Abstract
Systemic allergic reactions to insect stings affect up to 5% of the population during their lifetime, and up to 32% of beekeepers. Such reactions can be fatal, albeit very rarely, and fear of a further systemic reaction (SR) can lead to significant anxiety and quality of life impairment. A recent Cochrane systematic review confirmed that venom immunotherapy (VIT) is an effective treatment for people who have had a systemic allergic reaction to an insect sting. VIT reduces risk of a further SR (relative risk 0.10, 95% confidence interval 0.03–0.28), but VIT also reduces risk of a future large local reaction, and significantly improves disease-specific quality of life. However, health economic analysis showed that VIT is generally not cost effective for preventing future SRs; most people are stung infrequently, most SRs resolve without long-term consequences, and a fatal outcome is extremely rare. VIT only becomes cost effective if one is stung frequently (eg, beekeepers) or if quality of life improvement is considered. Thus, for most people with insect sting allergy, anxiety and quality of life impairment should be the overriding consideration when making treatment decisions, highlighting the importance of a patient-centered approach. Areas which need to be explored in future research include efforts to improve the safety and convenience of VIT such as the use of sublingual immunotherapy; quality of life effects of venom allergy in children and adolescents as well as their parents; and the optimal duration of treatment.
Keywords:
Background
A variety of stinging insects can cause allergic reactions in humans. The most common are: wasps (hornets [Vespa], yellow jackets [United States]/wasps [Europe] [Vespula], paper wasp [Polistinae]), bees (honey [Apis mellifera], bumble bees [Bombus]), and stinging ants (fire ants [Solenopsis], jack jumper and bull ants [Myrmecia], and Pachycondyla).Citation1 Their taxonomy is shown in . Venom from the jack jumper ant is the most allergenic of the venoms and causes the highest rate of anaphylaxis.Citation1 Honeybee stings are also generally held to be more severe than wasp stings. Bees inject 50–140 µg of venom.Citation2,Citation3 Due to a unique barbed stinging apparatus, the bee stinger is often still attached, and venom can continue to be injected for up to 1 minute following removal of the insect. However, bees cannot sting again, whereas wasps can sting multiple times delivering ~3 µg of venom with each sting.Citation1 Wasps are aggressive and sting to attack, whilst bees sting as a defensive maneuver. Most reactions manifest as a painful, erythematous swelling at the sting site.
Whilst most sting reactions are painful and annoying, they are easily dealt with and resolve quickly. Unfortunately, some people react more vigorously to stings and in such a way that requires treatment. This can be as a large local reaction (LLR) (>10 cm diameter), thought to represent a late-phase IgE mediated allergic reaction. This can sometimes be mistaken as cellulitis as it can last up to 24–48 hours.Citation5 Reactions which cause symptoms beyond the local site of sting are termed systemic reactions (SRs). Severe life-threatening SRs (anaphylaxis) can occur, and are termed venom anaphylaxis. If a patient experiences a LLR, there is 5%–10% risk of them developing a systemic allergic reaction to a sting in the future.Citation5–Citation7
Anaphylaxis to an insect sting is classified not only on the basis of cardiac or respiratory compromise, but also by any abdominal cramps or vomiting. This is unlike allergic reactions to food where gastrointestinal symptoms can be part of nonanaphylactic reactions, since the gastrointestinal tract is the site of allergen exposure.Citation8 Anaphylaxis to an insect sting can cause a terrifyingly rapid death, with initial cardiorespiratory arrest within 5–10 minutes of the sting in many such cases.Citation9 The possibility of such an event can lead to significant anxiety and social restrictions in people at risk of an allergic reaction to an insect sting.
Prevalence and incidence of venom allergy
Hypersensitivity to insect stings affects up to 5 %–7.5% of the population and up to 32% of beekeepers.Citation10 If a sting reaction is classified as anaphylaxis, the risk of anaphylaxis with a future sting is significantly raised, but is still less than 50% in most cases. The risk is greater in those with more severe previous venom anaphylaxis, and lower in children.Citation1,Citation11
The rates of venom allergy leading to hospital admission for anaphylaxis have risen since 1992.Citation12 Venom anaphylaxis admissions appear to be increasing in the UK at a rate of 11.5% (95% confidence interval [CI] 10.4–12.7) per year, in keeping with other forms of anaphylaxis.Citation12 However, fatal reactions are not increasing; the increase in admissions may be due to changes in awareness, health-seeking behavior, or coding. Hospital admission for venom anaphylaxis is very rare in the first 3 decades of life and rises with increasing age. Ninety-three episodes of fatal venom anaphylaxis occurred in the UK over a 10-year period at a mean age of 59 years, which converts to a fatality rate of 0.009 per 100,000 population per annum.Citation12 In the US, at least 4 0 people per year die from insect stings.Citation13 Overall, fatalities from sting anaphylaxis range from 0.03 to 0.48 per million inhabitants per year. Fatal venom anaphylaxis accounts for 20% of all anaphylaxis fatalities.Citation14
Whilst this mortality rate is important and anaphylaxis is relatively common, it is interesting to note that severe immediate outcomes are rare, with only 2 % of significant anaphylaxis involving cardiorespiratory arrest.Citation15 Long-term morbidity following venom anaphylaxis has not to our knowledge been reported beyond the psychological consequences of venom allergy. Insect stings can also be fatal without leading to an allergic reaction, especially if multiple stings are delivered.Citation16 SRs are more likely in men, older patients, patients with a raised baseline tryptase level,Citation17,Citation18 those taking angiotensin-converting-enzyme (ACE) inhibitorsCitation17,Citation18 or beta blockers,Citation19 and with physical exercise around the time of the sting, eosinophilia,Citation20 or nonsteroidal anti-inflammatory drug ingestion.Citation21
The life-time prevalence of an allergic reaction to stings in adults in America is documented as 19% via a self-reported telephone survey.Citation22 Of these patients, a previous allergic reaction to any substance raised the life-time prevalence of venom allergy to 41%, nearly 20% of whom had confirmed anaphylaxis. European data (NORA)Citation15 found that 20% of documented anaphylaxis was due to venom allergy in the European pediatric population. In adults, venom as the cause of anaphylaxis was 48.2%. Of these, 70.6% of stings were caused by wasps, 23.4% bee, and 4.1% hornet venom. Data for the UK show venom to be a less common cause of anaphylaxis: accounting for ~10% of hospital admissions for anaphylaxis at all ages, and very few cases in children.Citation12
Diagnosis of venom allergy
Diagnosis of any allergic reaction requires a clear, thorough medical history. However, in hymenoptera venom allergy, difficulty can occur in ascertaining the type of insect sting.Citation23 The issue is also clouded as the sensitization rate (positive tests to hymenoptera venom but with a negative history) is quite high; 25%–50% of people with positive specific IgE (sIgE) or intradermal tests for venom and a positive history of insect sting reaction have a positive sting challenge.Citation24 However, people with a clear documented history of a venom reaction can have negative tests, and occasionally testing can be positive to both honey bee and wasp venom.Citation4
Allergy skin testing
Skin prick testing (SPT) and intradermal testing should be performed at least 2 weeks after a sting reaction, ideally 1–2 months later in case of false negatives due to a refractory period after a sting. However, the sensitivity of SPT is lower than that of intradermal testing. Increasing doses of venom traditionally have been used for either SPT or intradermal testing; initially performed at low concentrations, the level of venom is increased until a positive reaction is exhibited.Citation25 The SPT should be performed at a concentration between 1.0 and 100 µg/mL, and the intradermal initial concentration should be in the range of 0.001–0.01 µg/mL. It should then increase in tenfold increments to a maximum concentration of 1.0 µg/mL.Citation17 Recent informationCitation26 suggests that simultaneous intradermal testing with different venoms is safe and efficient.
sIgE testing
In vitro sIgE testing for wasp and bee allergens can supplement the clinical history and skin testing. The sensitivity of venom sIgE, as for other allergens, is generally lower than that of skin (intradermal) testing. sIgE can be performed for the whole extract or for components in the venom. These components can be natural components (nApi m 4) or recombinant allergens (rApi m 1, rVes v 1, rVes v 5, rPol d 5).Citation27 The use of natural venom extracts can lead to clouding of the diagnostic process secondary to cross-reacting carbohydrate determinants (CCDs). Recombinant allergens can circumnavigate the issues of: double-positive tests to bee and wasps with an unclear history; patients with a negative test but a good clinical history where natural allergens may have degraded or denatured; and planning of immunotherapy. Commercially available microarray chips can measure multiple components at once, including rApi m 1, nApi m 4, rPol d 5, and rVes v 5, to aid in venom allergy diagnosis.Citation28 There is strong immunological cross-reactivity between species: between honey bee and wasp there is 50% sequence homology in the hyaluronidase enzymes.Citation29 Api m 2 and Ves v 2 (hyaluronidases), and Api m 5 and Ves v 3 (dipeptidyl-peptidases), are well-known to cross react.Citation30 This cross-reactivity is mainly due to CCDs exhibited in both allergen pairs,Citation31 though they also show protein cross-reactivity. CCD-free components for in vitro diagnostic testing are Api m 1, Pol d 1 and 5, and Ves v 1 and 5. The venom from bees and wasps contains many different components. A list of the major venom allergens from honey bees, bumble bees, and the main wasp species is shown in .
Table 1 Hymenoptera venom allergens
The major honeybee allergen is Api m 1, but unfortunately this has been shown to have low sensitivity,Citation33 though the sensitivity can alter due to the criteria of the patient population studied. In wasp-allergic patients, rVes v 5 has good diagnostic performance.Citation30 If used in conjunction with Ves v 1, the sensitivity was significantly increased.Citation34 The most reliable serological indicators to aid in diagnosis of true double-sensitization or cross-reactivity in patients with positive IgE results to both venom extracts are Api m 1 and Ves v 5.Citation35 In patients who are positive to both Polistes and Vespula, the use of Pol d 5 and Ves v 5, and Pol d 1 and Ves v 1 can be discriminatory.Citation36
Basophil activation test
Evidence is increasing that basophil activation tests can be used to discriminate clinical reactivity, and severity of clinical reactivity, in allergic disorders.Citation37,Citation38 With regard to venom allergy, one studyCitation39 suggested that basophil activation tests can be useful in the setting of negative intradermal test results to complement sIgE testing when the latter is positive. Another study suggested basophil activation tests may be useful for aiding the decision to cease immunotherapy.Citation40 The emerging role of basophil activation tests in the diagnosis and management of venom allergy requires further study.
Treatment of venom allergy
Acute treatment of venom allergic reactions
Acute treatment of allergic reactions to venom involves local care for LLRs including cold packs, antihistamines, NSAID gels, analgesia, or systemic corticosteroids as appropriate. SRs to venom are managed in the same way as other triggers of systemic allergic reactions. However, the rapid onset of venom allergic reactions, and the association with cardiovascular compromise, especially hypotension, mean that aggressive treatment with oxygen, intravenous fluids, and adrenaline needs to be started early.Citation41,Citation42 Appropriate posture may also be an important way of preventing adverse cardiac outcomes during a venom allergic reaction.Citation43 Emergency department studies suggest that low-dose peripheral adrenaline infusion may be the optimal way to manage venom anaphylaxis, and such infusions may need to be continued for many hours.Citation44 Older studies suggest that bolus adrenaline, even when administered intravenously, may have very limited and transient effects on cardiovascular parameters when used to treat venom anaphylaxis.Citation45 For bee stings, removal of the sting may reduce the total dose of venom and is an important part of first aid management – there is no clear evidence that any specific method for sting removal is superior.Citation46
Long-term management following a systemic venom allergic reaction
Following recovery from an acute SR to an insect sting, provision of an adrenaline auto-injector is recommendedCitation47 although this has not been shown to improve quality of life.Citation48 Other advice may include avoidance of high-risk activities/locations, methods for removal of stings should there be further insect stings,Citation46 and provision of antihistamine, bronchodilator medication, and corticosteroids. For the purpose of this review, we will focus our discussion on the role of venom immunotherapy (VIT) for prevention of further allergic reactions to insect stings and promoting improved quality of life in those with a past history of significant reaction.
History of VIT
Allergen immunotherapy for hay fever was discovered at St Mary’s Hospital in London by Leonard Noon in 1911, and the first randomized controlled trial of this treatment for hay fever, also at St Mary’s Hospital, proved its efficacy in 1954.Citation49,Citation50 The first report of VIT was described in 1925,Citation51 where the whole crushed body of a wasp was used. Whole-body extract VIT continued to be used for many yearsCitation52 until it was shown to be ineffective in a randomized controlled trial. It was not until 1978 that an effective modality of VIT was developed. This used venom extracted from venom sacs, and was shown to be highly effective in a randomized controlled trial.Citation53 Since that time, extracted venom has been used for immunotherapy with wasps, hornets, jumper ants, and honey bees. However, for fire ants, whole-body extract is still used in the US, and the latter practice has not yet been subjected to a randomized controlled efficacy trial.
In VIT, gradually increasing doses of insect venom are administered to induce immunological tolerance, typically by subcutaneous injection at an interval of several weeks for up to 5 years. Extrapolating from randomized controlled trial evidence in relation to hay fever, a treatment course of 3 years is recommended, although longer courses are recommended in some settings.Citation4,Citation54
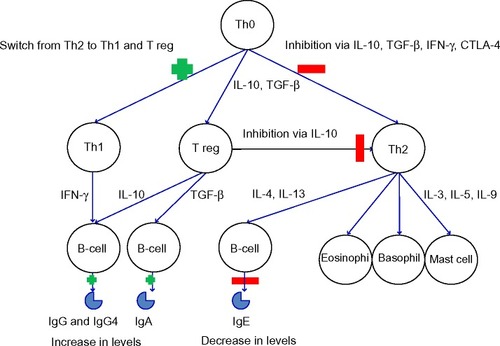
Immunotherapy works through complex immunological mechanisms. The initial mechanism of action is a mast cell and basophil desensitization, followed by changes in T-cells (including the formation of T regulatory cells to the allergen) and finally an alteration in B-cell, IgE (an initial rise, followed by a reduction over several months), mast cell, basophil, and eosinophil responses to the allergen.Citation55,Citation56 Monitoring during VIT has shown a drop in sIgE and a rise in IgG4.Citation57–Citation59 These immunological changes can be seen in .
Figure 2 Immunological changes in immunotherapy.
Abbreviations: CTLA-4, cytotoxic T-lymphocyte-associated protein 4; IL, interleukin; IFN-γ, Interferon gamma; Ig, Immunoglobulin; TGF-β, transforming growth factor beta; Th, T helper cells; T reg, regulatory T-cells.
VIT treatment protocols
VIT is commercially available for honeybee, paper wasp, and yellow jacket (“European”) wasps since these are the commonest causes of venom anaphylaxis worldwide. To our knowledge, immunotherapy products for ant allergy are not currently commercially available. This review will therefore focus on wasp and honeybee VIT using extracted venom. At present, the treatment offered for venom allergy is largely via subcutaneous immunotherapy (SCIT). Treatment regimes are diverse and the populations they have been tested on vary. This makes comparison between schedules difficult. However, the ability to tailor the schedule to each patient will improve efficacy and optimize safety.Citation10 Up-dosing treatment protocols for subcutaneous VIT can be divided into roughly four groups which are summarized in .Citation10,Citation60–Citation62 In general, rapid up-dosing is more convenient for patients but carries increased risk of a systemic allergic reaction to treatment.
Table 2 Typical VIT up-dosing protocols
Improvement of honeybee immunotherapy efficacy may be possible by increasing the quantities of major determinants such as Api m 3 and 10 in VIT preparation, or tailoring the allergenic component mix according to individual patient sensitization pattern. This could be done by improving the method of generating the venom preparation, spiking the venom with recombinant allergens, or tailoring the immunotherapy for different patients with a range of component allergens relevant for that patient.Citation32,Citation63,Citation64
VIT starting doses are often around 0.0001 µg per injection and rise to a maintenance does of 1 00 µg (approximately two bee stings or 30 wasp stings). In children, data from a small study suggest that a lower 50 µg maintenance dose provides effective protection from SRs with potentially improved safety profile.Citation65 Longer induction-phase treatments can be done as an outpatient; however, for rush therapy, hospital admission is advisable. Although the safety profile of semi-rush and ultra-rush VIT up-dosing protocols is good,Citation60,Citation62 in general, accelerated up-dosing immunotherapy protocols are associated with increased risk of systemic allergic reaction to VIT.Citation17
The maintenance dose interval has historically been set at 1 month but studies have shown that intervals of up to 3–4 months retain their efficacy.Citation66 In patients who experience SRs to stings during the traditional regime induction, accelerated induction protocols have been successfully used.Citation67 If a patient experiences a SR during maintenance VIT then a larger dose of 200 µg can be given, although switching to a lower maintenance dose for a prolonged period may be needed first.Citation68
Patient selection for VIT
VIT is recommended “as an option for the treatment of IgE-mediated bee and wasp venom allergy”.Citation69 However, guidelines vary in suggested indications for VIT. We present a summary of the major international guidelines for patient selection for VIT in . There is significant inconsistency between guidelines, especially in relation to patients with a history of less severe local reactions or SRs. There are variations in the relative role of attempts at risk stratification through frequency of stings/severity of incident reaction versus focusing on the quality of life impact of the allergy on patients when making treatment decisions. We will discuss these specific issues below, because recent findings shed some light on considerations to be made during patient selection for VIT, and suggest the need for revised and more consistent patient-selection guidance.
Table 3 Patient selection for VIT – discrepancies between guidelines
Recommendations for VIT patient selection from the European Academy of Allergy and Clinical Immunology (EAACI); American Academy of Allergy, Asthma and Immunology (AAAAI); National Institute for Health and Care Excellence (NICE); and the British Society of Allergy and Clinical Immunology (BSACI) are listed in .
Contraindications for VIT also vary between guidelines. Manufacturer-stated contraindications for Pharmalgen (ALK-Abelló, Horsholm, Denmark) include malignancy, severe asthma, immunological conditions, chronic heart and lung disease, severe hypertensions, beta blockers, tricyclic antidepressants, monoamine oxidase inhibitors, angiotensin-converting enzyme inhibitors, and initiation during pregnancy (though maintenance can be continued in pregnancy).Citation69
Efficacy of VIT: reduction in SRs
In the recent Cochrane systematic review, VIT was shown to significantly reduce risk of SR to a future sting in people who have already suffered at least one SR to a sting (relative risk [RR] 0.10, 95% CI 0.03–0.28).Citation17 From the seven trials (392 participants) included in the Cochrane review, 2.7% of patients post-VIT had a systemic allergic reaction on re-sting, compared to 39.8% untreated.Citation19 The review included both adults and children, allergic to bee, wasp, or ant venom, and follow-up varied from months to years with re-stings triggered by either accidental field stings or intentional supervised sting challenges. One trial used sublingual (SLIT) and six trials used subcutaneous VIT, with little evidence of difference in treatment outcomes. VIT is the only specific treatment for venom allergy which can provide a reduction in morbidity. Mortality is so rare that proving VIT reduces mortality is a challenging task; however, the Cochrane review found VIT was effective across the range of reaction severity suggesting that it is likely to reduce risk of fatal venom anaphylaxis as well as nonfatal venom anaphylaxis.
The effectiveness of VIT is apparent immediately once the maintenance dose is reached.Citation14 One study suggested a 10% reaction risk for each sting post-cessation of treatment, with a cumulative risk of a SR reaching a plateau of 20% after a decade posttreatment cessation.Citation71 In patients not responding to VIT, it has been found that basophil activation test response to venom in vitro is associated with treatment efficacy. In patients who reacted to venom stings after completing VIT, they were found to have higher basophil responses than those who tolerated stings post-VIT.Citation72
Efficacy of VIT: reduction in LLRs
The Cochrane review also found that VIT significantly reduces the risk of a LLR from venom (RR 0.41, 9 5% CI 0.24–0.69).Citation19 Both SCIT and SLIT VIT are effective in reducing LLRs as well as SRs.Citation19 The role of VIT for LLRs is controversial, given the relatively low risk of serious outcomes with future stings in those with a history of LLRs. Those with troublesome recurrent LLRs, or associated anxiety or quality of life impairment through concern about further LLRs or SRs, may be considered for VIT. Although VIT is not indicated for LLRs in the EAACI and BSACI guidelines, they are cited as a relative indication in the AAAAI practice parameter ().
Efficacy of VIT: quality of life
Allergy to insect venom has been documented to cause emotional distress in patients leading to a decrease in their quality of life.Citation73 VIT has been shown to improve the quality of life of people undergoing therapy above and beyond those who simply carry an adrenaline auto-injector (using a seven-point score where a score of 1–3 gave a positive view of treatment). The study found that patients with previous anaphylaxis had a more positive view of life after treatment (RR 2.01, 95% CI 1.43–2.82), as well as those patients with previous cutaneous SR (RR 2.24, 95% CI 1.13–4.43).Citation19 These differences were seen in women and men, patients with varying degrees of baseline anxiety, and those with recent stings or stings over 1 year ago.Citation48 It has been quoted that 72% of patients undergoing VIT derive benefit in their quality of life by undergoing treatment,Citation48 and those who do not derive a quality of life benefit are those who have no quality of life impairment at baseline (Dr Hanneke Oude-Elberlink, personal communication, January 2015). The Cochrane review found that VIT improves venom-specific quality of life by a mean 1.21 points (95% CI 0.75–1.67) on a seven-point scaleCitation19 (where a change of 0.5 points has been defined as the minimally important difference).Citation74 There is also evidence suggesting that the use of a sting challenge during the maintenance phase of VIT, when tolerated, can improve quality of life.Citation75,Citation76
Adverse events associated with VIT
Serum tryptase should be performed on patients undergoing VIT to rule out mastocytosis.Citation1 In patients with mastocytosis, due to their significant risk of SR even after VIT and risk of anaphylaxis from other causes, continued availability of an adrenaline auto-injector is recommended following VIT.Citation4 For all patients, there is a risk of a systemic adverse reaction during VIT; 9.3% suffered a SR in the randomized controlled trials included in the Cochrane review.Citation19 In a larger series of observational studies, the authors found 14.2% of people suffered a systemic allergic reaction due to a VIT injection for bee venom, and 2.8% for wasp venom.Citation19 NICE estimated that the treatment-related adverse reaction rate is 2% per injection in the initial phase and 0.26% per injection during the maintenance phase.Citation69 Adverse reactions are more likely with bee venom VIT, when there is a short time between sting and VIT, during up-dosing (initial) phase, in the elderly, in those using beta blockers, in clonal mast cell activation syndrome, in asthmatic children, and in those with elevated serum basal tryptase.Citation10,Citation77,Citation78 Elevated serum tryptase did not lead to an increased risk of VIT failure.Citation79
There does, however, seem to be debate about the link between length of maintenance treatment and VIT failure after a field sting.Citation79,Citation80 VIT failure was more likely to be linked to bee venom than wasp (16%–18% versus 4%–7.5%),Citation80,Citation81 ACE inhibitors, and systemic allergic reaction during dose increasing.Citation79 Sting challenges are reputed to historically reveal failure of VIT in up to 22% of patients undergoing therapy.Citation82
In the event of an LLR or SR during VIT, the recommendation is to reduce the dose to the last tolerated dose with careful dose escalation.Citation4 However, antihistamine given pre-injections in traditional, rush, and ultra-rush VIT reduces the incidence of local reactions, and may play a role in enhancing the efficacy of immunotherapyCitation83–Citation85 and should be used in patients who have experienced reactions during VIT. There are a small subsection of patients who do not respond to VIT at all. There are ongoing studies at present investigating changes in gene expression in relation to response to treatment. The belief is that genetic factors may influence a patient’s response to VIT and genetics may show the way to best tackle this problem.Citation86
Cost-effectiveness of VIT
Bee venom extracts from Pharmalgen (ALK-Abelló) cost £54.81 per induction and £15.94 per maintenance injection. Wasp induction costs £67.20 and £20.51 per injection during maintenance.Citation69 Whilst the cost varies per protocol, the average cost of a full treatment is £2,299.33 per patient.Citation64 Cost-effectiveness of Pharmalgen (ALK-Abelló)Citation70 was examined in a NICE health technology assessment, in providing VIT for patients who have suffered a SR to bee or wasp venom. Four randomized controlled trials and five “quasi-experimental” small studies of poor quality were found so the authors were unable to perform meta-analysis. The clinical evidence suggested that there was a decrease in reactions to stings post-immunotherapy, but there were associated adverse reactions, some of which were systemic although all were treatable. The assessment group developed an economic model to compare cost-effectiveness of VIT with other treatment options for venom allergy.
In general, they found that VIT is not cost-effective when only considering direct costs of outcomes.Citation87 This is because for most people, recurrent insect stings are rare, and even in those with a history of severe venom allergic reaction previously, the risk of fatal outcome following a sting is extremely low. However, immunotherapy was found to be cost-effective in patients at high risk of future stings (≥3.3 a year, for people such as bee keepers, their neighbors and children, roofers, and gardeners) and in those whom the quality of life indices show improvement with reduced anxiety. In those at high risk of a future sting (five or more stings per year) the incremental cost-effectiveness ratio of VIT with an adrenaline auto-injector and high-dose antihistamines is £23,368 per QALY (quality adjusted life year) gained when compared with high-dose antihistamine and adrenaline auto-injector alone, and £25,661 compared to avoidance advice only. These findings are summarized in .
Table 4 Summary of Pharmalgen VIT health economic assessmentCitation70
Regarding quality of life impact, even a very small improvement in anxiety associated with treatment (0.01 points on the EuroQol-5 Dimension Questionnaire [EQ-5D]) makes the VIT cost-effective in the base case scenario of one sting every 9 years. This suggests that most people with a history of insect sting allergic reaction who are concerned enough to seek treatment, and are appropriately informed of the prognosis and treatment of the condition, merit consideration of VIT. Thus, focusing immunotherapy on those who are concerned, those who have documented quality of life impairment related to fear of future sting reactions, and those who have frequent insect stings is at least as important as considerations of individual risk for severe reaction based on severity of prior reaction.Citation70
This new information, that quality of life impact is the key driver of cost in relation to venom allergy sheds some light on the discrepancies seen in the guidelines summarized in . In common with food allergy, the lives of individuals with venom allergy are overshadowed by the possibility of sudden, unexpected, rapidly fatal anaphylaxis. Such events are extremely rare, but fear of them is a major contributor to quality of life impairment and, in the case of venom allergy, quality of life can be improved by using VIT to reduce the risk of such an outcome.
If we refer back to , it is clear that VIT treatment guidelines are making an attempt to “risk stratify” patients on the basis that the decision whether or not to treat with VIT should be driven by risk of fatal or near-fatal anaphylaxis to a future sting. If one changes the paradigm, to one where quality of life is the key driver of venom allergy impact on individuals, then the “indications” for VIT change subtly. For example, the EAACI recommendation to use VIT for those with an urticarial reaction and quality of life impairment seems supported, but the AAAAI recommendation that VIT is not indicated in those aged ≤16 years with a cutaneous SR to an insect sting may not be appropriate for patients who are also suffering significant quality of life impact.
Future perspectives
Ongoing research efforts are studying ways to make VIT safer, more convenient, and more widely available without compromising treatment efficacy. highlights some uncertainties that need clarification in future research.
Table 5 Key areas of uncertainty for future research
Anti-Ige Therapy
The use of omalizumab has been described several time during VIT for cases of recurrent anaphylaxis during treatment.Citation88,Citation89 It has been shown to have good efficacy in improving VIT outcomes in these situations.
SLIT
The benefit of SLIT remains its ease of administration and excellent safety profile, which make it suitable for home use. Doses used are generally higher than for SCIT, which may increase the direct pharmacy cost, but there is a cost saving and likely quality of life benefit through reduced need for supervised administration of treatment in a medical setting.Citation90 An observational study of wasp SLIT in 2 1 patients showed good tolerability and suggested possible efficacy.Citation91 An observational study of bee venom SLIT suggested reduced LLR in the majority of patients treated.Citation92 A single randomized controlled trial of SLIT VIT was identified in the Cochrane review:Citation19 there was no evidence that SLIT was less effective than SCIT for reducing risk of SR or LLR in that review, although this preliminary conclusion was based on a small number of studies and participants. Given the importance of quality of life improvement as an objective in VIT, highlighted by the Health Technology Assessment Programme (HTA) health economic reviewCitation70 and the high burden of treatment from SCIT regimens, further investigation of SLIT as a treatment option in insect sting allergy is an important goal.
Peptide immunotherapy
Other novel techniques that have been investigated to the earliest level include the use of allergen-derived long synthetic peptides to bee venom.Citation93 These injections proved safe and instigated T-cell hyporesponsiveness to bee venom allergen. There was also a rise in specific IgG4. A small study into the use of peptide immunotherapy in mild bee allergy found changes in surrogate markers for successful immunotherapy were exhibited suggesting that there may be benefit from this method of treating bee allergy.Citation94 We are not aware of any further studies beyond the late 1990s into peptide immunotherapy for venom allergy.
Duration of treatment
The full length of maintenance VIT is often quoted as 3–5 years, with 5 years showing a better long-term suppression of risk.Citation80,Citation95 This was initially recommended because the SPT or sIgE generally was recorded as negative 3–5 years into treatment, and was extrapolated from studies of pollen immunotherapy,Citation14,Citation54 though there is debate about whether or not a negative intradermal test at the end of treatment is the right goal for treatment.Citation80,Citation96 Given the findings above, further research is needed to assess long-term quality of life in people treated for different durations. Lifelong VIT is recommended in some centers for high-risk patients such as those with mastocytosis or a baseline tryptase over 11.4 ng/mLCitation97 due to the significantly higher risk of SRs on VIT maintenance and the higher rate of treatment failure. However, this is not supported by UK guidelines due to lack of evidence.Citation4
The HTA health economic reviewCitation70 has highlighted the importance of quality of life improvement as the primary goal of treatment, except in those at high risk of recurrent stings such as beekeepers. This means that the duration of treatment needs to be a duration which leads to quality of life improvement and suggests that 3 years may be adequate in all those other than individuals at high risk of recurrent reactions. Furthermore, the role of sting challenge as a method for providing evidence to patients that they are no longer at high risk of sting anaphylaxis merits further investigation in this context.Citation75,Citation76
Conclusion
Wasp and bee sting allergy can cause rapid-onset fatal anaphylaxis. Thankfully, such events are extremely rare; however, fear of such an event is a major driver of quality of life impairment in people with insect sting allergy. Venom anaphylaxis admissions in the UK appear to be increasing at a rate of 11.5% (95% CI 10.4–12.7) per year, although fatalities remain stable.
VIT is a clinically effective treatment which should be offered to those at risk of a SR to an insect sting who have significant anxiety or quality of life impairment related to fear of future stings. A sting challenge during maintenance treatment may enhance the quality of life improvement seen with VIT. The decision whether to initiate VIT should be largely driven by considerations of quality of life impairment rather than an attempt to quantify risk of future SR, except in those such as bee keepers who suffer frequent stings.
More work is needed to clarify the role of SLIT VIT; the role of sting challenges during VIT treatment; the quality of life effect of VIT in children and adolescents, and their parents; and methods for determining the optimal duration of VIT treatment for different patient groups.
Disclosure
The authors report no conflicts of interest in this work.
References
- TanJWCampbellDEInsect allergy in childrenJ Paediatr Child Health2013499E381E38723586469
- HoffmanDRDoveDEJacobsonRSAllergens in Hymenoptera venom. XX. Isolation of four allergens from imported fire ant (Solenopsis invicta) venomJ Allergy Clin Immunol1988825 Pt 18188273192865
- HoffmanDRJacobsonRSAllergens in hymenoptera venom XII: how much protein is in a sting?Ann Allergy19845242762786711914
- KrishnaMTEwanPWDiwakarLDiagnosis and management of hymenoptera venom allergy: British Society for Allergy and Clinical Immunology (BSACI) guidelinesClin Exp Allergy20114191201122021848758
- GraftDFSchuberthKCKagey-SobotkaAA prospective study of the natural history of large local reactions after Hymenoptera stings in childrenJ Pediatr198410456646686716215
- MaurielloPMBardeSHGeorgitisJWReismanRENatural history of large local reactions from stinging insectsJ Allergy Clin Immunol1984744 Pt 14944986491095
- GoldenDBKellyDHamiltonRGCraigTJVenom immunotherapy reduces large local reactions to insect stingsJ Allergy Clin Immunol200912361371137519443022
- BilóBMRueffFMosbechHBonifaziFOude-ElberinkJNthe EAACI Interest Group on Insect Venom HypersensitivityDiagnosis of Hymenoptera venom allergyAllergy200560111339134916197464
- PumphreyRSLessons for management of anaphylaxis from a study of fatal reactionsClin Exp Allergy20003081144115010931122
- Antolín-AmérigoDMoreno AguilarCVegaAAlvarez-MonMVenom immunotherapy: an updated reviewCurr Allergy Asthma Rep201414744924934908
- GoldenDBKagey-SobotkaANormanPSHamiltonRGLichtensteinLMOutcomes of allergy to insect stings in children, with and without venom immunotherapyN Engl J Med2004351766867415306668
- TurnerPJGowlandMHSharmaVIncrease in anaphylaxisrelated hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012J Allergy Clin Immunol20151354956963.e125468198
- GraftDFInsect sting allergyMed Clin North Am200690121123216310531
- BilòMBAnaphylaxis caused by Hymenoptera stings: from epidemiology to treatmentAllergy201166Suppl 95353721668850
- WormMMoneret-VautrinASchererKFirst European data from the network of severe allergic reactions (NORA)Allergy201469101397140424989080
- XieCXuSDingFClinical features of severe wasp sting patients with dominantly toxic reaction: analysis of 1091 casesPloS One2013812e8316424391743
- GoldenDBMoffittJNicklasRAStinging insect hypersensitivity: a practice parameter update 2 011J Allergy Clin Immunol201112748528544.e1e2321458655
- RuëffFPrzybillaBBilóMBPredictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase-a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom HypersensitivityJ Allergy Clin Immunol200912451047105419895993
- BoyleRJElremeliMHockenhullJVenom immunotherapy for preventing allergic reactions to insect stingsCochrane Database Syst Rev201210CD00883823076950
- YavuzSTSahinerUMBuyuktiryakiBClinical features of children with venom allergy and risk factors for severe systemic reactionsInt Arch Allergy Immunol2013160331332123095437
- PucciSDe PasqualeTD’AlòSIlluminatiIMakrìEIncorvaiaCSystemic reactions to honeybee stings and nonsteroidal antinflammatory drugsAnn Allergy Asthma Immunol2014113223723824986037
- WoodRACamargoCAJrLiebermanPAnaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United StatesJ Allergy Clin Immunol2014133246146724144575
- BakerTWForesterJPJohnsonMLStolfAStahlMCThe HIT study: Hymenoptera Identification Test – how accurate are people at identifying stinging insects?Ann Allergy Asthma Immunol2014113326727024969241
- SturmGJKranzelbinderBSchusterCSensitization to Hymenoptera venoms is common, but systemic sting reactions are rareJ Allergy Clin Immunol2014133616351643.e124365141
- PortnoyJMMoffittJEGoldenDBStinging insect hypersensitivity: a practice parameter. The Joint Force on Practice Parameters, the American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and ImmunologyJ Allergy Clin Immunol19991035 Pt 196398010366310
- StrohmeierBAbererWBokanovicDKomerickiPSturmGJSimultaneous intradermal testing with hymenoptera venoms is safe and more efficient than sequential testingAllergy201368454254423405953
- TreudlerRSimonJCOverview of component resolved diagnosticsCurr Allergy Asthma Rep201313111011723076421
- Product Catalog 2012 – ImmunoCAP: Is it allergy?WalthamThermo Fisher Scientific2012 Available from: http://www.phadia.com/Global/A%20Document%20Library/Product%20Catalogues/ProdCat2012_ID_AI.pdfAccessed March 10, 2015
- ReismanREMüllerURWypychJILazellMIStudies of coexisting honeybee and vespid-venom sensitivityJ Allergy Clin Immunol19847322462526699307
- MüllerURJohansenNPetersenABFromberg-NielsenJHaeberliGHymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m1 and Ves v5Allergy200964454354819120073
- MittermannIZidarnMSilarMRecombinant allergen-based IgE testing to distinguish bee and wasp allergyJ Allergy Clin Immunol2010125613001307.e320466415
- SpillnerEBlankSJakobTHymenoptera allergens: from venom to “venome”Front Immunol201457724616722
- KorošecPValentaRMittermannILow sensitivity of commercially available rApi m 1 for diagnosis of honeybee venom allergyJ Allergy Clin Immunol2011128367167321481443
- SeismannHBlankSCifuentesLRecombinant phospholipase A1 (Ves v 1) from yellow jacket venom for improved diagnosis of hymenoptera venom hypersensitivityClin Mol Allergy20108720359368
- EberleinBKrischanLDarsowUOllertMRingJDouble positivity to bee and wasp venom: improved diagnostic procedure by recombinant allergen-based IgE testing and basophil activation test including data about cross-reactive carbohydrate determinantsJ Allergy Clin Immunol2012130115516122421265
- MonsalveRIVegaAMarquésLComponent-resolved diagnosis of vespid venom-allergic individuals: phospholipases and antigen 5s are necessary to identify Vespula or Polistes sensitizationAllergy201267452853622229815
- SantosAFDu ToitGDouiriADistinct parameters of the basophil activation test refect the severity and threshold of allergic reactions to peanutJ Allergy Clin Immunol201513511798625567046
- SantosAFDouiriABécaresNBasophil activation test discriminates between allergy and tolerance in peanut-sensitized childrenJ Allergy Clin Immunol2014134364565225065721
- BonadonnaPZanottiRMelioliGThe role of basophil activation test in special populations with mastocytosis and reactions to hymenoptera stingAllergy201267796296522676063
- González-de-OlanoDAlvarez-TwoseIMorgadoJMEvaluation of basophil activation in mastocytosis with Hymenoptera venom anaphylaxisCytometry B Clin Cytom201180316717521520404
- Working Group of the Resuscitation Council (UK)Emergency Treatment of Anaphylactic Reactions. Guidelines for Healthcare ProvidersLondonResuscitation Council (UK)2008 Available from: http://www.resus.org.uk/pages/reaction.pdfAccessed March 10, 2015
- MuraroARobertsGWormMAnaphylaxis: guidelines from the European Academy of Allergy and Clinical ImmunologyAllergy20146981026104524909803
- PumphreyRSHFatal posture in anaphylactic shockJ Allergy Clin Immunol2003112245145212897756
- BrownSGBlackmanKEStenlakeVHeddleRJInsect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitationEmerg Med J200421214915414988337
- SmithPLKagey-SobotkaABleeckerERPhysiologic manifestations of human anaphylaxisJ Clin Invest1980665107210806776143
- VisscherPKVetterRSCamazineSRemoving bee stingsLancet199634890233013028709689
- HowdlePNICEclinical guideline 1 34. Anaphylaxis: assessment to confirm an anaphylactic episode and the decision to refer after emergency treatment for a suspected anaphylactic episode [webpage on the Internet]LondonNational Institute for Health and Care Excellence2011 Available from: http://www.nice.org.uk/guidance/CG134Accessed March 10, 2015
- Oude ElberinkJNDe MonchyJGVan Der HeideSGuyattGHDuboisAEVenom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venomJ Allergy Clin Immunol2002110117418212110838
- NoonLProphylactic inoculation against hay feverLancet1911177458015721573
- FranklandAWAugustinRProphylaxis of summer hay-fever and asthma: a controlled trial comparing crude grass-pollen extracts with the isolated main protein componentLancet195426668211055105713164324
- BraunLNotes on desensitisation of a patient hypersensitive to bee stingsSouth Afr Med Rec192523408409
- JutelMAkdisCAImmunological mechanisms of allergen-specific immunotherapyAllergy201166672573221466562
- HuntKJValentineMDSobotkaAKBentonAWAmodioFJLichtensteinLMA controlled trial of immunotherapy in insect hypersensitivityN Engl J Med1978299415716178446
- DurhamSRWalkerSMVargaEMLong-term clinical efficacy of grass-pollen immunotherapyN Engl J Med1999341746847510441602
- EwanPWNew insight into immunological mechanisms of venom immunotherapyCurr Opin Allergy Clin Immunol20011436737411964715
- AkdisMAkdisCAMechanisms of allergen-specific immunotherapyJ Allergy Clin Immunol2007119478079117321578
- HayashiYHirataHWatanabeMUsefulness of specific-IgG4 to Hymenoptera venom in the natural history of hymenoptera stingsJ Investig Allergol Clin Immunol2014243192194
- CoxLNelsonHLockeyRAllergen immunotherapy: a practice parameter third updateJ Allergy Clin Immunol20111271 SupplS1S5521122901
- GoldbergAConfino-CohenRBee venom immunotherapy – how early is it effective?Allergy201065339139519839973
- BrownSGWieseMDvan EedenPUltrarush versus semirush initiation of insect venom immunotherapy: a randomized controlled trialJ Allergy Clin Immunol2012130116216822460067
- BrehlerRWolfHKüttingBSchnitkerJLugerTSafety of a two-day ultrarush insect venom immunotherapy protocol in comparison with protocols of longer duration and involving a larger number of injectionsJ Allergy Clin Immunol20001056 Pt 11231123510856159
- BirnbaumJCharpinDVervloetDRapid Hymenoptera venom immunotherapy: comparative safety of three protocolsClin Exp Allergy19932332262308472191
- KöhlerJBlankSMüllerSComponent resolution reveals additional major allergens in patients with honeybee venom allergyJ Allergy Clin Immunol20141335138313891389. e1e624440283
- GoldenDBNew directions in diagnostic evaluation of insect allergyCurr Opin Allergy Clin Immunol201414433433924915545
- KonstantinouGNManoussakisEDouladirisNA 5-year venom immunotherapy protocol with 50 µg maintenance dose: safety and efficacy in school childrenPediatr Allergy Immunol201122439339721235631
- SimioniLVianelloABonadonnaPEfficacy of venom immunotherapy given every 3 or 4 months: a prospective comparison with the conventional regimenAnn Allergy Asthma Immunol20131101515423244659
- GoldbergAConfino-CohenRRush venom immunotherapy in patients experiencing recurrent systemic reactions to conventional venom immunotherapyAnn Allergy Asthma Immunol200391440541014582821
- RuëffFWenderothAPrzybillaBPatients still reacting to a sting challenge while receiving conventional Hymenoptera venom immunotherapy are protected by increased venom dosesJ Allergy Clin Immunol200110861027103211742283
- AdlerAPharmalgen for the Treatment of Bee and Wasp Venom Allergy: Technology Appraisal Guidance [TA246] [webpage on the Internet]LondonNational Institute for Health and Care Excellence2012 [cited January 27, 2015]. Available from: https://www.nice.org.uk/guidance/ta246Accessed March 10, 2015
- HockenhullJElremeliMCherryMGA systematic review of the clinical effectiveness and cost-effectiveness of Pharmalgen® for the treatment of bee and wasp venom allergyHealth Technol Assess20121612IIIIV111022409877
- GoldenDBKagey-SobotkaALichtensteinLMSurvey of patients after discontinuing venom immunotherapyJ Allergy Clin Immunol20001052 Pt 138539010669863
- PeterneljASilarMErzenRKosnikMKorosecPBasophil sensitivity in patients not responding to venom immunotherapyInt Arch Allergy Immunol2008146324825418270492
- Oude ElberinkJNde MonchyJGGoldenDBBrouwerJLGuyattGHDuboisAEDevelopment and validation of a health-related quality-of-life questionnaire in patients with yellow jacket allergyJ Allergy Clin Immunol2002109116217011799384
- Oude ElberinkJNvan der HeideSGuyattGHDuboisAEImmunotherapy improves health-related quality of life of adult patients with dermal reactions following yellow jacket stingsClin Exp Allergy200939688388919364334
- KoschelDSSchmiesMWeberCNHöffkenGBalckFTolerated sting challenge in patients on Hymenoptera venom immunotherapy improves health-related quality of lifeJ Investig Allergol Clin Immunol2014244226230
- FischerJTeufelMFeidtAGielKEZipfelSBiedermannTTolerated wasp sting challenge improves health-related quality of life in patients allergic to wasp venomJ Allergy Clin Immunol2013132248949023639308
- YavuzSTSahinerUMBuyuktiryakiBPD41 – Risk factors for side effects during venom immunotherapy in children with hymenoptera venom allergyClin Transl Allergy20144P41
- RuëffFPrzybillaBBilóMBPredictors of side effects during the buildup phase of venom immunotherapy for Hymenoptera venom allergy: the importance of baseline serum tryptaseJ Allergy Clin Immunol20101261105111.e520542320
- RuëffFPrzybillaBBilóMBClinical effectiveness of hymenoptera venom immunotherapy: a prospective observational multicenter study of the European academy of allergology and clinical immunology interest group on insect venom hypersensitivityPLoS One201385e6323323700415
- LerchEMüllerURLong-term protection after stopping venom immunotherapy: results of re-stings in 200 patientsJ Allergy Clin Immunol199810156066129600496
- MüllerUHelblingABerchtoldEImmunotherapy with honeybee venom and yellow jacket venom is different regarding efficacy and safetyJ Allergy Clin Immunol19928925295351740583
- RuëffFPrzybillaBMüllerUMosbechHThe sting challenge test in Hymenoptera venom allergy. Position paper of the Subcommittee on Insect Venom Allergy of the European Academy of Allergology and Clinical ImmunologyAllergy19965142162258792917
- BerchtoldEMaibachRMüllerUReduction of side effects from rush-immunotherapy with honey bee venom by pretreatment with terfenadineClin Exp Allergy199222159651551035
- MüllerUHariYBerchtoldEPremedication with antihistamines may enhance efficacy of specific-allergen immunotherapyJ Allergy Clin Immunol20011071818611149995
- BrockowKKiehnMRiethmüllerCVielufDBergerJRingJEfficacy of antihistamine pretreatment in the prevention of adverse reactions to Hymenoptera immunotherapy: a prospective, randomized, placebo-controlled trialJ Allergy Clin Immunol199710044584639338537
- NiedoszytkoMGruchała-NiedoszytkoMJassemEGene expression analysis in allergology: the prediction of Hymenoptera venom allergy severity and treatment efficacyClin Transl Allergy2013313524160178
- BoyleRJDicksonRHockenhullJCherryMGElremeliMImmunotherapy for Hymenoptera venom allergy: too expensive for European health care?Allergy201368101341134224134604
- GaleraCSoohunNZankarNCaimmiSGallenCDemolyPSevere anaphylaxis to bee venom immunotherapy: efficacy of pretreatment and concurrent treatment with omalizumabJ Investig Allergol Clin Immunol2009193225229
- PalganKBartuziZGotz-ZbikowskaMTreatment with a combination of omalizumab and specific immunotherapy for severe anaphylaxis after a wasp stingInt J Immunopathol Pharmacol201427110911224674685
- CoxLCompalatiEKundigTLarcheMNew directions in immunotherapyCurr Allergy Asthma Rep201313217819523315329
- PatriarcaGNuceraERoncalloCSublingual desensitization in patients with wasp venom allergy: preliminary resultsInt J Immunopathol Pharmacol200821366967718831935
- SeverinoMGCortelliniGBonadonnaPSublingual immunotherapy for large local reactions caused by honeybee sting: a double-blind, placebo-controlled trialJ Allergy Clin Immunol20081221444818468672
- FellrathJMKettnerADufourNAllergen-specific T-cell tolerance induction with allergen-derived long synthetic peptides: results of a phase I trialJ Allergy Clin Immunol2003111485486112704369
- TarziMKlunkerSTexierCInduction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapyClin Exp Allergy200636446547416630151
- KeatingMUKagey-SobotkaAHamiltonRGYungingerJWClinical and immunologic follow-up of patients who stop venom immunotherapyJ Allergy Clin Immunol1991883 Pt 13393481890261
- GoldenDBFatal insect allergy after discontinuation of venom immunotherapyJ Allergy Clin Immunol2001107592592611344366
- EboDGVan VaerenberghMde GraafDCBridtsCHDe ClerckLSSabatoVIn vitro diagnosis of Hymenoptera venom allergy and further development of component resolved diagnosticsExpert Rev Clin Immunol201410337538424490811