma patients from clusters 1 and 2. Interestingly, in asthma patients from cluster 1, higher serum levels of IL-8, eotaxin, VEGF, CTACK, GROα, and HGF were present, indicating that different inflammatory and molecular mechanisms were activated. In addition, IL-17 had the tendency to be elevated in cluster 1 compared with cluster 2. Serum IL-17 is elevated in severe asthmaCitation11 and amplifies local airway inflammation by induction of IL-6 in bronchial epithelial cellsCitation17 or IL-8 in human airway epithelial cells.Citation18 IL-17 and Th2 cytokine-producing T-cells also promote asthmatic inflammation via the upregulation of eotaxin in bronchial epithelial cells.Citation19 In addition, in bronchial biopsies from asthmatic patients, there is a high expression of VEGF,Citation20 which is a key regulator of blood vessel growth in the airways of asthma patients via the promotion of proliferation and differentiation of endothelial cells and inducing vascular leakage and increased permeability.Citation21 One possible explanation for elevated inflammation-related cytokines and chemokines in the sera of asthma patients with lower FEV1 values and more frequent exacerbations may be that airway inflammation may lead to the generation of large amounts of cytokines, which may enter into the circulation, resulting in elevated serum concentrations. Therefore, the upregulation of certain cytokines and chemokines in the sera may be an indicator for physicians to optimize or initiate asthma treatment. Interestingly, asthma patients in cluster 1 and 2 were all mostly compliant with the medication therapy, indicating that the FEV1 improvement in asthma patients with clinically more severe asthma (cluster 1) might be due to treatment optimization and not to an improvement in medication compliance.
Although treatment management of asthma patients by measurement of sputum eosinophils could decrease the number of asthma exacerbations and steroid dose,Citation22 eosinophils may not be useful in the prediction of exacerbations for all asthma types because, besides eosinophilic asthma, neutrophilic, mixed granulocytic, and paucigranulocytic asthma subgroups also exist.Citation23 In addition, the induction of induced sputa may not be technically possible in all asthma patients. Analysis of serum eosinophils is a simple method that is routinely undertaken for asthma patients. However, in our study, serum eosinophils were not significantly elevated in patients with clinically more severe asthma (cluster 1) in contrast with the identified serum proteins, suggesting that they are also upregulated in exacerbated non-eosinophilic asthma. The higher levels of pro-inflammatory cytokines and chemokines in the serum indicate asthma patients with poorly controlled asthma. Therefore, measuring serum cytokines as a diagnostic tool might be useful for the optimization of asthma treatment. However, this study has not assessed the direct effect of asthma medications on serum cytokines. Therefore, the effect of asthma treatment on the serum cytokines upregulated in cluster 1 should be investigated in future studies. Less invasive methods for the assessment of local airway inflammation include analysis of nitratesCitation24 or pH valuesCitation25 in exhaled breath condensates, which are related to asthma control and may be interesting tools for asthma management in the future. These methods may also be combined with the measurement of serum cytokines and chemokines.
Repeated airway inflammation causes structural airway changes, known as airway remodeling, including smooth muscle hypertrophy, goblet cell hyperplasia, subepithelial fibrosis, and angiogenesis.Citation21 These structural changes influence the reversibility of airway obstruction and increase disease severity.Citation26 Inflammatory markers, which can predict the severity of airway inflammation, could be important for optimal asthma treatment decisions to avoid airway remodeling caused by chronic inflammation. We demonstrate that certain serum cytokines and chemokines could identify asthma patients with clinically more severe asthma and better treatment response.
Conclusion
Certain pro-inflammatory serum cytokines and chemokines are important markers for the severity and activity of asthma, asthma control, and treatment response. The assessment of systemic immune response by serum levels of cytokines and chemokines in asthma patients might be an important tool for monitoring asthma patients and for asthma therapy optimization.
Supplementary material
Table S1 Serum cytokine and chemokine levels in asthma patients belonging to cluster 1 or to cluster 2
Disclosure
The NM, CAA and GM are supported by the European Allergy and Asthma Center Davos (EACD), SNF grants No 32-132899 and the Christine Kühne Center for Allergy Research and Education (CK-CARE).
References
- LötvallJAkdisCABacharierLBAsthma endotypes: a new approach to classification of disease entities within the asthma syndromeJ Allergy Clin Immunol2011127235536021281866
- KimHYDeKruyffRHUmetsuDTThe many paths to asthma: phenotype shaped by innate and adaptive immunityNat Immunol201011757758420562844
- RobinsonDSHamidQYingSPredominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthmaN Engl J Med199232652983041530827
- WalkerCBauerWBraunRKActivated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophiliaAm J Respir Crit Care Med19941504103810487921434
- AkdisCAAkdisMMechanisms and treatment of allergic disease in the big picture of regulatory T cellsJ Allergy Clin Immunol20091234735746 quiz 747–74819348912
- PalomaresOYamanGAzkurAKAkkocTAkdisMAkdisCARole of Treg in immune regulation of allergic diseasesEur J Immunol20104051232124020148422
- WiehlerSProudDInterleukin-17A modulates human airway epithelial responses to human rhinovirus infectionAm J Physiol Lung Cell Mol Physiol20072932L505L51517545490
- HellingsPWKasranALiuZInterleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthmaAm J Respir Cell Mol Biol2003281425012495931
- MeyerNZimmermannMBürglerSIL-32 is expressed by human primary keratinocytes and modulates keratinocyte apoptosis in atopic dermatitisJ Allergy Clin Immunol20101254858865.e1020227751
- KimJHKimJEChoiGSHimHYYeYMParkHSSerum cytokines markers in toluene diisocyanate-induced asthmaRespir Med201110571091109421439806
- AgacheICiobanuCAgacheCAnghelMIncreased serum IL-17 is an independent risk factor for severe asthmaRespir Med201010481131113720338742
- MeyerNChristophJMakriniotiHInhibition of angiogenesis by IL-32: possible role in asthmaJ Allergy Clin Immunol20121294964973.e722336080
- PukelsheimKStoegerTKutschkeDGangulyKWjstMCytokine profiles in asthma families depend on age and phenotypePLoS One2010512e1429921179211
- JuniperEFO’ByrnePMGuyattGHFerriePJKingDRDevelopment and validation of a questionnaire to measure asthma controlEur Respir J199914490290710573240
- BalzarSStrandMNakanoTWenzelSESubtle immunodeficiency in severe asthma: IgA and IgG2 correlate with lung function and symptomsInt Arch Allergy Immunol200614029610216557027
- AndersonGPEndotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous diseaseLancet200837296431107111918805339
- BurglerSOuakedNBassinCDifferentiation and functional analysis of human T(H)17 cellsJ Allergy Clin Immunol20091233588595595.e1e719178935
- JonesCEChanKInterleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cellsAm J Respir Cell Mol Biol200226674875312034575
- WangYHVooKSLiuBA novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthmaJ Exp Med2010207112479249120921287
- HoshinoMNakamuraYHamidQAGene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthmaJ Allergy Clin Immunol200110761034103811398081
- DetorakiAGranataFStaibanoSRossiFWMaroneGGenoveseAAngiogenesis and lymphangiogenesis in bronchial asthmaAllergy201065894695820415716
- GreenRHBrightlingCEMcKennaSAsthma exacerbations and sputum eosinophil counts: a randomised controlled trialLancet200236093471715172112480423
- SimpsonJLScottRBoyleMJGibsonPGInflammatory subtypes in asthma: assessment and identification using induced sputumRespirology2006111546116423202
- MalinovschiAPizzimentiSSciasciaSHefflerEBadiuIRollaGExhaled breath condensate nitrates, but not nitrites or FENO, relate to asthma controlRespir Med201110571007101321277184
- KostikasKPapaioannouAITanouKExhaled NO and exhaled breath condensate pH in the evaluation of asthma controlRespir Med2011105452653221051211
- Tillie-LeblondIde BlicJJaubertFWallaertBScheinmannPGossetPAirway remodeling is correlated with obstruction in children with severe asthmaAllergy200863553354118394127
