Abstract
It is increasingly recognized that large proportions of patients with asthma remain poorly controlled with daily symptoms, limitation in activities, or severe exacerbations despite traditional treatment with inhaled corticosteroids and other agents. This suggests that there is considerable scope for the refinement of traditional guidelines on the use of inhaled therapies in asthma and also a need for the development of novel therapeutic agents, particularly for the treatment of severe asthma. This review aims to discuss a range of emerging treatment approaches in asthma. Firstly, we will set the scene by highlighting the importance of achieving good asthma control in a patient-focused manner and discussing recent work that has furthered our understanding of asthma phenotypes and paved the way for patient-specific treatments. Secondly, we will review new strategies to better use the existing therapies such as inhaled corticosteroids and long-acting β2-agonists that remain the mainstay of treatment for most patients. Finally, we will review the novel therapies that are becoming available, both pharmacological and interventional, and discuss their likely place in the management of this complex disease.
Introduction
Asthma is largely regarded as a chronic inflammatory respiratory disease manifesting in variable airflow obstruction and symptoms of cough, wheeze, and dyspnea. This definition continues to undergo refinement in concert with developments in pathophysiology, immunology, and pharmacology.Citation1 The infiltration of airway tissues with increased numbers of eosinophils, a hallmark of allergic disease, is also seen in asthma.Citation2 Although debate continues as to the role of these inflammatory cells in mediating the expression of asthma, little doubt remains to the long-known efficacy of glucocorticoids in reducing both the blood and airway eosinophilia with consequent improvements in symptoms and lung function,Citation3,Citation4 and an attenuation in its decline.Citation5
Most patients with asthma achieve good disease control with principal use of inhaled corticosteroids and long-acting β2-adrenoceptor agonists (LABAs) that are the mainstay of asthma therapy. These therapies, however, unfortunately fail to afford a significant proportion of patients with good control of their symptoms or prevent severe exacerbations.
There are a number of possible reasons for this limitation in the efficacy of traditional therapies, including 1) a failure of traditional guidelines to reflect patients’ own priorities of asthma control; 2) inappropriate timing of the introduction of treatments or the use of inadequate doses; 3) a poor understanding of different asthma subgroups, which due to their distinct pathophysiology have different pharmacological responses; 4) limitations in the ability of patients to adhere to prescribed regimens; and 5) a proportion of patients genuinely having severe disease who are resistant to treatment (difficult-to-treat asthma). This review will outline a number of strategies that are now recommended to address these limitations in asthma management. We will highlight the important goals in asthma management, offer definitions of difficult-to-treat asthma, and present the background to the development of our understanding of asthma phenotypes before reviewing specific management strategies and novel therapeutic options.
Importance of asthma control and preventing exacerbations
The goal of achieving good asthma control has become increasingly important with the recognition of its increasing prevalence in the general population.Citation6 Given that not all emergency visits for asthma or exacerbations can be avoided, they represent a high cost in terms of a poorer quality of life, days off work or school, and the consequent financial loss while poor symptom control invariably gives rise to increased health care utilization. Emergency hospital admissions, for example, are significantly costly, accounting for £61 million of the estimated £1 billion cost of asthma to the NHS each year in the UK.Citation7 In addition, although severe asthma accounts for <5% of asthma in the general population, it consumes a disproportionate share of resources,Citation8 with associated health care expenditures in severe asthma being more than 6 times those of mild asthma.Citation9 That said, it is increasingly recognized that although severe exacerbations and hospital admissions represent the extreme of asthma morbidity, many patients with relatively mild asthma continue to experience unacceptable levels of daily symptoms, which significantly impacts their day-to-day activities and health status.Citation10,Citation11 Consequently, current asthma treatment guidelines (BTS/SIGN 2009,Citation12 ATS/ERS joints statement,Citation13 and GINACitation14) have been updated to highlight the importance of adjusting asthma therapy with the aim of attempting to prevent severe exacerbations and admissions while also minimizing daily symptoms and maximizing quality of life. They also recognize the importance of considering the likelihood of future deteriorations in asthma control when reviewing treatment regimens for individual patients.Citation15
Difficult-to-treat asthma
Severe asthma represents an extreme in the spectrum of the asthma population in those (5%–10%) who despite a period of extensive re-evaluation of diagnosis and management cannot be controlled with a combination of high-dose inhaled corticosteroids together with LABAs.
Although no universally agreed definition exists, various labels, such as refractory asthma (),Citation16 severe asthma, and therapy-resistant asthma, have all been ascribed to this aspect of asthma. Difficult-to-treat asthma may be characterized by poor symptom control, persistent airflow obstruction, and/or recurrent exacerbations, including fatal or near-fatal episodes, despite high medication requirements to maintain disease control, which itself too often complicates the illness.Citation16 The concept of difficult-to-treat asthma is highlighted here because this group of patients is likely to be the group for which novel approaches, including specific targeted therapies, are likely to be particularly needed. In these patients, existing therapies either do not achieve adequate control or do so only at high doses, leading to unacceptable side effects. The lack of a consistent definition and the wide range of clinical presentations reflect the heterogeneous nature of asthma in generalCitation17,Citation18 and difficult-to-treat asthma in particular.Citation1,Citation2 A sound understanding of this heterogeneity is crucial for the successful development and appropriate utilization of new therapies for difficult-to-treat asthma and will now be discussed (see ).
Table 1 Refractory asthma: workshop consensus for typical clinical featuresTable Footnote*
Asthma heterogeneity and phenotyping
Characterizing patients with asthma offers particular benefits in treating moderate to severe asthma providing not only prognostic information but also targeted therapeutic opportunities and helping to formulate a tailored approach to management. Historically, various methods of characterizing the heterogeneity of asthma have been considered based on specific features such as the age of onset, nature of airflow obstruction, pattern of exacerbations, or inflammatory profile.Citation1,Citation17,Citation19 These systems of asthma classification are limited by inconsistency of method, subjective bias, and a failure to address the multiple dimensions of the disease, leading to models of little clinical significance. These limitations have led to increasing interest in more careful attempts at classification using multidimensional analytical techniques to identify specific asthma phenotypes.
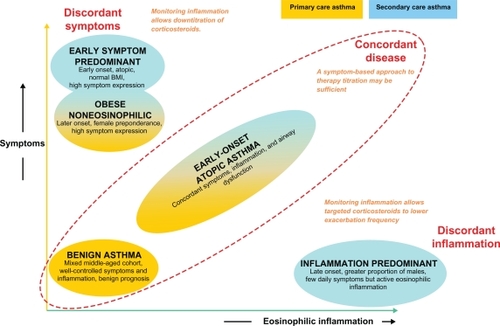
We have recently described the results of a study using the technique of cluster analysis within a population of patients with severe asthma treated in our clinic compared with a second population managed in primary care. Cluster analysis is a multivariate statistical tool that seeks to organize information about variables so that heterogeneous groups of subjects can be classified into relatively homogeneous subgroups or ‘clusters’. By including large numbers of variables, this approach may reveal associations that were not previously evident and lead to the identification of distinct novel phenotypes. Our results () identified two clusters (early-onset atopic asthma and obese, noneosinophilic asthma) that were common to both asthma populations. In contrast, two clusters characterized by marked discordance between symptom expression and eosinophilic airway inflammation (early-onset, symptom-predominant asthma and late-onset, inflammation-predominant asthma) were specific to refractory asthma. The identification of these separate phenotypes of asthma and in particular the recognition of the disparity between symptoms and inflammation highlight the need for the development of different management strategies for different groups of patients. A management strategy based on targeting airway inflammation may be particularly helpful for patients with inflammation-predominant asthma,Citation15 for example, and will be discussed later. Further consideration of the complexity of the different aspects of airways disease has led to the recent suggestion that traditional labels applied to airways disease (including asthma and chronic obstructive pulmonary disease [COPD]) be replaced by an A to E alphabetical assessment tool using factors potentially responsible for morbidity: airway hyperresponsiveness, bronchitis, cough reflex hypersensitivity, and damage to the airway and surrounding lung, and extrapulmonary factors.Citation20 This may offer a useful checklist to remind the clinician of the need to relate clinical presentation with underlying pathophysiology, and in doing so to select the most appropriate treatment strategy. For some patients, particularly those with difficult-to-treat asthma, this may be a novel pharmacological agent, whereas, for others, more judicious use of existing treatments may be sufficient to achieve good control.
Improving the use of existing asthma therapies: patient-focused management strategies
Self-management plans
Patients with asthma deserve both oral and written advice helping them to recognize the signs of asthma and what to do when it is worsening.
With this in mind, one of the most significant advances in patient-focused developments in asthma therapy has been the way medications are employed. In particular, and in common with most chronic diseases, empowering patients to take responsibility through education is considered to be necessary to help patients gain the skills and confidence to control their asthma. A practical way of doing this is to provide patients with a written asthma self-management or ‘action’ plan, an example of which is given in . This has seen distinct benefits both in economic terms and in reductions in morbidity in a recent Cochrane review.Citation21
Table 2 Example of adult asthma self-management plan: what to do and when
It is well recognized that patients frequently fail to fully appreciate the severity of their asthma symptoms.Citation22 Prior to most exacerbations is a period of deteriorating control usually seen 10–3 days prior to a more significant decline in symptom control.Citation23 This pattern of escaping asthma control underwrites the utility of self-management plans, which, although based on an objective test such as peak flow measurements, often parallel a decline with symptoms of increasing wheeze and dyspnea. It allows patients, in particular those with concordant airway inflammation and symptoms, the prospect of recognizing escaping control and initiating early treatment with inhaled corticosteroids, rapidly suppressing airway inflammation that occurs during the evolution of an exacerbation, preventing its development, and thereby reducing mortality and morbidity by preventing hospital admissions.Citation24
There is, however, a substantial variation in the structure and implementation of self-management plans,Citation25 and factors facilitating their long-term use are not fully understood.Citation26
Stepwise incremental management
Traditionally, inhaled therapies have been used in a symptom-guided strategy, which remains the most widely accepted and practised strategy in optimizing asthma control. Although inhaled corticosteroidsCitation27 have been shown to be arguably the most important therapeutic and beneficial intervention in patients with airway disease, the importance of LABAs cannot be understated.
The Gaining Optimal Asthma Control (GOAL)Citation10 study, one of the largest studies utilizing both combination and separate therapies, explored the potential of achieving total control of asthma symptoms. In this study, treatment was optimized in those with uncontrolled asthma symptoms, by increasing combination therapy at three monthly reviews until all asthma-related symptoms were abolished based on widely accepted guidelines.
Reconfirming earlier findings from similar trials of the importance of LABAs,Citation28 it demonstrated that good control was achieved more rapidly by this strategy and at a lower corticosteroid dose with the fixed-dose inhaled corticosteroid and LABA combination product (seretide) than fluticasone alone despite total control not being achieved in the majority of patients (41% versus 28%, seretide versus fluticasone). Exacerbations occurred at much lower rates overall in this cohort (<0.5 mean exacerbations/patient/year in both groups), further suggesting that most of these patients had concordant disease appropriate for this symptom-driven type of management. Other phenotypes, however, run the potential risk of over- or undertreatment.
The advantage of combination therapy in being able to control asthma at lower doses of corticosteroids while reducing the total number of inhalers has since led to its widespread adoption in most management guidelines. There has been recent concern that though LABAs improve lung function and symptoms in asthma, their use may be associated with more severe symptoms and increased mortalityCitation29,Citation30 during severe exacerbations of asthma. It is not clear, however, whether the link between LABAs and worsening asthma exacerbations is causal or rather whether the use of LABAs reflects more severe asthma or may reduce adherence to corticosteroids by improving symptoms without addressing underlying airway inflammation. Nevertheless, these concerns have led to a decision by the US Food and Drug Administration to issue a ‘black box’Citation31 warning to confine the use of LABAs to patients who remain poorly controlled despite inhaled corticosteroids and to recommend in pediatric and adolescent patients that LABAs should only be prescribed in a combination inhaler to ensure adherence to both medications.
Single maintenance and reliever therapy
Inherent in persistent asthma is the periodic need for reliever medication for symptoms that may invite overreliance on short-acting β2-agonists at the expense of reduced adherence to inhaled corticosteroid therapy (ICS).
Formoterol, an LABA, uniquely offers both immediate (within 1–3 min) and sustained bronchodilationCitation32 equivalent to salbutamol, allowing its use in combination preparations to be used in single maintenance and reliever therapy (SMART) recognized by international guidelines.
Although short-acting bronchodilators provide rapid relief of symptoms such as dyspnea associated with allergen-induced bronchoconstriction, they fail to address the accompanying eosinophilic inflammation known to precede exacerbations in asthma.Citation33 One potential advantage of the SMART strategy is that patients will simultaneously receive additional doses of inhaled corticosteroids alongside a bronchodilator when they use their combined inhaler for symptom relief. This may target anti-inflammatory treatment to periods of poor control when it is most needed, aside from the convenience the strategy may give to patients.
The FACET studyCitation28 demonstrated that both budesonide and formoterol had complementary effects on reducing exacerbations in adults and provided greater improvements in symptoms at low doses. Pharmacologically, both budesonide and formoterol reduce the secretion of granulocyte-macrophage colony-stimulating factor, counteracting the capacity of formoterol alone to induce interleukin-8 (IL-8) production, which may itself facilitate improved asthma control.Citation34 Corticosteroids also promote increased expression of β2-receptors through gene transcription, Citation35,Citation36 protecting against the loss of LABA response, which is essential when used in rescue therapy.
Several studies have consequently sought to demonstrate the added advantages of SMART over conventional maintenance therapy, though with mixed results. Exacerbations (the clinical endpoint of most comparison trials of SMART) have been significantly reduced when higher doses of ICS have been employed in the flexible dosing of budesonide/formoterol therapy. O’Byrne et alCitation37 showed that although patients on SMART averaged 50% higher mean daily ICS doses than patients using traditional fixed combination therapy with short-acting β2-agonists, they used significantly less ICS overall than those on higher-dose ICS alone. SMART also conferred at least a 45% reduction in severe exacerbations compared with other treatment arms. Other studies using similar combination therapies in fixed doses have found similar reductions in exacerbations. Bousquet et alCitation38 in a study involving 2309 patients across 17 countries, compared the use of formoterol/budesonide as maintenance and reliever therapy with sustained high-dose salmeterol/fluticasone. No significant difference in the primary endpoint of time to first exacerbations was seen, though there was a modest reduction in the total number of exacerbations again despite being on a lower dose of inhaled corticosteroid. A retrospective analysis of several studies of the use of SMART has raised concerns that this strategy fails to provide good day-to-day symptom control for the majority of patientsCitation39 in addition to the anti-inflammatory mode of action being cast into doubt.Citation40
Although not demonstrably improving asthma control above other combination therapies, it does allow patients a reduction in inhalers, which may play a helpful role in improving adherence,Citation41 particularly in those who are poorly adherent to ICS.Citation42
Airway inflammometry
The use of noninvasive biomarkers in monitoring airway inflammation has provided an alternative method to patient-driven symptom management for the assessment and management of asthma.
InflammometryCitation43 provides a direct measure to identify the need for corticosteroids in relation to underlying airway inflammation, allowing judicious use of these agents to improve symptoms and reduce exacerbations and medication side effects. The use of nebulized hypertonic saline to induce sputum has provided a simple, safe, noninvasive airway sampling techniqueCitation44 to assess airway inflammation and has now secured a place in managing patients with chronic coughCitation45 and refractory asthmaCitation12,Citation46,Citation47 where it has shown particular benefit, though its use is still mainly limited to tertiary centers.
Induced sputum eosinophil counts
Healthy subjects usually have a sputum eosinophil count of <1.9%, but this is commonly elevated in up to 60% of patients with asthma. Additionally, infiltration of the airway mucosa with activated eosinophils is observed in postmortem examinations of patients who have died of acute severe asthma.Citation48 Early studies published in the Lancet in 1958 had shown the importance of an airway eosinophilia in predicting the clinical response to prednisolone with a reduction in eosinophils seen in sputum smears.Citation49 The response to corticosteroids is further explained by the increased eosinophil apoptotic rate observed.Citation50
A cutoff of 3% for sputum eosinophilia has been shown to identify individuals with corticosteroid-responsive asthmaCitation51 and, utilizing this, weCitation46 successfully demonstrated the benefits of a management strategy directed at normalizing eosinophilic airway inflammation over a standard symptom-based management strategy. This was based on the hypothesis that low sputum eosinophil counts would predict few exacerbations.
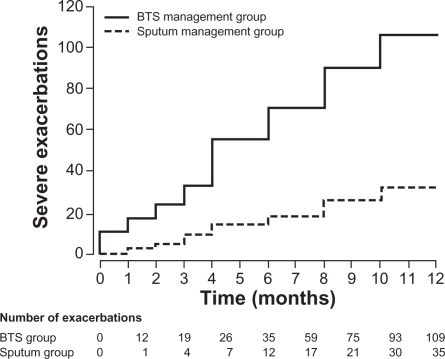
We randomized 74 attending outpatients with moderate to severe asthma into treatments based on standard guidelines (BTS) or to a management strategy directed at maintaining the sputum eosinophil count at or below 3% using anti-inflammatory therapy, both inhaled and oral. If the sputum eosinophil count was <1%, irrespective of asthma control, anti-inflammatory treatment was reduced. If the eosinophil count was 1%–3%, no changes to anti-inflammatory treatment were made, and if the eosinophil count was >3%, anti-inflammatory treatment was increased. Bronchodilator treatment was modified according to individual patients’ symptoms, rescue β2-agonists use, and peak expiratory flow readings compared with baseline using the same measures as in the standard management group (). There were significantly fewer severe exacerbations in the sputum management group in contrast to the BTS management group (35 versus 109 total exacerbations, respectively, P = 0.01) and fewer rescue courses of oral corticosteroids (24 versus 73, P = 0.008). Additionally, we demonstrated a significant reduction in the inhaled corticosteroid dose in those managed by sputum guidelines compared with baseline, in contrast to an increase in dose in the BTS group. This demonstrated that the sputum management strategy allowed appropriate targeting of anti-inflammatory treatment where it was most needed and an avoidance of inappropriately high doses in patients who were unlikely to benefit. Accepting that widespread inflammometry using induced sputum is not available, this tailored approach offers significant proven benefits to patients with severe asthma, particularly in avoiding harmful unwarranted treatment.
Figure 2 Cumulative asthma exacerbations in the BTS management group and the sputum management group.
Copyright © 2010, Elsevier Limited. Reproduced with permission from Green RH, Brightling Ce, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360(9347):1715–1721.
Fraction of nitric oxide in exhaled air
Alternative noninvasive measurements of airway inflammation have since been sought to offer supportive bedside measures easily and readily available outside of large hospitals or research centers. Against this background, with its reproducibility and noninvasive nature, the measurement of fraction of exhaled nitric oxide (FeNO), which is elevated in the presence of airway inflammation, has attracted interest furthered by its relationship, albeit loose, to sputum eosinophils. Although confounded by factors such as atopyCitation52 and respiratory viral infections,Citation53 and widely varying agreed cutoff values and confidence intervals (CIs),Citation54,Citation55 FeNO appears to be a useful screening and management tool in asthma in defining lower airway pathology, being rarely present in nonasthmaticsCitation54,Citation56 and lowered by use of corticosteroids.Citation57
Two main trials in adults have evaluated the use of FeNO in conjunction with clinical parameters to titrate inhaled corticosteroid dose. Shaw et alCitation58 recruited 128 patients from primary care, randomized them to two groups, and evaluated them over a 1-year period. ICS was adjusted according to FeNO levels, where above 26 parts/billion (ppb) it was increased and if it was <16 ppb or <26 ppb on two consecutive occasions, treatment was decreased. Additional bronchodilator therapy was used to control symptoms. No significant differences were observed between the two treatment arms. The FeNO management strategy resulted in 0.33 exacerbations/patient/year (0.69) in the FeNO group and 0.42 (0.79) in the control group (mean difference −21%; 95% CI: −57%–43%; P = 0.43). The study did not, however, have sufficient power to demonstrate a more modest effect on exacerbation frequency.
Smith et alCitation56 used FeNO in managing an ICS downtitration and compared it with conventional guidelines based on a protocol algorithm and an FeNO cutoff of 35 ppb equivalent. This also resulted in a 40% ICS dose reduction without change in the exacerbation frequency. Although results from these and other studiesCitation59 using different FeNO cutoff levels suggest a beneficial effect in using FeNO in managing asthma, it has yet been shown to improve asthma control. Moreover, trials in pediatric asthma populations included in a recent Cochrane reviewCitation60 potentially resulted in higher doses of inhaled corticosteroids in children. Although FeNO has shown promise as a tool in the diagnosis and treatment of asthma, further studies incorporating individualized FeNO profiles into treatment algorithms are needed.
The recognition and management of nonadherence
Despite all the advances in expensive biological therapeutics, identifying nonadherence is of important diagnostic value in severe asthma. Worryingly, several studies have found that a significant proportion of patients with difficult asthma are poorly adherent to inhaled and oral corticosteroid therapy. Gamble et alCitation61 found that 35% of patients collected less than half of their prescription, and 88% admitted poor adherence with inhaled therapy after initial denial. We have also shown that patients with severe asthma who adhere poorly to inhaled corticosteroids have worse asthma control and higher ITU admissions.Citation62 Not infrequently, patients also forget or fail to understand proper inhaler technique,Citation63 which should be demonstrated at least once and perhaps on repeated occasions, as adherence is learned behavior that can be improved with practice and reinforcement. Reasons for poor adherence are numerous,Citation64,Citation65 though strategies to prevent and correct nonadherence are difficult and there is no convincing evidence for their success in asthma.Citation66 Suggested approaches are to improve patient education by targeted interventions, and there is some evidence to support the introduction of asthma self-management plans as already outlined in improving adherence.Citation67 The use of combination inhalers as single inhaler therapy as described above is attractive, and there is some evidence to suggest that this approach may increase the likelihood of adherence to inhaled corticosteroids,Citation42 although further work is needed to confirm this. Finally, for some patients with particularly severe asthma who are thought to be nonadherent to treatment and who are at risk of near-fatal attacks, we and others have used short courses of intramuscular triamcinolone to demonstrate a good steroid response and exclude true corticosteroid resistance.Citation4 Improvement in asthma control following systemic corticosteroids in this way may increase patients’ awareness of their poor asthma control and motivate them to start taking their treatment regularly to maintain improvements in their symptoms.Citation4,Citation68,Citation69
Novel therapeutic options
Despite effectively reducing exacerbations, systemic corticosteroids have significant adverse effects in asthma that prohibit their long-term use and have driven the search for alternative therapies with acceptable risk-to-benefit ratios not achievable with commonly used immunosuppressants such as methotrexate, azathioprine, gold, or cyclosporine.Citation63
Much recent work has therefore been concerned with the introduction of novel therapies targeting specific components of the anti-inflammatory pathway usually via a systemic approach. These include monoclonal antibodies (mAbs) targeting IgE, tumor necrosis factor-alpha (TNF-α) or IL-5, and thermal bronchoplasty and antifungal agents. In view of their invasive nature, risk of adverse effects, and expense, these treatments are likely to have narrow, well-defined roles in asthma management and will usually be reserved for patients with difficult-to-treat asthma. The evidence supporting their use will now be discussed.
Anti-IgE therapy
Allergens are one of the many multiple triggers in asthma,Citation70 and through their tendency for IgE production give rise to airway inflammation, which is an important aspect of allergic asthma and exacerbations.
The first of the European Community Respiratory Health Surveys, a cross-sectional, multicenter study, identified the rates of atopy as defined by an elevated specific IgE to common aeroallergens as varying from 4% to 61%, depending on the country examined.Citation71
The IgE receptor FcɛRI is significantly upregulated on eosinophils, mast cells, macrophages, and dendritic cells in patients with rhinitis and allergic asthma. Mast cell activation through IgE releases a variety of proinflammatory cytokines including IL-4, IL-13, and IL-5 all contributing to the inflammatory and bronchoconstrictive response seen in asthma.
Omalizumab, a recombinant humanized monoclonal antibody, is the first treatment to specifically bind IgE and block its effects. It targets the Fc region that attaches to the high-affinity receptor FcɛRI, binding free IgE, significantly reducing circulating free IgE levels, and downregulating the receptor.
In a 28-week, randomized, placebo-controlled trial, the Investigation of Omalizumab in Severe Asthma Treatment (INNOVATE) studyCitation72 demonstrated the efficacy of omalizumab in those with severe persistent allergic asthma as add-on therapy, showing significant improvements in quality of life across all domains and a 26% reduction in exacerbation rates when corrected for baseline exacerbations compared with placebo (P = 0.002).
Earlier studies in patients with sputum eosinophilia elucidated their mechanisms of action in the depletion of IgE from airway tissue with marked reductions in airway eosinophilia as measured by sputum and bronchial biopsies.Citation73
Patients with recurrent exacerbations appear to benefit most from this treatment. A recent Cochrane reviewCitation74 concluded highly significant reductions in frequency and duration of exacerbations, including a 90% reduction in hospitalization, together with a reduction in the use of both inhaled steroids and rescue drugs. Compliance issues are also minimized with its administration as 2–4 weekly subcutaneous injections with the dose based on the patients’ serum total IgE level and body weight.
Omalizumab treatment is generally well tolerated with few adverse effects, although anaphylaxis attributed to its administration has been quoted as being between 0.1% and 0.2% in clinical trials and postmarketing survelliance.Citation75 In addition, clinical trial data suggest that, numerically, more malignancies were reported in patients receiving omalizumab compared with control (0.5% versus 0.2%). This difference was not statistically significant, but the long-term risk of additional malignancies is unknown. The major limitation to the use of omalizumab is its cost, which restricts its use in patients with severe atopic uncontrolled asthma despite adequate doses of inhaled corticosteroids and LABAs who demonstrate persistent symptoms and a degree of airflow obstruction and who are sensitized to a perennial allergen. In addition, patients’ total IgE levels should be in the range of 30–1500 IU/mL, and some patients will have a total IgE greater than the maximum recommended for dosing in relation to their body weight. A recent audit in our clinic showed that only 34 out of 251 patients with difficult-to-control asthma were eligible for treatment based on these licensing criteria.Citation76 Furthermore, in the UK, guidance from the National Institute for Health and Clinical Excellence now limits its prescription to patients who meet the licensing criteria and who have also had two or more hospital admissions or one admission plus two A&E attendances for asthma exacerbations. Using these additional criteria, only 6.2% of our patients were eligible for treatment with omalizumab. Nevertheless, if funding is available, this does appear to be a promising therapy for patients with severe atopic disease and may be particularly helpful to patients who have additional atopic diseases alongside asthma, such as severe rhinitis.Citation77
Antitumor necrosis factor-α therapy
In patients with rheumatoid arthritis, TNF-α, with its ability to promote inflammation, is markedly increased in the synovial fluid, and treatment with TNF-α mAbs has resulted in substantial improvement in disease activity scores.Citation78 Both macrophage and mast cells release TNF-α in allergic responses via IgE-dependent mechanismsCitation79 and can also induce its own production via an autocrine mechanism. The TNF-α axis is found to be upregulated in patients with severe asthmaCitation80,Citation81 and is consequently thought to play a key role in the pathogenesis of inflammatory disorders. This recognition has led to trials of anti-TNF-α therapy in patients with asthma. Despite initially promising results, subsequent studies have not only shown a marked heterogeneous response, suggesting benefit to a small subgroup, but also highlighted concerns about its safety.
Using the most widely studied anti-TNF agent in asthma, etanercept, Berry et alCitation80 demonstrated significant improvements in airways hyperresponsiveness and quality of life and reduced expression of membrane-bound TNF-α by peripheral blood monocytes in patients with refractory asthma treated for 10 weeks. Morjaria et alCitation82 found small but similar significant improvements in patients’ asthma control questionnaire responses but failed to replicate other earlier findings in an unselected refractory asthma population. The largest and longest study to date, using golimumab for 52 weeks and involving 231 patients, found no benefit compared with placebo.Citation83
Unfortunately, anti-TNF antibody agents may increase the risk of serious infections and malignancies in patients with rheumatoid arthritis,Citation84 though larger, more carefully selected studies may be required before this therapy is abandoned.
Anti-IL-5 therapy
IL-5 has long been regarded as an important cytokine responsible for eosinophil differentiation, maturation, migration, and survival. mAbs directed against this cytokine offering the prospect of abolishing exacerbations without entertaining the significant problems associated with corticosteroid therapy seemed possible.
The initial enthusiasm for anti-IL-5 was, however, tampered by the apparent failure of eosinophilic suppression through anti-IL-5 to confer clinical benefit in the asthmatic late response. Leckie et alCitation85 showed that anti-IL-5 blockade effectively suppressed blood and sputum eosinophilia in the mild asthmatic cohort, but in studying airway hyper-responsiveness as an outcome failed to appreciate that these measures are not closely associated with eosinophilic airway inflammation. This suggests that the choice of an alternative outcome measure may have demonstrated significant patient benefits. The demonstration that a sputum and bronchial submucosal eosinophilia occurs in eosinophilic bronchitis, a condition that presents with chronic cough without airway hyperresponsiveness,Citation86 further supports this view, as did earlier studies demonstrating significant reductions in eosinophilic exacerbations using management strategies that control airway inflammation.Citation46
In a double-blind, placebo-controlled trial, the largest to date, involving patients with refractory asthma and eosinophilic inflammatory phenotypes, we have shown the profound effect of mepolizumab on suppressing both blood and sputum eosinophils.Citation87 This effect consequently reduced asthma exacerbations significantly, with a reduction in episodes requiring high-dose oral corticosteroids (2 versus 3.4 exacerbations/subject/year; P = 0.02) as well as improved quality of life. There were no improvements in symptoms or forced expiratory volume in 1 second consistent with previous studies,Citation88 further illustrating the disassociation between eosinophilic airway inflammation and day-to-day symptoms and lung function while confirming the increased risk of exacerbations seen alongside uncontrolled airway eosinophilia. Nair et alCitation89 also evaluated a similar steroid-dependant cohort involving 20 patients; 9 were treated with mepolizumab 750 mg administered over 5 monthly infusions and 11 patients received placebo. Again, the mepolizumab group experienced a significant decrease in asthma exacerbations and were able to reduce their prednisolone dose significantly.
Further work is ongoing with the larger multicenter study Dose Ranging Efficacy and Safety with Mepolizumab in Severe Asthma (DREAM)Citation90 underway, but the likely role for this treatment will be in a selected group. Importantly, characterizing patients with inflammometry provides targeted treatment and is likely to be particularly helpful in selecting patients for treatment with anti-iL-5, because those patients with inflammation-predominant disease are particularly likely to benefit.
Antifungal therapy
With up to 60% of patients with asthma being atopic to common aeroallergens, sensitization to fungi appears to be an emerging phenotype conferring an increased risk of hospital and ITU admissions.Citation91 Exposure to allergenic fungi is ubiquitous in the aerospora, which we have shown to be present in sputum from colonized airways of patients with asthma,Citation92 further increasing the body of evidence about the link between fungal sensitization and severe asthma.Citation93
Several trials in patients with allergic bronchopulmonary aspergillosisCitation94 have established the role of antifungal therapy, but only recently have trials in asthma been undertaken. The Fungal Asthma Sensitization Trial (FAST)Citation95 studied patients with severe asthma who were sensitized by a skin prick or radioallergosorbent testing to one or more fungal allergens and did not fulfill the criteria for allergic bronchopulmonary mycosis. Treatment with oral itraconazole 200 mg twice daily/placebo for 32 weeks resulted in clinically significant improvements of asthma quality of life scores as well as rhinitis and morning peak flows.
Interestingly, the precise mechanisms of antifungal action in asthma remain unknown. Although generally accepted to modulate the immunological response, concerns remain about the azole–corticosteroid interaction as seen in earlier trials with adrenal suppression.Citation94 Although active against some species of Aspergillus, itraconazole is not active against all of the fungal species that the human airway is constantly subjected to. Its microbiological activity is further limited by variable absorption and need for monitoringCitation96 in contrast to newer triazoles that have better oral bioavailability. Further trials in this interesting area are warranted.
Thermal bronchoplasty
Targeted treatment of the airway smooth muscle hypertrophy seen in chronic asthma, aside from other changes of airway remodeling including goblet cell hyperplasia, increased mucus secretion, and increased vascularization, has been made possible with a novel technique utilizing radiofrequency ablation. Treatment involves delivering thermal energy through use of a standard bronchoscope into which a catheter containing an expandable basket is inserted. When extended, this comes into circumferential contact with the walls of targeted airways, thus depleting smooth muscle mass with the hope of attenuating the bronchoconstrictor response.
In clinical studies, thermal bronchoplasty has been shown to reduce parameters of airways hyperresponsiveness and minimize exacerbations. The randomized, double-blind, sham-controlled AIR 2 studyCitation97 built on earlier promising resultsCitation98 and demonstrated a clinically significant improvement in asthma quality of life in up to 80% of patients over those treated with the sham protocol, as well as a 36% reduction in exacerbations with benefits persisting at 1 year.
The benefits were overshadowed early on, however, with a higher rate of exacerbations in the treatment arm up to 6 weeks after therapy (6% more than placebo). All other adverse events were not significantly different. These exacerbations required hospital admissions and steroids, which may have confounded the improved asthma control seen later on. More important was the profound placebo effect, with 64% of sham subjects achieving changes in Asthma Quality of Life Questionnaire of 0.5 or greater.
This procedure offers a novel way to reduce the expression of smooth muscle in patients not controlled with combinations of anti-inflammatory and bronchodilators. This suggests that its greatest potential is in the long-lasting duration of effect in contrast to other therapies, but longer follow-up studies are required to evaluate this.
Conclusion
Treatments for asthma are rapidly evolving with the development of both novel pharmacological therapies and the establishment of new management strategies with which to deploy existing therapies. With these developments has come the recognition that previous goals of therapy can be improved to focus not only on preventing death and severe exacerbations but also on improving day-to-day symptom control and quality of life. Individual treatments and management strategies in general are likely to be most successful where they offer convenience for patients and/or aid adherence. Many of these patient-focused strategies are readily available. Although sputum cell analysis had previously been limited as a research tool, it is gradually gaining widespread acceptance as an invaluable biomarker in clinical practice in concert with the developments in bedside inflammometry using FeNO.
Although novel biological therapies offer a useful adjunct for those patients who are unresponsive to conventional treatment, the varied responses to these agents emphasize the need for careful patient selection. This highlights the vital importance of accurate phenotyping of the asthma population not only to ensure that each individual patient receives the most appropriate therapy but also to maximize the likelihood of the successful development of additional new drugs.
Disclosure
The authors report no conflicts of interest in this work.
References
- McFaddenERJrA century of asthmaAm J Respir Crit Care Med2004170321522115280175
- WardlawAJBrightlingCGreenRWoltmannGPavordIEosinophils in asthma and other allergic diseasesBr Med Bull2000564985100311359633
- DjukanovicRHomeyardSGratziouCThe effect of treatment with oral corticosteroids on asthma symptoms and airway inflammationAm J Respir Crit Care Med199715538268329117012
- ten BrinkeAZwindermanAHSterkPJRabeKFBelEH“Refractory” eosinophilic airway inflammation in severe asthma: effect of parenteral corticosteroidsAm J Respir Crit Care Med2004170660160515215154
- O’ByrnePMPedersenSLammCJTanWCBusseWWSevere exacerbations and decline in lung function in asthmaAm J Respir Crit Care Med20091791192418990678
- BousquetJBousquetPJGodardPDauresJPThe public health implications of asthmaBull World Health Organ200583754855416175830
- Asthma UKHigh cost of asthma in West Midlands Available from: http://www.asthma.org.uk/news_media/media_releases/high_cost_of_asthm_3.html. Updated 2009 Oct 22.
- GodardPChanezPSiraudinLNicoloyannisNDuruGCosts of asthma are correlated with severity: a 1-yr prospective studyEur Respir J2001191616711843329
- Serra-BatllesJPlazaVMorejonEComellaABruguesJCosts of asthma according to the degree of severityEur Respir J1998126132213269877485
- BatemanEDBousheyHABousquetJCan guideline-defined asthma control be achieved? The Gaining Optimal Asthma Control studyAm J Respir Crit Care Med2004170883684415256389
- RabeKFVermeirePASorianoJBMaierWCClinical management of asthma in 1999: the Asthma Insights and Reality in Europe (AIRE) studyEur Respir J200016580280711153575
- SIGN and the British Thoracic Society (BTS)British Guideline on the Management of AsthmaEdinburgh (UK)SIGN and the British Thoracic Society (BTS)2010 Guideline No. 101,ISBN 978 1 90581 28 52008 May, Revised 2009 Jun.
- ReddelHKTaylorDRBatemanEDAn official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practiceAm J Respir Crit Care Med20091801599919535666
- Global Initiative for Asthma (GINA), National Heart Lung and Blood Institute (NHLBI)Global Strategy for Asthma Management and PreventionBethesda (MD)Global Initiative for Asthma (GINA), National Heart, Lung and Blood Institute (NHLBI) Available from: http://www.ginasthma.com. Updated 2010 Jan 12.
- HaldarPPavordIDShawDECluster analysis and clinical asthma phenotypesAm J Respir Crit Care Med2008178321822418480428
- Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. American Thoracic SocietyAm J Respir Crit Care Med200016262341235111112161
- GreenRHBrightlingCEBraddingPThe reclassification of asthma based on subphenotypesCurr Opin Allergy Clin Immunol200771435017218810
- BraddingPGreenRHSubclinical phenotypes of asthmaCurr Opin Allergy Clin Immunol2010101545919907311
- WenzelSEAsthma: defining of the persistent adult phenotypesLancet2006368953780481316935691
- PavordIDWardlawAJThe A to E of airway diseaseClin Exp Allergy2010401626720205696
- GibsonPGPowellHWilsonAJSelf-management education and regular practitioner review for adults with asthmaCochrane Database Syst Rev20033CD00111712535399
- KendrickAHHiggsCMWhitfieldMJLaszloGAccuracy of perception of severity of asthma: patients treated in general practiceBMJ199330769014224248374455
- TattersfieldAEPostmaDSBarnesPJExacerbations of asthma: a descriptive study of 425 severe exacerbations. The FACET International Study GroupAm J Respir Crit Care Med1999160259459910430734
- AbramsonMJBaileyMJCouperFJAre asthma medications and management related to deaths from asthma?Am J Respir Crit Care Med20011631121811208619
- HoltSMasoliMBeasleyRThe use of the self-management plan system of care in adult asthmaPrim Care Respir J2004131192716701633
- RingNMalcolmCWykeSPromoting the use of personal asthma action plans: a systematic reviewPrim Care Respir J200716527128317710351
- HaahtelaTJarvinenMKavaTComparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthmaN Engl J Med199132563883922062329
- PauwelsRALofdahlCGPostmaDSEffect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticos-teroids Establishing Therapy (FACET)N Engl J Med199733720140514119358137
- NelsonHSWeissSTBleeckerERYanceySWDorinskyPMThe Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterolChest20061291152616424409
- WeatherallMWijesingheMPerrinKHarwoodMBeasleyRMeta-analysis of the risk of mortality with salmeterol and the effect of concomitant inhaled corticosteroid therapyThorax2010651394320029037
- US Food and Drug Administration, FDALong-Acting Beta-Agonists (LABAs): New Safe Use RequirementsSilver Spring (MD)FDA Available from: http://www.fda.gov/Safety/MedWatch/SafetyInfor-mation/SafetyAlertsforHumanMedicalProducts/ucm201003.htm. Accessed 2010 Oct 22.
- van NoordJASmeetsJJRaaijmakersJABommerAMMaesenFPSalmeterol versus formoterol in patients with moderately severe asthma: onset and duration of actionEur Respir J199698168416888866595
- JatakanonALimSBarnesPJChanges in sputum eosinophils predict loss of asthma controlAm J Respir Crit Care Med20001611647210619799
- KornSHJerreABrattsandREffects of formoterol and budesonide on GM-CSF and IL-8 secretion by triggered human bronchial epithelial cellsEur Respir J20011761070107711491146
- BaraniukJNAliMBrodyDGlucocorticoids induce beta2-adrenergic receptor function in human nasal mucosaAm J Respir Crit Care Med199715527047109032216
- MakJCNishikawaMBarnesPJGlucocorticosteroids increase beta 2-adrenergic receptor transcription in human lungAm J Physiol19952681 Pt 1L41L467840227
- O’ByrnePMBisgaardHGodardPPBudesonide/formoterol combination therapy as both maintenance and reliever medication in asthmaAm J Respir Crit Care Med2005171212913615502112
- BousquetJBouletLPPetersMJBudesonide/formoterol for maintenance and relief in uncontrolled asthma vs high-dose salmeterol/fluticasoneRespir Med2007101122437244617905575
- BatemanEDReddelHKErikssonGOverall asthma control: the relationship between current control and future riskJ Allergy Clin Immunol2010125360060820153029
- PavordIDJefferyPKQiuYAirway inflammation in patients with asthma with high-fixed or low-fixed plus as-needed budesonide/formoterolJ Allergy Clin Immunol200912351083108919368965
- BosleyCMParryDTCochraneGMPatient compliance with inhaled medication: does combining beta-agonists with corticosteroids improve compliance?Eur Respir J1994735045098013609
- SovaniMPWhaleCIOborneJPoor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help?Br J Gen Pract200858546374318186995
- PavordIDShawDEGibsonPGTaylorDRInflammometry to assess airway diseasesLancet200837296431017101918805315
- PavordIDPizzichiniMMPizzichiniEHargreaveFEThe use of induced sputum to investigate airway inflammationThorax19975264985019227713
- BrightlingCEWardRGohKLWardlawAJPavordIDEosinophilic bronchitis is an important cause of chronic coughAm J Respir Crit Care Med1999160240641010430705
- GreenRHBrightlingCEMcKennaSAsthma exacerbations and sputum eosinophil counts: a randomised controlled trialLancet200236093471715172112480423
- JayaramLPizzichiniMMCookRJDetermining asthma treatment by monitoring sputum cell counts: effect on exacerbationsEur Respir J200627348349416507847
- HoustonJCde NavasquezSTrounceJRA clinical and pathological study of fatal cases of status asthmaticusThorax19538320721313102418
- BrownHMTreatment of chronic asthma with prednisolone; significance of eosinophils in the sputumLancet1958270591245124713612182
- KankaanrantaHLindsayMAGiembyczMAZhangXMoilanenEBarnesPJDelayed eosinophil apoptosis in asthmaJ Allergy Clin Immunol20001061778310887309
- HunterCJBrightlingCEWoltmannGWardlawAJPavordIDA comparison of the validity of different diagnostic tests in adults with asthmaChest200212141051105711948032
- FranklinPJTaplinRStickSMA community study of exhaled nitric oxide in healthy childrenAm J Respir Crit Care Med1999159169739872820
- KharitonovSAYatesDBarnesPJIncreased nitric oxide in exhaled air of normal human subjects with upper respiratory tract infectionsEur Respir J1995822952977538934
- MalmbergLPPelkonenASHaahtelaTTurpeinenMExhaled nitric oxide rather than lung function distinguishes preschool children with probable asthmaThorax200358649449912775859
- OlinACBakeBTorenKFraction of exhaled nitric oxide at 50 mL/s: reference values for adult lifelong never-smokersChest200713161852185617565022
- SmithADCowanJOFilsellSDiagnosing asthma: comparisons between exhaled nitric oxide measurements and conventional testsAm J Respir Crit Care Med2004169447347814644933
- KharitonovSAYatesDRobbinsRALogan-SinclairRShinebourneEABarnesPJIncreased nitric oxide in exhaled air of asthmatic patientsLancet199434388901331357904001
- ShawDEBerryMAThomasMThe use of exhaled nitric oxide to guide asthma management: a randomized controlled trialAm J Respir Crit Care Med2007176323123717496226
- de JongsteJCCarraroSHopWCBaraldiEDaily telemonitoring of exhaled nitric oxide and symptoms in the treatment of childhood asthmaAm J Respir Crit Care Med20091792939718931330
- PetskyHLCatesCJLiAKynastonJATurnerCChangABTailored interventions based on exhaled nitric oxide versus clinical symptoms for asthma in children and adultsCochrane Database Syst Rev20094CD00634019821360
- GambleJStevensonMMcCleanEHeaneyLGThe prevalence of nonadherence in difficult asthmaAm J Respir Crit Care Med2009180981782219644048
- MurphyAProeschelALinnettMEBraddingPGreenRHOral abstract sessionsAllergy201065177
- LeungDYBloomJWUpdate on glucocorticoid action and resistanceJ Allergy Clin Immunol2003111132212532089
- van der PalenJKleinJJvan HerwaardenCLZielhuisGASeydelERMultiple inhalers confuse asthma patientsEur Respir J19991451034103710596686
- BouletLPPerception of the role and potential side effects of inhaled corticosteroids among asthmatic patientsChest199811335875929515829
- BenderBMilgromHRCNonadherence in asthmatic patients: is there a solution to the problem?Ann Allergy Asthma Immunol1997793177185 quiz1851869305223
- JansonSLMcGrathKWCovingtonJKChengSCBousheyHAIndividualized asthma self-management improves medication adherence and markers of asthma controlJ Allergy Clin Immunol2009123484084619348923
- MashBBheekieAJonesPWInhaled vs oral steroids for adults with chronic asthmaCochrane Database Syst Rev20011CD00216011279754
- McGivneySAOgiralaRGEffect of high-dose intramuscular triamcinolone in older adults with severe, chronic asthmaLung1994172273788114514
- WardlawAJSilvermanMSivaRPavordIDGreenRMulti-dimensional phenotyping: towards a new taxonomy for airway diseaseClin Exp Allergy200535101254126216238783
- SunyerJJarvisDPekkanenJGeographic variations in the effect of atopy on asthma in the European Community Respiratory Health StudyJ Allergy Clin Immunol200411451033103915536406
- HumbertMBeasleyRAyresJBenefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATEAllergy200560330931615679715
- DjukanovicRWilsonSJKraftMEffects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthmaAm J Respir Crit Care Med2004170658359315172898
- WalkerSMonteilMPhelanKLassersonTJWaltersEHAnti-IgE for chronic asthma in adults and childrenCochrane Database Syst Rev20062CD00355916625585
- Genentech, IncXolair(R) (omalizumab for subcutaneous use) package insertSouth San Francisco, CA2003
- HargadonBHaldarPShawDEligibility for treatment with omalizumab among patients attending the Glenfield Hospital Refractory Asthma ClinicThorax200661Suppl 2ii57ii133
- AdelrothERakSHaahtelaTRecombinant humanized mAb-E25, an anti-IgE mAb, in birch pollen-induced seasonal allergic rhinitisJ Allergy Clin Immunol2000106225325910932067
- EpsteinFHChoyEHSPanayiGSCytokine pathways and joint inflammation in rheumatoid arthritisN Engl J Med200934412907916
- OhkawaraYYamauchiKTannoYHuman lung mast cells and pulmonary macrophages produce tumor necrosis factor-alpha in sensitized lung tissue after IgE receptor triggeringAm J Respir Cell Mol Biol1992743853921382477
- BerryMAHargadonBShelleyMEvidence of a role of tumor necrosis factor α in refractory asthmaN Engl J Med2009354769770816481637
- HowarthPHBabuKSArshadHSTumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthmaThorax200560121012101816166100
- MorjariaJBChauhanAJBabuKSPolosaRDaviesDEHolgateSTThe role of a soluble TNFalpha receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trialThorax200863758459118245148
- WenzelSEBarnesPJBleeckerERA randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthmaAm J Respir Crit Care Med2009179754955819136369
- BongartzTSuttonAJSweetingMJBuchanIMattesonELMontoriVAnti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trialsJAMA2006295192275228516705109
- LeckieMJten BrinkeAKhanJEffects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic responseLancet200035692482144214811191542
- GibsonPGDolovichJDenburgJRamsdaleEHHargreaveFEChronic cough: eosinophilic bronchitis without asthmaLancet198918651134613482567371
- HaldarPBrightlingCEHargadonBMepolizumab and exacerbations of refractory eosinophilic asthmaN Engl J Med20093601097398419264686
- Flood-PagePSwensonCFaifermanIA study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthmaAm J Respir Crit Care Med2007176111062107117872493
- NairPPizzichiniMMKjarsgaardMMepolizumab for prednisone-dependent asthma with sputum eosinophiliaN Engl J Med20093601098599319264687
- Dose Ranging Efficacy and Safety with Mepolizumab in Severe Asthma (DREAM) Available from: clinicaltrials.gov/ct2/show/NCT01000506. Updated 2010 Jul 15.
- BlackPNUdyAABrodieSMSensitivity to fungal allergens is a risk factor for life-threatening asthmaAllergy200055550150410843433
- FairsAAgbetileJHargadonBIgE sensitisation to aspergillus fumigatus is associated with reduced lung function in asthmaAm J Respir Crit Care Med2010 201001-0087OC.
- DenningDWO’DriscollBRHogaboamCMBowyerPNivenRMThe link between fungi and severe asthma: a summary of the evidenceEur Respir J200627361562616507864
- WarkPAGibsonPGWilsonAJAzoles for allergic bronchopulmonary aspergillosis associated with asthmaCochrane Database Syst Rev20033CD00110812917898
- DenningDWO’DriscollBRPowellGRandomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) studyAm J Respir Crit Care Med20091791111818948425
- HopeWWBillaudEMLestnerJDenningDWTherapeutic drug monitoring for triazolesCurr Opin Infect Dis200821658058618978525
- CastroMRubinASLavioletteMEffectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trialAm J Respir Crit Care Med2010181211612419815809
- PavordIDCoxGThomsonNCSafety and efficacy of bronchial thermoplasty in symptomatic, severe asthmaAm J Respir Crit Care Med2007176121185119117901415