Abstract
Purpose
To investigate the clinical and cost effectiveness of switching real-life asthma patients from other types of inhalers to the Easyhaler® (EH) for the administration of inhaled corticosteroids (ICS).
Patients and methods
Historical, matched-cohort study of 1,958 asthma patients (children and adults) treated in UK primary-care practices, using data obtained from the Optimum Patient Care Research Database and Clinical Practice Research Datalink. Other inhalers (OH) included pressurized metered-dose inhalers, breath-actuated inhalers, and dry-powder inhalers, delivering beclomethasone, budesonide, fluticasone, or ciclesonide. Patients remaining on OH unchanged (same drug, dosage, and device; n=979) were matched 1:1 with those switched to the EH (beclomethasone or budesonide) at the same or lower ICS dosage (n=979), based on age, sex, year of index patient review/switch, most recent ICS drug, dosage, and device, and the number of severe exacerbations and average daily short-acting β2 agonist (SABA) dosage in the preceding year. Clinical outcomes and health care costs were compared between groups for 12 months before and after the switch. Co-primary clinical outcomes were: 1) risk domain asthma control (RDAC) – no asthma-related hospitalization, acute oral steroid use, or lower respiratory tract infection (LRTI); 2) exacerbation rate (American Thoracic Society [ATS] definition) – where exacerbation is asthma-related hospitalization or acute oral steroid use; 3) exacerbation rate (clinical definition) – where exacerbation is ATS exacerbation or LRTI; and 4) overall asthma control (OAC) – RDAC plus average salbutamol-equivalent SABA dosage ≤200 μg/day. Non-inferiority (at least equivalence) of EH was tested against OH for the four co-primary outcomes in order (hierarchical approach) by comparing the difference in proportions of patients [EH-OH] achieving asthma control or having no exacerbations in the outcome year, using a limit of 10% difference.
Results
Non-inferiority was shown for the EH for all four co-primary outcomes. There were no significant differences between groups for RDAC or exacerbation rates, but EH patients were significantly more likely to achieve OAC (adjusted odds ratio [95% confidence interval]: 1.26 [1.05, 1.52]), as significantly more EH than OH patients had an average SABA dosage of ≤200 μg/day (52% versus 47%, respectively; P<0.001). Mean asthma-related health care costs increased from baseline to outcome years in both groups, but SABA costs increased significantly more in OH than EH patients (mean difference £5.5/patient/year) and consultation costs decreased significantly more in EH than OH patients (mean difference £13.5/patient/year).
Conclusion
Typical asthma patients may be switched from other ICS devices to the Easyhaler® with no reduction in clinical effectiveness or increase in cost.
Introduction
The Easyhaler® (EH; Orion Pharma UK Ltd, Newbury, Berkshire, England) is a dry-powder inhaler (DPI) that may be prescribed for the treatment of asthma.Citation1 In experimental studies and randomized controlled clinical trials, the EH has been shown to be at least equivalent in lung deposition and/or clinical efficacy to other DPIs or pressurized metered-dose inhalers (pMDI) for a variety of asthma medications, including inhaled corticosteroids (ICS),Citation2–Citation10 short-acting β2 receptor agonists (SABA),Citation11–Citation18 and long-acting β2 receptor agonists (LABA).Citation19
In the UK, the EH is available for the delivery of beclomethasone, budesonide, formoterol, or salbutamol.Citation1 Physicians may choose the EH over another type of inhaler for a number of reasons. First, unlike some other DPIs in common use, which require inspiratory flow rates of 45–60 L/minute for optimal drug delivery,Citation20–Citation23 the EH generates a consistent therapeutic dose at inspiratory flow rates as low as 28 L/minute.Citation12,Citation23,Citation24 Even young children with asthmaCitation11,Citation12 and elderly patients with chronic obstructive pulmonary disease (COPD)Citation25 can usually manage inspiratory flow rates of at least 28 L/minute.
Second, the EH generates a consistent emitted dose and fine-particle fraction across a range of inspiratory flow rates from 30 to 90 L/minute, in contrast to a widely used DPI which showed greater variability in emitted dose at any flow rate and a fine-particle dose that was flow-rate dependent.Citation23,Citation26 Thus, the patient is more likely to receive the same dose of drug every time with the EH, even at low or variable inspiratory flow rates.
Third, patient satisfaction generally is better with the EH than with other inhalers. In numerous studies comparing the EH with other inhalers, most people with asthma or parents of children with asthma expressed a preference for the EH.Citation2–Citation6,Citation27 In a meta-analysis of nine clinical trials, the EH was clearly preferred over the pMDIs and other DPIs evaluated. In particular, the EH was favored by patients for its ease of use, learning how to use, dosing, and inhaling through the device.Citation28 One of the features for which the EH repeatedly fared better among patients was the perception of drug inhalation, or receiving the powder from the device and thus controlling inhalation of the powder.Citation27,Citation29
A fourth consideration is that the EH is less expensive than most other DPIs. Thus, there are several potential advantages to switching asthma patients on ICS therapy from another type of inhaler to the EH. However, the clinical effectiveness of such a switch has not been investigated in a large and diverse population of asthma patients, ie, in real-life asthma care – where critical inhalation errors are both common and various.Citation20,Citation21,Citation29–Citation34
Critical inhalation errors are defined as any error in the handling or use of the inhaler that is likely to significantly impair the delivery of adequate medication, and they have been documented for all inhaler types.Citation30 Because they reduce the delivered dose of medication, critical errors compromise asthma control and thus increase the costs of asthma management.Citation30 As demonstration of correct inhaler technique is typically a prerequisite for inclusion in controlled clinical trials evaluating the efficacy of inhaled medications or comparing different inhalers,Citation20,Citation31,Citation35,Citation36 the applicability of such trials to asthma patients in real life is limited. In fact, it has been observed that typical asthma patients make many errors – even with their regular inhaler – which may negate the benefits documented in controlled clinical trials.Citation30–Citation33
The technical differences among the various ICS devices, even within the same class of device (such as DPIs), are sufficient that a switch from one type of inhaler to another increases the potential for critical handling or inhalation errors.Citation22,Citation29,Citation31,Citation34 Switching a patient from one inhaler to another without an accompanying face-to-face consultation may further compromise asthma control,Citation37,Citation38 yet switching by electronic review or correspondence occurs with some frequency in clinical practice.
Hence, we investigated the results of switching from any other inhaler to the EH for the delivery of ICS therapy in a large and diverse population of asthma patients (children and adults) in UK primary-care practice. We focused the comparisons on the inhaler devices by limiting the study to asthma patients who were relatively stable on their current ICS therapy. Our hypothesis was that switching these patients from another type of inhaler to the EH in a real-life setting would nevertheless result in a significant reduction in clinical effectiveness and thus a significant increase in the costs of on-going asthma therapy.
Material and methods
Study design and patients
We conducted an historical, matched-cohort study of asthma patients in the UK treated in primary-care practice and prescribed ICS therapy. We obtained the patient data from two large, anonymized UK primary-care databases: the Optimum Patient Care Research Database (OPCRD)Citation39 and the Clinical Practice Research Datalink (CPRD),Citation40 formerly called the General Practice Research Database (GPRD). Both databases were examined for suitable patients spanning the period from January 2005 to December 2012. We took care to avoid duplication of individual patients by cross-referencing the databases using patient characteristics.
Qualifying patients comprised two groups: 1) those remaining on any other inhaler (OH) than the EH for the duration of the investigation period; and 2) those who were switched from an OH to the EH. For each patient, we studied the medical records for two consecutive years: a baseline year preceding the index prescription date (IPD) on which the physician either continued the patient on an OH or switched the patient to the EH, and an outcome year following the IPD ().
Figure 1 Schematic of the study design.
Abbreviations: EH, Easyhaler®; ICS, inhaled corticosteroid; OH, other inhaler.
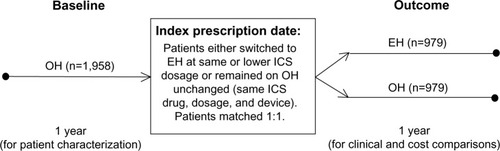
In order to ensure that the OH group was limited to patients whose physician clearly intended to continue the patient on the current ICS therapy unchanged, the OH group was restricted to patients who, at IPD, remained on the same drug, dosage, and device as their most recent prescription. But in order to ensure as large a study group as possible, we placed no such restrictions on the much smaller pool of potential EH patients, who were permitted a change of ICS drug (to beclomethasone or budesonide) and a decrease in ICS dosage, in addition to the change of device (from OH to EH) at IPD. The study population included smokers and ex-smokers in addition to nonsmokers and patients whose smoking status was not recorded. However, patients were excluded if they were prescribed a fixed-dose combination inhaler during their baseline year, they had more than one ICS switch at IPD, or they did not have complete data for both the baseline and outcome years.
To ensure that we were comparing the two treatments in similar patients, we matched the patients remaining on an OH with those switched to the EH in a ratio of 1:1, based on several demographic and clinical characteristics: age, sex, year of IPD, most recent ICS prescription (drug, dosage, and device), and the number of severe exacerbations and average daily SABA dosage during the baseline year (). Thus, within the demographic categories, EH and OH patients were matched on the drugs and dosages required to achieve comparable asthma control during their baseline year.
Table 1 Matching criteria
Clinical outcomes
As definitions of asthma control and acute exacerbation differ among organizations and studies, we examined several clinical outcome measures, encompassing various combinations of asthma-related hospital attendance, use of acute oral steroids, general practitioner (GP) consultations for lower respiratory tract infections (LRTI) requiring antibiotic therapy, average daily SABA usage, and changes in asthma therapy, in addition to controller–reliever ratios during the year of interest. In all, eight simple or composite measures of clinical effectiveness were compared between treatment groups: four co-primary outcomes () and four secondary outcomes (). For the purpose of investigating the non-inferiority (clinical equivalence) of EH against OH, the four co-primary outcomes were analyzed using a four-tier hierarchical approach, as described in the statistical analysis section.
Table 2 Co-primary measures of clinical effectiveness
Table 3 Secondary measures of clinical effectiveness
Health economic outcomes
We examined asthma treatment costs in both groups for the baseline and outcome years. Total asthma-related health care costs were calculated from the costs of asthma drugs, primary-care asthma consultations, and respiratory-related hospital attendance/admission. Drug costs included ICS, oral corticosteroids for acute use, SABA, LABA, leukotriene receptor antagonists (LTRA), theophylline, antibiotics prescribed for LRTI, and other respiratory drugs.
Quantities of resources used were obtained from the patient databases and multiplied by unit costs to produce total costs. Unit costs for asthma drugs were obtained from the Prescription Service of the National Health Service (NHS) Business Services Authority via the Dictionary of Medicines and Devices.Citation41 Drug unit prices were converted to 2010 prices for statistical analysis. Unit costs for GP consultations were derived from the Personal Social Services Research Unit report: Unit Costs of Health and Social Care 2011,Citation42 assuming an average consultation duration of 11.7 minutes. Hospital usage costs were obtained from the NHS Reference Costs 2010–2011.Citation43
Statistical analysis
We carried out all analyses using SPSS Statistics version 20 (IBM Corporation, Armonk, NY, USA), SAS version 9.3 (SAS Institute Inc., Cary, NC, USA), and Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA, USA). We defined statistically significant results as P<0.05, and trends as P≥0.05 but <0.10. We report mean values with their standard deviations (SD), median values with their interquartile ranges (IQR; percentiles 25 and 75), and adjusted odds/rate ratios and differences in proportions with their 95% confidence intervals (CI). Dosages for SABA drugs are reported as salbutamol-equivalents and dosages for ICS drugs are reported as beclomethasone dipropionate (BDP)-equivalents.
First, we conducted exploratory data analysis for all variables of interest, for both the baseline and outcome years. As a conservative approach, we considered differences between treatment groups as possibly important if P<0.10. We examined variables meeting this criterion for co-linearity and clinical importance to select those used as potential confounders (Figure S1) in the regression modeling of outcomes.
Next, we performed multivariate analyses using the full data set and each data split to identify baseline variables that are predictive (P<0.05) of each outcome variable during the outcome year. These baseline variables were considered as potential confounders in the regression modeling of outcomes. Spearman correlation coefficients were calculated among all potential confounders to determine strengths of linear relationships between variables. Correlation coefficients were considered, in conjunction with clinical interpretation, to identify pairings of variables that may present co-linearity issues at the modeling stage. We then used scatter plots and error-bar plots to further investigate nonlinear relationships. When unadjusted and adjusted results were subsequently compared, no disparities were found in the direction of any differences between treatment groups before and after adjusting for confounders.
Clinical outcomes
Non-inferiority analysis
Our primary goal was to determine whether the EH is clinically non-inferior (at least equivalent) to the OH in this diverse patient population. To that end, we used a four-tier hierarchical approach for analysis of the co-primary clinical effectiveness measures. The four co-primary outcomes were assessed in pre-determined order, as presented in , using pre-specified acceptance limits. In order to claim non-inferiority for an outcome on a lower level, it must first be demonstrated for the preceding outcome(s) in the hierarchy. If any outcome failed to meet the pre-specified limit, then any subsequent primary outcome(s) would be changed to a secondary outcome, which we analyzed singly.
The pre-specified acceptance limit was the demonstration of non-inferiority of the EH compared with the OH as follows: a) for the measures of asthma control, the difference in the proportions of patients (EH – OH) achieving asthma control has a lower 95% CI of ≥−10%; and b) for the exacerbation rates, the difference in proportions of patients having no exacerbations has an upper 95% CI of ≤10%. Subsequent power calculations using an equivalence model confirmed that, with group numbers of 979 each, there was sufficient power (99%) to detect a 10% difference in proportions between EH and OH.
Odds/rate ratios and other summary statistics
We compared the adjusted odds of achieving risk domain asthma control between matched treatment groups using conditional binary logistic regression models. Asthma control status (controlled/uncontrolled) was used as the dependent variable, with treatment and potential confounding factors as explanatory variables. The exacerbation rates (both definitions) were compared between treatment groups using a conditional Poisson regression model to obtain an estimate of relative exacerbation rates. The model used empirical standard errors (for more conservative CI estimations) and adjustments were made for potential baseline confounders. We compared the adjusted odds of achieving overall asthma control between matched treatment groups using conditional binary logistic regression models, as described above for risk domain asthma control.
For the secondary outcomes, we compared the adjusted odds of achieving treatment stability (both definitions) between matched treatment groups using conditional binary logistic regression models. Treatment stability status (stable/unstable) was used as the dependent variable, with treatment and potential confounding factors as explanatory variables. The adjusted odds of being in a higher SABA usage category were compared between matched treatment groups using conditional ordinal logistic regression models. The SABA category () was used as the dependent variable, with treatment and potential confounding factors as explanatory variables.
In addition, we compiled summary statistics for the controller–reliever ratio by treatment group. The controller–reliever ratio was categorized (<0.5 and ≥0.5), and patient numbers and percentages determined for each category. Unadjusted conditional logistic regression (stratified by matching) was used to generate P-values for both the summary and categorized data.
Health economic outcomes
After calculating total asthma-related health care costs, asthma drug costs, consultation costs, and hospitalization costs for each treatment group for the baseline and outcome years, we compared summary costs between matched treatment groups using conditional logistic regression. These analyses were repeated for changes in costs between baseline and outcome years for each treatment group.
Results
Patients and devices
Of the 3,706,575 patients initially identified who had repeat prescriptions for an ICS, 1,029 were switched from an OH to the EH at the same or lower ICS dosage. After 1:1 matching, 979 patients remained in the EH group and 979 uniquely matched patients were in the OH group. Of this total, 281 OH patients (28.7%) and 643 EH patients (65.7%) had a face-to-face consultation at IPD; the remaining 698 OH patients (71.3%) and 336 EH patients (34.3%) had just an electronic review at IPD.
The baseline characteristics of both patient groups are summarized in Tables S1 and S2. Despite patient matching, some statistically significant differences remained. More EH than OH patients had significant co-morbidities, as expressed by a Charlson co-morbidity index (CCI)Citation44 score >0 (51.5% and 41%, respectively; Table S1), but there were no significant differences between treatment groups in the incidence of gastroesophageal reflux disease, cardiac disease, or ischemic heart disease (data not shown). The incidence of rhinitis was higher in OH than EH patients (30% and 24%, respectively; Table S1), but as CCI score and rhinitis were included as confounding factors (Figure S1), these baseline rates and differences were accounted for in the outcome analyses.
More importantly, patients in the EH group had fewer ICS prescriptions and inhalers, lower average daily ICS dosages, and lower controller–reliever ratios, but more asthma-related consultations with their primary-care physicians and a higher rate of asthma-related hospital outpatient department attendance than patients in the OH group. There were no significant differences in SABA prescriptions or daily SABA usage between groups, but LABA usage was significantly lower in the EH than the OH group. Given that there were no significant differences between treatment groups for any of the four co-primary clinical effectiveness measures (Table S2), these findings suggest different patterns of self-management between the two groups; in particular, the patients who would be switched to the EH at IPD appeared to use less ICS or be less compliant with their ICS prescriptions and thus sought physician services more often.
Most of the devices compared with the EH were pMDIs (62.3% of all devices); breath-actuated inhalers (BAIs) represented 18.6% of devices, and DPIs other than the EH comprised the remaining 19.1% (Table S3). Thus, 81% of EH patients switched from a non-DPI to the EH and the other 19% switched from another type of DPI to the EH. Beclomethasone was the predominant ICS (84.5%) used in both groups prior to IPD, but thereafter patients in the EH group were fairly equally divided between beclomethasone and budesonide. Budesonide, fluticasone, and ciclesonide accounted for <20% of all ICS prescriptions in the OH group. In the EH group, 325 patients (33.2%) had a change of ICS drug and 286 patients (29.2%) had a decrease in ICS dosage at IPD.
Also of note, only 53 patients (2.7%) were also prescribed a long-acting muscarinic antagonist (LAMA): 31 EH patients (3.2%) and 22 OH patients (2.2%). Of those on LAMA therapy, 45 patients (2.3%) were ≥40 years of age and current or former smokers: 26 EH patients (2.7%) and 19 OH patients (1.9%). Thus, the likely incidence of COPD instead of, or in addition to, asthma in this study population was <3%.
Clinical outcomes
The EH was shown to be non-inferior to the OH for all four co-primary clinical outcomes: the differences in proportions of patients achieving asthma control had lower 95% CIs of >−10% and the differences in proportions of patients having no severe exacerbations had upper 95% CIs of <10% (). After adjusting for baseline confounders, there were no significant differences between treatment groups for risk domain asthma control or severe exacerbations (both definitions) during the outcome year, but patients in the EH group were significantly more likely to achieve overall asthma control than were those in the OH group ().
Table 4 Comparison of co-primary measures of clinical effectiveness between the matched treatment groups during the outcome year
The difference between risk domain and overall asthma control lies in SABA usage, specifically the average daily prescribed SABA dosage (). During the outcome year, EH patients were significantly less likely than OH patients to be in one of the higher SABA usage categories (). During the baseline year, the number of patients on a salbutamol-equivalent SABA dosage of ≤200 μg/day was identical (560 patients, or 57%; Table S2) in both treatment groups, SABA dosage being a matching criterion. However, while average daily SABA dosages generally increased in both treatment groups between baseline and outcome years (), significantly more EH than OH patients remained on a SABA dosage of ≤200 μg/day (52% and 47%, respectively; ).
Figure 2 Comparison of average daily SABA dosages between baseline and outcome years for the matched treatment groups.
Abbreviations: EH, Easyhaler®; OH, other inhalers; SABA, short-acting β2 receptor agonist.
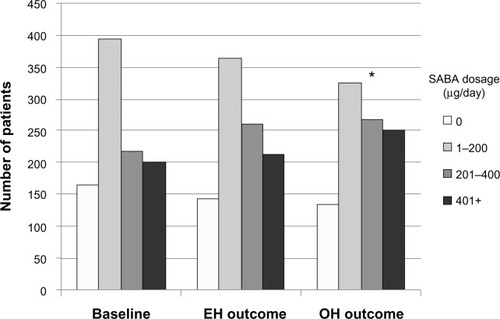
Table 5 Comparison of secondary measures of clinical effectiveness between the matched treatment groups during the outcome year
Table 6 Disaggregated components of the clinical effectiveness measures between the matched treatment groups during the outcome year
The importance of these differences in SABA usage is further reflected in the changes in risk domain and overall asthma control status between baseline and outcome years. Whereas the number of patients achieving risk domain asthma control increased slightly in both groups, from 66%–67% at baseline (Table S2) to 72%–73% in the outcome year (), the number of patients achieving overall asthma control dropped substantially over the same period. Approximately 60% of patients achieved overall asthma control at baseline (Table S2), but only 36% (OH) and 41% (EH) achieved overall asthma control during the outcome year (P=0.016; ). As SABA usage is the single factor that differentiates risk domain and overall asthma control, these small differences evidently played a significant, albeit indirect, role in the clinical effectiveness of the EH, in that the EH patients used significantly less SABA for comparable asthma control.
The other secondary clinical outcomes are summarized in . Patients in the EH group were significantly less likely to achieve treatment stability (definition 1) than those in the OH group, but there was no significant difference in the odds of achieving treatment stability (definition 2) between groups. The key difference between these two indices is that a change in ICS drug or device is included in the first but not in the second definition of treatment stability. Significantly more EH than OH patients had a change in ICS drug or device during the outcome year (), which may explain the difference in outcomes for the two treatment stability definitions. Even so, only 17.5% of EH patients had a change in ICS drug and 22% a change in ICS device during the outcome year. Thus, the EH evidently was well accepted by patients for the year after the switch.
While significantly more EH than OH patients had an increase in ICS dosage during their outcome year (12.8% and 7.8%, respectively; ), 29% of EH patients had undergone a decrease in ICS dosage at IPD. From these data, it appears possible that some EH patients may have been inappropriately stepped down at IPD; if so, then the increase in ICS dosage during the outcome year may simply have been a return to an effective ICS dosage in those patients. In both treatment groups, ICS dosages generally increased between baseline and outcome years (), but ICS dosages remained significantly higher in the OH than the EH patients (Tables S2 and S4).
Figure 3 Comparison of average daily ICS dosages between baseline and outcome years for the matched treatment groups.
Abbreviations: BDP, beclomethasone dipropionate; EH, Easyhaler®; ICS, inhaled corticosteroid; IPD, index prescription date; OH, other inhalers.
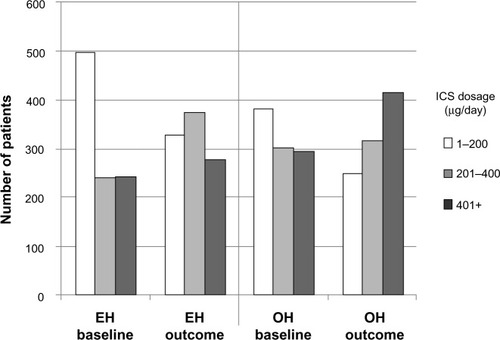
Just as in the baseline year, EH patients had significantly fewer ICS prescriptions and inhalers, lower average daily ICS dosages, and less LABA usage than OH patients (Table S4). However, there were no longer any significant differences between treatment groups in controller–reliever ratios or primary-care asthma consultations, which suggests better self-management in the EH patients during the outcome year compared with the baseline year. The incidence of asthma-related hospital outpatient attendance, however, remained significantly different between treatment groups and essentially unchanged from baseline. No other types of respiratory-related hospital attendance/admission were significantly different between treatment groups (Table S4).
Health economic outcomes
There was no significant difference in total asthma-related health care costs between treatment groups during the baseline year (). There were, however, significant differences between groups in specific cost components. Asthma drug costs were significantly lower in the group who would be switched to the EH at IPD, owing in large part to the lower number of ICS inhalers and thus ICS costs in these patients. However, these lower drug costs evidently were offset by the significantly higher number, and thus cost, of primary-care asthma consultations and hospital outpatient attendances in the EH group. The number and cost of SABA inhalers were comparable between treatment groups.
Table 7 Comparison of asthma-related health care costs (£/patient/year) between matched treatment groups for baseline and outcome years, including changes (Δ) in costs from baseline to outcome years
During the outcome year, total asthma-related health care costs were significantly lower in the EH group, driven largely by lower asthma drug costs (ICS and SABA), as asthma-related consultations and costs were no longer significantly different from those in the OH group (). Asthma-related hospital outpatient attendance costs remained significantly different between groups and were essentially unchanged from baseline.
Mean asthma-related health care costs increased from baseline to outcome years in both treatment groups, with the exception of primary-care asthma consultation costs, which decreased in both groups. The changes in costs from baseline to outcome years were not significantly different between groups for total asthma-related health care costs, asthma drug costs, ICS costs, or hospital outpatient attendance costs (). However, SABA costs increased significantly more in the OH than the EH group (mean difference of £5.5/patient/year) and asthma consultation costs decreased significantly more in the EH than the OH group (mean difference of £13.5/patient/year). Thus, switching to the EH in this patient population was not more costly than remaining on the same ICS drug, dosage, and device, and for some measures it reduced the costs of on-going asthma therapy, for equivalent clinical effectiveness.
Discussion
The aim of our study was to critically evaluate the clinical and cost effectiveness of switching real-life asthma patients from any other type of ICS device to the EH. Our findings showed that both aspects of our hypothesis – that such a switch would result in a significant reduction in clinical effectiveness and thus a significant increase in the costs of asthma therapy – were disproven.
By examining a large and diverse population of asthma patients – from young children to elderly patients, and including smokers, patients with co-morbidities, and those with poor treatment compliance – we sought to represent the challenges typically faced in primary-care asthma management and the circumstances under which a physician might switch a patient from one ICS device to another. In an effort to include as many EH patients as possible within the limits of our study parameters, the EH was compared not only with other DPIs but also with pMDIs and BAIs, and across four different ICS drugs and a range of ICS dosages that in about one-third of patients were substantially different between matched cohorts after the switch.
Arguably, more specific associations of clinical importance may have been identified if our inclusion criteria had been more refined. For example, a comparison limited to the EH with other DPIs only (no pMDIs or BAIs) may have been of value, as might limiting the investigation to one ICS drug delivered by EH or OH. However, doing so within our present study framework would have reduced group numbers considerably; for example, a comparison of the EH with other DPIs would have reduced the group numbers to only 187 patients each.
One of the strengths of our study is that we were able to include almost 980 patients in each treatment group, and we examined the outcome of the switch against a backdrop of real-life asthma management. In addition, our study reviewed data for a full 12 months before and after the switch. In contrast, most randomized controlled clinical trials typically have very strict selection criteria which result in a homogeneous but often poorly representative study population, relatively small numbers of patients, and relatively short data collection periods.Citation4–Citation6,Citation35,Citation36 By comparing various other ICS devices with the EH, and by retrospectively and remotely examining the outcome of the switch, we documented the results when primary-care physicians switched their asthma patients from another ICS device to the EH, regardless of which device the patient was currently using. In this respect, our study design put the EH to a very challenging test.
By the same token, one of the drawbacks of a study of this size and scope is that small baseline differences inevitably remained between treatment groups, despite patient matching on several clinically relevant variables. One such difference was the incidence of potentially important co-morbidities, as expressed by the CCI. However, the higher incidence of co-morbidities in the EH group may strengthen the case for the EH, although both of the exacerbation rate ratios (ATS/ERS and clinical definitions) in the outcome year were adjusted for CCI score (), thus reducing the impact of this baseline difference.
Another difference worth noting is the incidence of hospital outpatient attendance during the baseline year, which, while low in both groups, was higher in the EH group. However, this finding may be noteworthy only for its role as a red herring: the rates of asthma-related accident and emergency attendance and inpatient admission were lower than that of outpatient attendance in both groups and were comparable between groups, all of which suggests that some patients, particularly in the EH group, may have been using the hospital outpatient department as a de facto primary-care clinic.
The two matched treatment groups in our study had comparable levels of asthma control and exacerbation rates during their baseline year, but there were some apparent differences in the patterns of medication usage and physician contact which suggest that the patients who would be switched to the EH were not as compliant with their controller medications as those who would remain on the same ICS device for the outcome year. Whether or not patient compliance or adherence to their ICS prescription was a factor in the physicians’ decision to switch patients to the EH cannot be determined with our study design. However, based on published comparisons of patient preferences for specific inhalers,Citation2–Citation6,Citation27–Citation29 it is possible that some patients were switched to the EH because they were not satisfied with, or not correctly using, their current ICS device.
During the outcome year, the EH compared favorably with the other devices in clinical effectiveness, even though 29% of EH patients underwent a decrease in ICS dosage and 33% a change of ICS drug, in addition to the change of device, at the index prescription date and 34% were switched to the EH without a face-to-face consultation. The importance of direct physician contact when switching inhalers cannot be overemphasized. In fact, the role of health care professionals in ensuring correct inhaler use has been described as critical, both in achieving correct inhaler technique initially and in maintaining correct inhaler use over time.Citation30
Approximately one in three of the EH patients in our study were switched without a face-to-face consultation, and possibly even without the patient’s knowledge and consent in some cases. Thus, patient training with the new device was not always ideal. This seemingly small fact adds weight to the effectiveness of the EH when compared with the reference group: patients who remained on the same ICS device they had been using for some time going into the outcome year. Whether or not their inhaler technique was optimal, the patients in the OH group were at least familiar with their device, whereas the patients in the EH group were using the EH for the first time at the start of the outcome year. Granted, the patients switching to the new device may have been more likely to read the product information sheet that came with the device and, at least initially, follow the instructions with care. However, in-person instruction and demonstration of correct inhaler use is documented to be superior to simply reading instructions in the patient information leaflet.Citation29,Citation34
All of the patients in both groups had been on ICS therapy for months or years prior to the index prescription date and so were experienced with general asthma inhaler use. Even so, the technical differences among the various ICS devices are sufficient that a switch from one type of inhaler to another increases the potential for critical handling or inhalation errors,Citation22,Citation29,Citation31,Citation34 and switching devices without an accompanying consultation may further compromise asthma control.Citation37,Citation38 In this light, the EH might be considered to have fared remarkably well. Not only was non-inferiority shown for the EH compared with the other devices for all four co-primary clinical outcome measures, but patients switched to the EH were significantly more likely to achieve overall asthma control: absence of asthma-related hospital attendance/admission, GP consultations for LRTI requiring antibiotic therapy, and acute courses of oral steroids, and an average salbutamol-equivalent SABA dosage of ≤200 μg/day.
Significantly more EH than OH patients had a change in ICS drug or device during the outcome year, which significantly decreased the proportion of EH patients achieving treatment stability, but only 22% of EH patients had a change of ICS device during their outcome year; 78% of EH patients continued to use the EH. The positive change in controller–reliever ratios between baseline and outcome years in the EH group further supports a conclusion of satisfaction with the new device in the majority of EH patients.
Simply put, when the units of controller and reliever medications are equal, the controller–reliever ratio is 0.5; if reliever use exceeds controller use, then the ratio drops below 0.5. During the baseline year, significantly more EH than OH patients had a controller–reliever ratio <0.5, which indicates a lower level of controller use and/or a greater reliance on reliever use in the patients who would be switched to the EH. However, this difference did not persist into the outcome year. While the controller–reliever ratios remained essentially unchanged between baseline and outcome years in the OH group, the percentage of EH patients whose controller–reliever ratio was ≥0.5 rose from 64% to 72%, even though SABA usage increased over the same period. This finding cannot be fully explained given our study design, but coupled with the decrease in primary-care asthma consultations in the EH group between baseline and outcome years, it is consistent with the better patient satisfaction documented for the EH, and thus more regular use of controller medications, which ultimately contributes to better asthma control.
Both treatment groups showed an increase in average daily ICS and SABA usage between baseline and outcome years, but the increases were greater in the OH group. These changes were reflected in the costs of asthma therapy. There were no significant differences in total asthma-related health care costs between groups during the baseline year, but total costs were significantly lower in the EH than the OH group during the outcome year, largely because of the greater increase in SABA usage in the OH group and the greater decrease in primary-care asthma consultations in the EH group between baseline and outcome years. These differences, while relatively small in monetary terms, may be considered particularly significant when framed in human terms: greater overall asthma control and less need for primary-care asthma consultations, for comparable or lower cost, in the patients switched to the EH.
Conclusion
Switching typical asthma patients from another type of ICS device to the EH at the same or lower ICS dosage, even in the absence of direct physician contact, was achieved without a compromise in clinical effectiveness or increase in cost. In fact, patients switched to the EH were significantly more likely to achieve overall asthma control and had comparable or lower asthma-related health care costs in relation to patients who remained on an ICS device other than the EH.
Supplementary materials
Figure S1 Potential confounders examined in the initial analysis.
Notes: aThe equations of Roberts et alCitation1 were used for patients >18 years of age and the equations of Rosenthal et alCitation2 were used for patients 6–18 years of age; bas described by Aylin et al.Citation3

Table S1 Baseline patient characteristics: demographics and co-morbidities
Table S2 Baseline patient characteristics: disease severity and therapies
Table S3 Baseline patient characteristics: ICS drugs, dosages, and devices
Table S4 Patient characteristics during the outcome year: disease severity and therapies
References
- RobertsCMMacRaeKDWinningAJReference values and prediction equations for normal lung function in a non-smoking white urban populationThorax19914696436501948793
- RosenthalMCramerDBainSHLung function in white children aged 4 to 19 years: II Single breath analysis and plethysmographyThorax19934888038088211869
- AylinPBottleAJenMHHSMR mortality indicatorsLondonDoctor Foster Research2010 Available from http://www.nhs.uk/scorecard/Documents/HSMR%20methodology%2009%20November.pdfAccessed on March 15, 2013
Disclosure
This study was sponsored by Orion Pharma UK Ltd; however, the funders had no role in the conduct of the study, interpretation of study results, nor preparation of the manuscript.
DP has consultant arrangements with Almirall, AstraZeneca (AZ), Boehringer Ingelheim (BI), Chiesi, GlaxoSmithKline (GSK), Merck, Mundipharma, Medapharma, Novartis, Napp, Nycomed, Pfizer, Sandoz, and Teva. He or his research team has received grants and support for research in respiratory disease from the following organizations in the last 5 years: UK National Health Service, Aerocrine, AZ, BI, Chiesi, GSK, Merck, Mundipharma, Novartis, Nycomed, Orion, Pfizer, and Teva. He has spoken for Almirall, AZ, Activaero, BI, Chiesi, Cipla, GSK, Kyorin, Novartis, Merck, Mundipharma, Pfizer, and Teva. He has shares in AKL Ltd, which produces phytopharmaceuticals. He is the sole owner of Research in Real Life (RiRL) and its subsidiary social enterprise, Optimum Patient Care.
VT, JvZ, SG, and CH are employees of RiRL, which has conducted paid research in respiratory disease for the following organizations in the last 5 years: Aerocrine, Almirall, AZ, BI, Chiesi, GSK, Meda, Merck, Mundipharma, Novartis, Nycomed, Orion, Pfizer, Takeda, Teva, and Zentiva. CK declares that she has no conflicts of interest in relation to this article.
References
- Electronic Medicines Compendium (eMC)SurreyDatapharm Communications Ltd2012 Available from: http://www.medicines.org.uk/emc/searchresults.aspx?term=Easyhaler&searchtype=QuickSearchAccessed December 8, 2013
- JägerLLaurikainenKLeinonenMSilvastiMBeclomethasone dipropionate Easyhaler is as effective as budesonide Turbohaler in the control of asthma and is preferred by patients. German Study GroupInt J Clin Pract200054636837211092110
- WettengelRLaurikainenKSilvastiMToivanenPSauterKTherapeutic equivalence and acceptability of two multidose powder inhalers in the treatment of asthmaRespiration2000671778210705267
- SchweisfurthHMalinenAKoskelaTToivanenPRanki-PesonenMGerman Study GroupComparison of two budesonide powder inhalers, Easyhaler and Turbuhaler, in steroid-naïve asthmatic patientsRespir Med200296859960612195841
- TukiainenHRytiläPHämäläinenKMSilvastiMSKeski-KarhuJFinnish Study GroupSafety, tolerability and acceptability of two dry powder inhalers in the administration of budesonide in steroid-treated asthmatic patientsRespir Med200296422122912000000
- VantoTHämäläinenKMVahteristoMWilleSNjåFHyldebrandtNStudy GroupComparison of two budesonide dry powder inhalers in the treatment of asthma in childrenJ Aerosol Med2004171152415120009
- PoukkulaAAlankoKKilpiöKComparison of a Multidose Powder Inhaler Containing Beclomethasone Dipropionate (BDP) with a BDP Metered Dose Inhaler with Spacer in the Treatment of Asthmatic PatientsClin Drug Investig1998162101110
- HämäläinenKMGrananderMToivanenPMalinenAAssessment of the systemic effects of budesonide inhaled from Easyhaler and from Turbuhaler in healthy male volunteersRespir Med2001951186386911716199
- HirstPHBaconREPitcairnGRSilvastiMNewmanSPA comparison of the lung deposition of budesonide from Easyhaler, Turbuhaler and pMDI plus spacer in asthmatic patientsRespir Med200195972072711575892
- LähelmäSKirjavainenMKelaMEquivalent lung deposition of budesonide in vivo: a comparison of dry powder inhalers using a pharmacokinetic methodBr J Clin Pharmacol200559216717315676038
- MalmströmKSorvaRSilvastiMApplication and efficacy of the multi-dose powder inhaler, Easyhaler, in children with asthmaPediatr Allergy Immunol1999101667010410920
- KoskelaTMalmströmKSairanenUPeltolaSKeski-KarhuJSilvastiMEfficacy of salbutamol via Easyhaler unaffected by low inspiratory flowRespir Med200094121229123311192960
- HaahtelaTVidgrenMNybergAKorhonenPLaurikainenKSilvastiMA novel multiple dose powder inhaler. Salbutamol powder and aerosol give equal bronchodilatation with equal dosesAnn Allergy19947221781828109809
- NieminenMMVidgrenMLaurikainenKEasyhaler, a novel multiple dose powder inhaler: clinically equivalent to salbutamol metered dose inhaler and easier to useRespiration199461137418177971
- VidgrenMArppeJVidgrenPPulmonary deposition and clinical response of 99mTc-labelled salbutamol delivered from a novel multiple dose powder inhalerPharm Res1994119132013247816763
- VidgrenMSilvastiMKorhonenPKinkelinAFrischerBSternKClinical equivalence of a novel multiple dose powder inhaler versus a conventional metered dose inhaler on bronchodilating effects of salbutamolArzneimittelforschung199545144477893268
- DirekwatanachaiCTeeratakulpisarnJSuntornlohanakulSComparison of salbutamol efficacy in children – via the metered-dose inhaler (MDI) with Volumatic spacer and via the dry powder inhaler, Easyhaler, with the nebulizer – in mild to moderate asthma exacerbation: a multicenter, randomized studyAsian Pac J Allergy Immunol2011291253321560485
- DelvadiaRHindleMLongestPWByronPRIn vitro tests for aerosol deposition II: IVIVCs for different dry powder inhalers in normal adultsJ Aerosol Med Pulm Drug Deliv201326313814422947131
- DubakieneRNargelaRSakalauskasRVahteristoMSilvastiMLähelmäSClinically equivalent bronchodilatation achieved with formoterol delivered via Easyhaler and AerolizerRespiration200673444144816432294
- LaubeBLJanssensHMde JonghFHEuropean Respiratory SocietyInternational Society for Aerosols in MedicineWhat the pulmonary specialist should know about the new inhalation therapiesEur Respir J20113761308133121310878
- HawksworthGMJamesLChrystynHCharacterization of the inspiratory manoeuvre when asthmatics inhale through a Turbohaler pre- and post-counselling in a community pharmacyRespir Med200094550150410868715
- TaylorAGustafssonPDo all dry powder inhalers show the same pharmaceutical performance?Int J Clin Pract Suppl200571216279997
- PalanderAMattilaTKarhuMMuttonenEIn vitro comparison of three salbutamol-containing multidose dry powder inhalersClin Drug Invest20002012533
- BelowABickmannDBreitkreutzJAssessing the performance of two dry powder inhalers in preschool children using an idealized pediatric upper airway modelInt J Pharm20134441–216917423333708
- MalmbergLPRytiläPHapponenPHaahtelaTInspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: age and gender rather than severity mattersInt J Chron Obstruct Pulmon Dis2010525726220714380
- ChrystynHCloser to an ‘ideal inhaler’ with the Easyhaler: an innovative dry powder inhalerClin Drug Investig2006264175183
- GinerJTorrejónMRamosAPatient preference in the choice of dry powder inhalersArch Bronconeumol2004403106109 Spanish14998473
- AhonenALeinonenMRanki-PesonenMPatient satisfaction with Easyhaler® compared with other inhalation systems in the treatment of asthma: A meta-analysisCurr Therap Res20006126173
- RönmarkEJögiRLindqvistACorrect use of three powder inhalers: comparison between Diskus, Turbuhaler, and EasyhalerJ Asthma200542317317815962873
- PriceDBosnic-AnticevichSBriggsAInhaler Error Steering CommitteeInhaler competence in asthma: common errors, barriers to use and recommended solutionsRespir Med20131071374623098685
- MolimardMRaherisonCLignotSDepontFAbouelfathAMooreNAssessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary careJ Aerosol Med200316324925414572322
- MolimardMLe GrosVImpact of patient-related factors on asthma controlJ Asthma200845210911318350401
- MalotLMolimardMAbouelfatahAAssessment of the handling of inhaler devices: an observational study of children in primary careArch Pediatr2007141011901195 French17658246
- SchulteMOsseiranKBetzRHandling of and preferences for available dry powder inhaler systems by patients with asthma and COPDJ Aerosol Med Pulm Drug Deliv200821432132818823257
- PriceDBjermerLHaughneyJReal-Life Asthma Strategies: The Missing Piece in the JigsawWest SussexRespirator Effectiveness Group2013 Available from: http://www.effectivenessevaluation.org/wp-content/uploads/2013/03/Real-life-Asthma-Strategies-The-Missing-Piece-in-the-Jigsaw_13Feb13.pdfAccessed May 16, 2013
- PriceDHillyerEVvan der MolenTEfficacy versus effectiveness trials: informing guidelines for asthma managementCurr Opin Allergy Clin Immunol2013131505723242115
- ThomasMPriceDChrystynHLloydAWilliamsAEvon ZiegenweidtJInhaled corticosteroids for asthma: impact of practice level device switching on asthma controlBMC Pulm Med20099119121204
- DoyleSLloydAWilliamsAWhat happens to patients who have their asthma device switched without their consent?Prim Care Respir J201019213113920174771
- Optimum Patient Care Research DatabaseCambridgeConscious Care Ltd2007 Available from: http://www.optimumpatientcare.org/Html_Docs/OPCRD.htmlAccessed February 2, 2014
- National Institute for Health Research [webpage on the Internet]Clinical Practice Research DatalinkEnglandCrown2014 Available from: http://www.cprd.com/intro.aspAccessed February 2, 2014
- The NHS dictionary of medicines and devices (dm+d) [website on the Internet] Available from: http://www.dmd.nhs.uk/Accessed February 2, 2014
- CurtisLUnit Costs of Health and Social Care 2011CanteburyPersonal Social Services Research Unit, The University of Kent2011 Available from: http://www.pssru.ac.uk/pdf/uc/uc2011/uc2011.pdfAccessed February 2, 2014
- GOV.UKNHS Reference Costs 2010–2011EnglandGOV.UK2011 Available from: https://www.gov.uk/government/publications/2010-11-reference-costs-publicationAccessed February 2, 2014
- AylinPBottleAJennMHHSMR mortality indicatorsLondonDoctor Foster Research2010 Available from: http://www.drfosterhealth.co.uk/docs/HSMR-methodology-Nov-2010.pdfAccessed March 15, 2013
