Abstract
Current evidence indicates an inverse association between Helicobacter pylori and asthma and allergy. H. pylori is a Gram-negative bacterium which represents the major cause of peptic ulcer and gastric cancer, and preferentially elicits a T helper (Th)-1 response. Many H. pylori factors, such as the neutrophil-activating factor of H. pylori (HP-NAP), are able to drive Th-1 polarization and to display a powerful inhibition of allergic Th-2 response. This article proposes an overview of the actual knowledge about the effects of H. pylori on asthma and allergy. Special attention has been drawn to HP-NAP as a potential novel strategy for the prevention and treatment of asthma and atopy.
Introduction
The prevalence of airway allergic disease such as asthma has over the years increased in developed countries. The causes of this increase remain largely unknown. Proposed associations include changes in smoking habits,Citation1 exposure to food-borne and orofecal infections,Citation2,Citation3 types of dwellings,Citation4 ownership of furry animals,Citation5 number of siblings, family income/education level,Citation6 and the presence of particulates in diesel exhaust.Citation7 The inverse association between family size and manifestations of allergy has been consistently found,Citation8–Citation11 and there is also a much-published potential link between allergy and childhood infection, especially with Helicobacter pylori.Citation12–Citation14
Until the late 1980s, interest in the role of infections in allergic diseases focused principally upon the process of primary allergic sensitization. The literature of the time contained several observations which argued for a role for infections, including the ability of bacterial-derived immunostimulants such as pertussigen to selectively improve priming for immunoglobulin (Ig)E antibody production,Citation15 and the potential of lipopolysaccharide to bypass tolerance to mucosally applied allergens. Also, other studies reported that respiratory viral infections such as influenza could subvert the generation of protective “inhalation tolerance” to aeroallergens.Citation16 More recently, signals such as enterotoxins from skin-dwelling bacteria have been invoked as important contributors to the pathogenesis of atopic dermatitis.Citation17 However, it was also clear from other observations that microbial exposure per se could not be considered in generic terms as “pro-atopic”. For example, other microbial-derived agents exemplified by the components of Freund’s adjuvant displayed atopy-antagonistic activity,Citation18 and stimuli derived from normal gut flora were demonstrated to be necessary to facilitate the expression of oral tolerance to fed allergens,Citation19 and also inhalation tolerance to aeroallergens.Citation20 These observations suggested that microbial-derived stimuli had potential to modulate the etiology and pathogenesis of atopic diseases in dichotomous ways, their ultimate effects perhaps being context-dependent.
In this review we will focus our attention on the unambiguous effects of H. pylori on asthma and atopy.
The role of Helicobacter pylori in asthma and allergy
H. pylori, a Gram-negative bacillus that colonizes the human stomach, is the main cause of peptic ulceration, gastric lymphoma, and gastric adenocarcinoma, the second leading cause of death from cancer worldwide. The World Health Organization classifies H. pylori as a human carcinogen for distal gastric cancer, and eradicating the bacterium in high risk populations reduces incidence of gastric cancer.Citation21 H. pylori also may contribute to other conditions, including iron and vitamin B12 deficiencies, idiopathic thrombocytopenic purpura, and growth retardation in children. H. pylori colonization occurs in childhood and persists throughout life, causing disease mainly in adults.Citation22,Citation23
In 1989, Strachan proposed the “hygiene hypothesis”, stating that the exposure to infectious agents and living in an unhygienic environment might “educate” the immune system and thus protect against the development of allergic diseases.Citation24 The idea originated from epidemiological observations suggesting a general hypothesis that infections in early childhood acquired from older siblings might confer protection against the development of atopic diseases such as atopic eczema, allergic rhinoconjunctivitis, and asthma. Subsequent research into the association between childhood infections and atopic sensitization or atopic disease have offered conflicting results. Indeed, our understanding of the timing, the mechanism, and the specific infections that might carry antiallergenic potential are by no means satisfactory.Citation25,Citation26 The T helper (Th)-1/Th-2 paradigm of adaptive immune responses provided the initial immunological backbone for the hygiene hypothesis.Citation27–Citation29 On the basis of the cytokine production patterns, T cell responses may be divided into counter-regulatory Th-1 and Th-2 subtypes. Th-2 responder phenotype is associated with atopic sensitization and atopic disease. Indeed, inflammation of the Th-2 type appears to be active in the initial stage of the pathogenesis of atopic eczema,Citation30,Citation31 allergic rhinoconjunctivitis,Citation32,Citation33 and asthma.Citation34,Citation35
In detail, the histopathological characteristics of bronchial asthma, even a mild one, are represented by inflammatory infiltrates consisting of T lymphocytes and accumulation of activated eosinophils, epithelial shedding, and basal membrane thickening. Immunological and molecular studies of bronchial biopsies and bronchoalveolar lavage samples obtained in baseline disease or taken after natural or “experimentally” induced asthma exacerbations have shown that a complex and fascinating inflammatory mechanism sustains the pathogenesis of bronchial asthma, including the participation of different types of Th cells and peculiar cytokine and chemokine networks.Citation36 In allergic asthmatic patients, allergen exposure induces a predominant activation of Th-2 lymphocytes in the airways, able to over-express several Th-2 cytokines, such as interleukin (IL)-4 and IL-5.Citation34,Citation37 Moreover, the degree of IL-5 expression at the bronchial level is associated with the disease severity both in atopic and in nonatopic asthma.Citation38 IL-5 and granulocyte macrophage colony-stimulating factor (GM-CSF) can be considered the most important cytokines for eosinophil accumulation in asthmatic inflammation. Th-2 cytokines in bronchial asthma are produced not only by CD4+ but also by CD8+ T cells, which contribute to the genesis of asthma and to the clinical expression of the disease.Citation39 In H. pylori infection, a predominant activation of Th-1 cells, with the production of interferon (IFN)-γ, IL-12, IL-18, IL-23, and tumor necrosis factor (TNF)-α, occurs in vivo in the stomach of humans and in animal models, and the inhibition of the allergic Th-2 inflammation by Th-1 responses can explain the inverse relationship between H. pylori and asthma.Citation40
Mechanism of action of Helicobacter pylori
H. pylori colonizes the human stomach in childhood and persists for decades.Citation23 This implies near perfect adaptation to the niche and an ability to evade the human immune response. Its spiral shape and flagella allow it to corkscrew through the gastric mucus gel, and numerous adhesins enable selective adherence to the epithelium. H. pylori has multiple mechanisms for protection against gastric acid;Citation41 notably, 15% of its protein content comprises preformed cytoplasmic urease that, when the external pH is less than 6.5, neutralizes the periplasm, allowing maintenance of the cytoplasmic membrane potential.Citation42
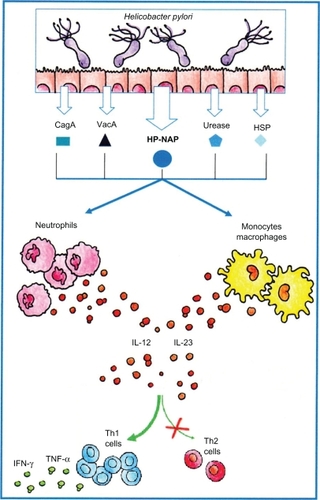
Like many human commensal bacteria, H. pylori has evolved specific mechanisms to avoid stimulating the immune response. For example, innate immune recognition by several Toll-like receptors (TLRs) is attenuated for H. pylori.Citation43,Citation44 Despite this, colonization is associated with inflammatory and mucosa infiltration of polymorphonuclear leukocytes, macrophages, and Th-1 lymphocytes, with active production of IL-12 and IFN-γ.Citation45 Such an immune response is expected to play a role in the pathogenesis of H. pylori-associated diseases in humans.Citation45,Citation46 Accordingly, a Th-1-directed immune response, induced by H. pylori infection, increases gastric inflammation and atrophy, whereas Th-2 redirection reduces them.Citation47,Citation48 Different pathways are responsible for the predominant H. pylori-induced mucosal Th-1 response.Citation45,Citation46 Stimulation of human neutrophils, monocytes, and dendritic cells with H. pylori neutrophil-activating protein (HP-NAP) strongly upregulates both IL-12 and IL-23 production, via TLR2 activation. In the gastric mucosa of H. pylori-infected patients, a considerable proportion of Th cells is specific for different H. pylori antigens, including HP-NAP, CagA, urease, VacA, and heat shock proteins, and HP-NAP drives the production of high levels of IFN-γ and TNF-α by gastric Th cells, thus promoting a polarized Th-1 response ().Citation45–Citation49
Figure 1 Schematic representation of Helicobacter pylori-driven inhibition of allergic Th-2 inflammation via activation of Th-1 responses. Following H. pylori infection, HP-NAP and other H. pylori factors induce the production of IL-12 and IL-23 that both promote the preferential development of Th-1 cells and repress the Th-2 allergic response.
Protective properties of Helicobacter pylori on asthma and allergies
Asthma, a chronic inflammatory disease of the airways is a multifaceted disorder characterized by airway hyperresponsiveness to a multiplicity of specific and nonspecific stimuli, and mucus hypersecretion by goblet cells.
The severity and incidence of asthma have increased drastically in the developed nations over recent decades. Although the underlying reason is still unknown, clinical, epidemiological, and experimental evidence indicate that infectious diseases can influence the development of allergic disorders.Citation24 Accordingly, an inverse correlation has been demonstrated between the onset of allergic disorders and the incidence of infections. This may be the result of an inhibition of allergic Th-2 inflammation exerted by Th-1 responses; the latter are elicited by infectious agents and are able to induce the production of IFN-γ, IL-12, IL-18, and IL-23.Citation50 This view is supported by studies showing that development of asthma can be prevented in animals by administering live or killed bacteria or their components, which induce Th-1 responses.Citation51 Also, we demonstrated that H. pylori inhibited Th-2 responses in asthmatic patients.Citation49 Interestingly, on the basis of large epidemiological studies, recently, a consistent negative association between H. pylori infection and the presence of allergic disorders, such as asthma and rhinitis, has been proposed.Citation52 summarizes some recent studies in which the relationships of H. pylori with asthma, atopy, allergic rhinitis, and/or eczema were examined.Citation2,Citation8,Citation53–Citation60 In general, the cross-sectional studies, involving a variety of populations and somewhat differing definitions of atopy and asthma, show significant inverse relationships of these conditions with H. pylori. The published case-control studies, in general much smaller in scale, do not show any significant direct or inverse relationships.
Table 1 Major studies showing a negative association between Helicobacter pylori and asthma/atopic diseases
Although it is an undoubtedly interesting theory, no convincing molecular mechanism has been proposed to support it. Our studies, carried out with H. pylori may help in understanding this complex issue. We have shown that addition of HP-NAP (a dodecamer formed by four-helix bundled subunits with a hollow central part) to allergen-induced T-cell lines derived from allergic asthmatic patients led to a drastic increase in IFN-γ-producing T cells and to a decrease in IL-4-secreting cells, thus resulting in a redirection of the immune response from a Th-2 to a Th-1 phenotype.Citation49 Furthermore, in the gastric mucosa of H. pylori-infected patients a remarkable proportion of Th cells showed significant proliferation to different H. pylori antigens, including HP-NAP; upon HP-NAP stimulation, Ag-specific gastric Th cells produced large amounts of IFN-γ and TNF-α, and displayed a powerful cytotoxic activity, thus showing a polarizing Th-1 effector phenotype.
Likewise, HP-NAP stimulation of neutrophils, monocytes, and dendritic cells resulted in a remarkable upregulation of cytokines, including IL-12 and IL-23, contributing to the induction of an IL-12- and IL-23-enriched milieu, which has the potential to drive the differentiation of antigen-stimulated T cells towards a polarized Th-1 phenotype ().Citation56,Citation61
An issue to be considered in studies showing negative associations between H. pylori and various atopic and allergic diseases is that H. pylori positivity is linked with more crowded living conditions and poor hygiene in infancy. Since these factors also are associated with other childhood infections, H. pylori status may simply be a marker for these. However, the negative association with childhood asthma is stronger for cagA+ H. pylori strains.Citation56
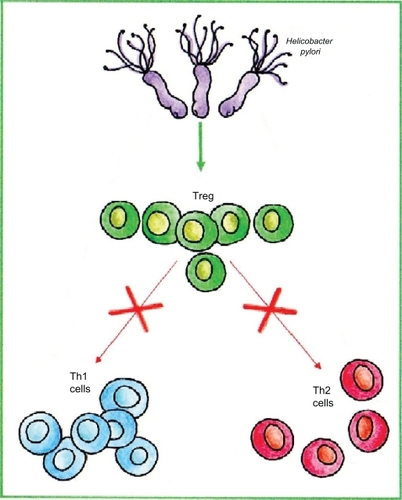
Another hypothetical explanation for the inverse association between H. pylori and asthma is that the high levels of regulatory T cells (Tregs) associated with H. pylori infection may contribute to prevention of allergic diseases, and H. pylori-free humans are thus more susceptible to these diseases (). In support of this, H. pylori-positive people have higher levels of gastric Tregs than those without the organism,Citation62,Citation63 and more importantly also, circulating Tregs are increased in number.Citation64 In addition, in mice experimentally infected with H. pylori, systemic Tregs are increased, and these suppress other immune responses, one effect of which is to facilitate H. pylori colonization.Citation65 The excess Tregs may have immunosuppressive activity in humans as well: among H. pylori-positive persons, those with fewer Tregs are more likely to have peptic ulcersCitation62 and so presumably have more intense gastritis. Finally, in cagA+ H. pylori colonization, mucosal Tregs may be more numerous, and mucosal levels of the immunomodulatory cytokine IL-10 may be higher than in cagA− colonization.Citation66 If the same phenomenon applies to circulating Tregs, it could potentially explain the stronger, negative association with childhood asthma of cagA+ strains.Citation56 Taken together, these studies imply a theoretical plausible link between H. pylori, Tregs, and reduction in risk of allergic diseases. However, interventional studies in relevant animal models and in humans are needed to verify the hypothesis.
Effect on immune system and lung function
So far we have considered what happens in the stomach and at the systemic level, following H. pylori colonization; but a very pertinent question about the link between asthma and H. pylori is whether H. pylori may have some effects in the lung region. To answer this question we created a mouse model of allergic asthma,Citation67 and demonstrated that in vivo administration of HP-NAP prevents the typical eosinophil accumulation in the lung, as well as the increase of serum IgE. These results suggest the possibility that HP-NAP might be a part of the molecular mechanism underlying the negative association between H. pylori infection and allergy, corroborating the epidemiological observations with a plausible scientific explanation. To address whether HP-NAP, on the basis of its immune-modulating activity, could be beneficial for the prevention and treatment of bronchial asthma, it was administered via the intraperitoneal or the intranasal route using a mouse model of allergic asthma induced by inhaled ovalbumin (OVA). Groups of nine C57BL/6j, wild type or tlr2−/−, mice were treated with OVA alone, or with OVA plus HP-NAP intraperitoneally or mucosally administered. In both systemic and mucosal protocols, mice were treated with OVA according to a standardized procedure consisting of a first phase of sensitization with OVA intraperitoneally and a second phase of induction of the allergic response with aerosolized OVA on day 8, followed by repeated aerosol challenge with the allergen on days 15–18. Control animals were injected with phosphate-buffered saline (PBS) alone and then exposed to aerosolized PBS. In the systemic protocol, mice were treated with intraperitoneal HP-NAP on day 1, whereas in the mucosal protocol mice received intranasal HP-NAP on days 7 and 8.Citation67 After priming and a repeated aerosol challenge with OVA, Th-2 responses were induced in the mouse lung. Accordingly, following OVA treatment, eosinophils were recruited and activated in bronchial airways, and serum IgE levels increased. Both systemic and mucosal administration of HP-NAP strongly inhibited the development of airway eosinophilia and bronchial inflammation. Likewise, HP-NAP treatment strongly affected the lung cytokine release, reducing the production of IL-4, IL-5, and GM-CSF. Systemic HP-NAP also significantly resulted in both the reduction of total serum IgE and an increase of IL-12 plasma levels. However, no suppression of lung eosinophilia and bronchial Th-2 cytokines was observed in TLR2 knock-out mice following treatment with the TLR2 ligand HP-NAP.Citation67 The results obtained in our studies suggest that HP-NAP might be the key element responsible for the decrement of allergy frequency in H. pylori-infected patients.
Conclusion
H. pylori and humans have coevolved for at least 50,000 years and probably for much longer. As such, H. pylori colonization has been essentially universal, and the usual pattern of inflammation has likely been pan-gastric. H. pylori is the main cause of peptic ulceration, gastric lymphoma, and gastric adenocarcinoma.
The loss of this ancient, dominant, and persistent member of the normal biota of humans would be predicted to have consequences, and now there is much information about the beneficial and deleterious aspects of this change on the health and disease of the gastrointestinal tract. However, increasing evidence is pointing to extra-intestinal manifestations of the disappearance of H. pylori, including asthma. An inverse association of H. pylori and childhood asthma, allergic rhinitis, and atopy is becoming increasingly obvious.
This phenomenon might be explained by the inhibition of the allergic Th-2 inflammation by the Tregs that could be present during H. pylori infection and could suppress the atopy-associated Th-2 response.
However, a realistic hypothesis, based on clinical and experimental evidence in humans and animal models,Citation45,Citation49,Citation67 is that the allergic Th-2 response is redirected by the Th-1 response elicited by H. pylori, that is able to induce the production of IFN-γ, IL-12, IL-18, and IL-23. Several studies were devoted to the definition of new immune modulating factors able to inhibit Th-2 responses, and different compounds have been proposed for the treatment and prevention of asthma and atopic diseases, including several TLR ligands mimicking the effects of microbial components such as dsRNA, CpG-oligodeoxynucleotides, and imidazoquinolines.Citation68–Citation70 In detail, it has been shown that HP-NAP, by acting on both neutrophils and monocytes following the engagement of TLR2, significantly contributes to create an IL-12- and IL-23-enriched milieu, and as such it represents a key bacterial factor able to drive the differentiation of antigen-stimulated T cells toward a polarized Th-1 phenotype. HP-NAP has the potential to redirect the in vitro allergen-specific T-cell response from a predominant Th-2 to a Th-1 response. Also, HP-NAP administration in vivo resulted in inhibition of the typical Th-2-mediated bronchial inflammation of allergic bronchial asthma. Thus, altogether, these results support the view that the increased prevalence and severity of asthma and allergy in Western countries may be related, at least in part, to the decline of H. pylori infection, which is able to induce a long-lasting Th-1 background, and suggest also that H. pylori compounds such as HP-NAP could be important candidates for novel strategies of the prevention and treatment of asthma and allergic diseases.
Acknowledgements
The authors thank the Italian Ministry of University and Research for their support of their studies, and Dr Chiara Della Bella for the artwork.
Disclosure
Mario M D’Elios, Amedeo Amedei, Gianfranco del Prete, and Marina de Bernard are applicants of EU Patent 05425666.4 for HP-NAP as a potential therapeutic agent in asthma, allergic and infectious diseases, and cancer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- DijkstraLHouthuijsDBrunekreefBRespiratory health effects of the indoor environment in a population of Dutch childrenAm Rev Respir Dis19901425117211782240840
- MatricardiPMRosminiFRiondinoSExposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological studyBMJ2000320723241241710669445
- MatricardiPMRosminiFPanettaVHay fever and asthma in relation to markers of infection in the United StatesJ Allergy Clin Immunol2002110338138712209083
- WickmanMNordvallSLPershagenGHouse dust mite sensitization in children and residential characteristics in a temperate regionJ Allergy Clin Immunol199188189952071788
- StrachanDPCareyIMHome environment and severe asthma in adolescence: a population based case-control studyBMJ19953117012105310567580660
- LitonjuaAACareyVJWeissSTRace, socioeconomic factors, and area of residence are associated with asthma prevalencePediatr Pulmonol199928639440110587412
- TakafujiSSuzukiSKoizumiKDiesel-exhaust particulates inoculated by the intranasal route have an adjuvant activity for IgE production in miceJ Allergy Clin Immunol19877946396452435776
- BodnerCAndersonWJReidTSChildhood exposure to infection and risk of adult onset wheeze and atopyThorax200055538338710770819
- PeatJKvan den BergRHGreenWFChanging prevalence of asthma in Australian childrenBMJ19943086944159115968025424
- von MutiusEMartinezFDFritzschCSkin test reactivity and number of siblingsBMJ199430869306926958142793
- WickensKCraneJPearceNThe magnitude of the effect of smaller family sizes on the increase in the prevalence of asthma and hay fever in the United Kingdom and New ZealandJ Allergy Clin Immunol19991043 Pt 155455810482827
- KosunenTUHook-NikanneJSalomaaAIncrease of allergen-specific immunoglobulin E antibodies from 1973 to 1994 in a Finnish population and a possible relationship to Helicobacter pylori infectionsClin Exp Allergy200232337337811940066
- StrachanDPFamily size, infection and atopy: the first decade of the ‘hygiene hypothesis’Thorax200055Suppl 1S2S1010943631
- SheikhAStrachanDPThe hygiene theory: fact or fiction?Curr Opin Otolaryngol Head Neck Surg200412323223615167035
- MunozJJPeacockMGAction of pertussigen (pertussis toxin) on serum IgE and Fce receptors on lymphocytesCell Immunol199012723273362139364
- HoltPGVinesJBilykNEffect of influenza virus infection on allergic sensitization to inhaled antigen in miceInt Arch Allergy Appl Immunol19888611211233372041
- SichererSHLeungDYMAdvances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insectsJ Allergy Clin Immunol2005116115316315990789
- IshizakaKRegulation of IgE synthesisAnnu Rev Immunol198421591826085750
- SudoNSawamuraS-ATanakaKThe requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance inductionJ Immunol19971594173917459257835
- HoltPGSedgwickJDSuppression of IgE responses following antigen inhalation: a natural homeostatic mechanism which limits sensitization to aeroallergensImmunol Today198781415
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 1994 Jun 7–14IARC Monogr Eval Carcinog Risks Hum19946112417715068
- BlaserMJAthertonJCHelicobacter pylori persistence: biology and diseaseJ Clin Invest2004113332133314755326
- BanatvalaNMayoKMegraudFJenningsRDeeksJJFeldmanRAThe cohort effect and Helicobacter pyloriJ Infect Dis199316812192218515114
- StrachanDPHay fever, hygiene, and household sizeBMJ19892996710125912602513902
- KempABjörksténBImmune deviation and the hygiene hypothesis: a review of the epidemiological evidencePediatr Allergy Immunol2003142748012675752
- KilpiTKeroJJokinenJCommon respiratory infections early in life may reduce the risk of atopic dermatitisClin Infect Dis200234562062611810601
- MosmannTRCherwinskiHBondMWTwo types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteinsJ Immunol19861367234823572419430
- Del PreteGFde CarliMMastromauroCPurified protein derivative of Mycobacterium tuberculosis and excretorysecretory antigen(s) of Toxocara canis expand in vitro human T cells with stable and opposite (type 1 T helper or type 2 T helper profile of cytokine productionJ Clin Invest19918813463501829097
- RomagnaniSTh1 and Th2 in human diseasesClin Immunol Immunopathol1996803 Pt 12252358811042
- ReinholdUKukelSGoedenBFunctional characterization of skin-infiltrating lymphocytes in atopic dermatitisClin Exp Immunol19918634444481721013
- LeungDYBieberTAtopic dermatitisLancet2003361935215116012531593
- Del PreteGFde CarliMD’EliosMMAllergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disordersEur J Immunol1993237144514498100770
- MaggiEBiswasPDel PreteGAccumulation of Th-2-like helper T cells in the conjunctiva of patients with vernal conjunctivitisJ Immunol19911464116911741825106
- RobinsonDSHamidQYingSPredominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthmaN Engl J Med199232652983041530827
- RobinsonDHamidQBentleyAActivation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthmaJ Allergy Clin Immunol19939223133248349942
- ChatilaTALiNGarcia-LloretT-cell effector pathways in allergic diseases: transcriptional mechanisms and therapeutic targetsJ Allergy Clin Immunol2008121481282318395547
- RomagnaniSParronchiPD’EliosMMAn update on human Th1 and Th2 cellsInt Arch Allergy Immunol19971131–31531569130508
- KonOMKayABT cells and chronic asthmaInt Arch Allergy Immunol19991182–413313510224360
- BettsRJKemenyDMCD8 T cells in asthma: friend or foe?Pharmacol Ther2009121212313118940198
- ReibmanJMarmorMFilnerJAsthma is inversely associated with Helicobacter pylori status in an urban populationPLoS One2008312e406019112508
- SachsGWeeksDLMelchersKScottDRThe gastric biology of Helicobacter pyloriAnnu Rev Physiol20036534936912471160
- WeeksDLEskandariSScottDRSachsGA H+-gated urea channel: the link between Helicobacter pylori urease and gastric colonizationScience2000287545248248510642549
- BäckhedFRokbiBTorstenssonEGastric mucosal recognition of Helicobacter pylori is independent of Toll like receptor 4J Infect Dis2003187582983612599057
- LeeSKStackAKatzowitschEAizawaSISuerbaumSJosenhansCHelicobacter pylori flagellins have very low intrinsic activity to stimulate human gastric epithelial cells via TLR5Microbes Infect20035151345135614670447
- D’EliosMMManghettiMde CarliMTh1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer diseaseJ Immunol199715829629678993017
- D’EliosMMAmedeiABenagianoMHelicobacter pylori, T cells and cytokines: the “dangerous liaisons”FEMS Immunol Med Mic2005442113119
- FoxJGBeckPDanglerCAConcurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophyNat Med20006553654210802709
- D’EliosMMManghettiMde CarliMDifferent cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcerEur J Immunol1997277175117559247587
- AmedeiACapponACodoloGThe neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responsesJ Clin Invest200611641092110116543949
- HerzULacyPRenzHErbKThe influence of infections on the development and severity of allergic disordersCurr Opin Immunol200012663264011102765
- WohllebenGErbKJImmune stimulatory strategies for the prevention and treatment of asthmaCurr Pharm Design2006122532813292
- BlaserMJChenYReibmanJDoes Helicobacter pylori protect against asthma and allergy?Gut200857556156718194986
- JunZJLeiYShimizuYHelicobacter pylori seroprevalence in patients with mild asthmaTohoku J Exp Med2005207428729116272799
- TsengKWLamWKChanKNHelicobacter pylori seroprevalence in asthmaRespir Med200094875675910955750
- McCuneALaneAMurrayLReduced risk of atopic disorders in adults with Helicobacter pylori infectionEur J Gastroenterol Hepatol200315663764012840675
- ChenYBlaserMJInverse associations of Helicobacter pylori with asthma and allergyArch Intern Med2007167882182717452546
- von HertzenLCLaatikainenTMakelaMJInfectious burden as a determinant of atopy – a comparison between adults in Finnish and Russian KareliaInt Arch Allergy Immunol20061402899516554659
- RadonKWindstetterDEckartJFarming exposure in childhood, exposure to markers of infections and the development of atopy in rural subjectsClin Exp Allergy20043481178118315298556
- LinnebergAOstergaardCTvedeMIgG antibodies against microorganisms and atopic disease in Danish adults: the Copenhagen Allergy StudyJ Allergy Clin Immunol2003111484785312704368
- JansonCAsbjornsdottirHBirgisdottirAThe effect of infectious burden on the prevalence of atopy and respiratory allergies in Iceland, Estonia, and SwedenJ Allergy Clin Immunol2007120367367917586034
- TrinchieriGInterleukin-12 and the regulation of innate resistance and adaptive immunityNature Rev Immunol20033213314612563297
- RobinsonKKenefeckRPidgeonELHelicobacter pylori induced peptic ulcer disease is associated with inadequate regulatory T cell responsesGut200857101375138518467372
- LundgrenAStrömbergESjölingAMucosal FOXP3-expressing CD4+ CD25 high regulatory T cells in Helicobacter pylori-infected patientsInfect Immun200573152353115618192
- WangSKZhuHFHeBSCagA+ H pylori infection is associated with polarization of T helper cell immune responses in gastric carcinogenesisWorld J Gastroenterol200713212923293117589941
- RadRBrennerLBauerSCD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivoGastroenterology2006131252553716890606
- HarrisPRWrightSWSerranoCHelicobacter pylori gastritis in children is associated with a regulatory T-cell responseGastroenterology2008134249149918242215
- CodoloGMazziPAmedeiAThe neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthmaCell Microbiol200810112355236318671823
- BallHAvan ScottMRRobinsonCBSense and antisense: therapeutic potential of oligonucleotides and interference RNA in asthma and allergic disordersClin Rev Allergy Immunol200427320721715630157
- VollmerJKriegAMImmunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonistsAdv Drug Deliv Rev200961319520419211030
- MoisanJCamaterosPThuraisingamTTLR7 ligand prevents allergen-induced airway hyperresponsiveness and eosinophilia in allergic asthma by a MYD88-dependent and MK2-independent pathwayAm J Physiol Lung Cell Mol Physiol20062905987995