Abstract
Background
Olive pollen is an important cause of respiratory allergy in the Middle East. In this study, the clinical characteristics of adults and children with confirmed allergic rhinitis (AR; with or without asthma) in Jordan were described, and the use of sublingual immunotherapy (SLIT) in a real-life clinical setting was assessed.
Methods
This retrospective observational study evaluated the clinical features of olive-induced allergy and the use of an SLIT solution of standardized extracts toward Ole e 1 given in a pre- and coseasonal scheme with a daily dose of 300 index of reactivity for two consecutive seasons. Inclusion criteria were as follows: ≥5 years of age, AR, proven olive sensitization, and at least 2 years follow-up after SLIT initiation. The following data were recorded at SLIT initiation: clinical characteristics, rhinitis and asthma symptom scores, and concomitant symptomatic medications. During follow-up and at the end of each season, the following data were recorded: symptom progression/scores, any changes to symptomatic medications, and treatment compliance. The secondary objective was to determine any effect on quality of life, use of concomitant AR medications, and treatment compliance.
Results
Eighty-six patients with seasonal AR were included in this analysis (52.3% with coexisting asthma). Between the initiation of treatment and the end of second pollen season, symptoms of AR and asthma were decreased by 79.5% and 41.7%, respectively, with an improvement in quality of life score in 71.5% of the patients (P<0.0001 for all). Physicians reported that after 2 years of SLIT, there was an improvement in the symptoms of both AR (95.2%) and asthma (93.3%), with 98.8% of the patients showing good treatment compliance. A reduction in symptomatic medications was also found. SLIT was well tolerated with no systemic reactions being reported.
Conclusion
In children and adults with olive-associated respiratory allergy in Jordan, the use of a pre- and coseasonal SLIT with a 300 index of reactivity daily dose is effective in reducing the clinical burden of AR and asthma with no tolerability issues.
Introduction
Seasonal exposure to olive pollen (Olea europaea) is increasingly recognized as an important cause of allergic rhinitis (AR), particularly in the Mediterranean region and the Middle East.Citation1 A range of causative allergens have been characterized (Ole e 1–10) with Ole e 1 being considered the most common sensitizing allegen.Citation2–Citation4 Although data are limited, it has been reported that olive-induced allergy is associated with more severe symptomology in comparison with other nongrass allergiesCitation5 and that quality of life (QoL) is lower in patients with olive-associated AR (and olive-associated asthma) than in patients with diseases caused by other common allergens.Citation6
Sublingual immunotherapy (SLIT) is an established recommended treatment for AR, with a broad, robust evidence base.Citation7–Citation12 One such therapy is Staloral® (Stallergenes, Antony, Paris, France), which is a sublingual solution; the efficacy and safety of this solution against a wide range of respiratory allergens have been reported in a large number of placebo-controlled and open-label trials, with additional data available from observational studies.Citation13 The sublingual solution contains standardized extracts of target allergens (eg, against hose dust mites and grass or tree pollens) at various concentrations, expressed as the index of reactivity (IR), with the final composition and concentration tailored toward the sensitizing allergen. In many of the clinical studies and in routine clinical practice, a standardized 300 IR daily dose is used.Citation13,Citation14 This solution is administered in a pre- and coseasonal scheme in which treatment is started before the onset of the pollen season and is continued until the end of the season, corresponding to 4–6 months of use each year across 2 years.Citation13,Citation14
In contrast to other perennial or seasonal causes of AR, clinical data on the benefits of SLIT in olive-associated AR are far more limited. In an early placebo-controlled study, Vourdas et alCitation15 investigated the use of a sublingual solution (containing Ole e 1 extract) in children with AR and/or mild asthma due to sensitization to olive pollen. They found that SLIT reduced dyspnea and conjunctivitis scores and cutaneous allergen reactivity compared with placebo.Citation15 However, few subsequent studies have evaluated SLIT in olive-associated AR, and when so, such studies have been small, and/or of short duration.Citation16 Patients with olive sensitivity usually form a minority of a larger study cohort.Citation5,Citation17
In Jordan, as in other Mediterranean countries, seasonal olive-induced allergy is considered an important cause of AR although the prevalence of this condition is uncertain. In view of this and the limited data on the use of SLIT in olive-induced allergy, we conducted a retrospective analysis of patients with confirmed olive-induced allergy treated with a 300 IR sublingual solution to examine the clinical features of olive-associated respiratory allergy and the impact of therapy in a real-life clinical setting.
Methods
Study design
This study was a multicenter, retrospective, open-label, non-controlled, real-life observational study, conducted in Jordan, in patients with proven clinical allergy to olive tree pollen. In this study, we evaluated the use of a standardized SLIT regimen using a high dose of sublingual solution of olive pollen allergen (Staloral), across two consecutive seasons over a 3-year period (between 2010 and 2012). The sublingual solution consists of a standardized preparation of Ole e 1, administered following an initial dose titration as a single-strength 300 IR dose taken once daily, in a pre- and coseasonal scheme. In this preparation, a 300 IR daily maintenance dose corresponds to ~30 µg of Ole e 1 allergen each day.Citation13,Citation18 In Jordan, for each season, treatment was started in January, February, or March and stopped at the end of the olive pollen season, that is, in June. All treatments were provided in accordance with the relevant health care provision for individual patients and were funded either directly by the patient or via health care insurance (national or private). The sponsor of this study was not involved in funding any treatment.
The primary objective was to determine the effectiveness of this SLIT as determined by its impact on AR and asthma symptom scores and assessment of symptoms by the physician. The secondary objective was to determine any effect on QoL, the use of concomitant AR medications, and treatment compliance.
Patient selection
Participating physicians were all experienced in the management of the selected seasonal allergy and the use of SLIT in everyday clinical practice. Each was asked to review his or her medical record systems to identify potentially suitable male and female subjects aged ≥5 years with olive pollen-induced rhinitis (with or without coexisting asthma) for inclusion in this study. Subjects were required to have symptoms of AR and confirmatory positive skin prick tests to olive pollens, with at least 2-year follow-up after initiation of SLIT and with adequate medical documentation of all relevant parameters to provide reliable data collection.
As per the trial design (a retrospective observational study), formal sample size was not calculated; however, the aim was to recruit at least twice as many subjects treated with the olive sublingual solution as that recruited by Vourdas et al (n=34), the largest randomized controlled trial performed specifically in patients with olive-induced allergy.Citation15
Study assessments
For each subject, the treating physician completed a case report form, based on his or her medical records. Data were collected and analyzed for three time points: SLIT initiation (V1), after 1 year of treatment (V2), and after 2 years of treatment (V3). For V1, a full allergy history with confirmatory clinical and laboratory examinations of each subject was collected. For each time point, symptoms were recorded on the basis of a 15-item allergic respiratory symptom questionnaire across three domains: rhinitis (eight questions), asthma (three questions), and QoL (four questions), with each question being scored on a scale from 0 to 3. With this questionnaire, a symptom score can be calculated for each domain for each time point (ie, V1, V2, and V3). Data regarding concomitant medication use in the previous season were also collected for these time points. Physician assessment of rhinitis and asthma symptom improvement was recorded at V2 and V3, based on whether they felt the condition had improved, was unchanged, or had deteriorated, in comparison with previous seasons. At these visits, treatment compliance was also recorded. At V2 and V3, any treatment-associated adverse events reported by the patient were also recorded in the case report form.
The study was conducted in accordance with the Declaration of Helsinki and guidelines on good clinical practice.Citation19,Citation20 According to the local ethical boards criteria, no ethical approval was required for this retrospective anonymous analysis. Written informed consent was obtained from all patients before being enrolled in this study, and in the case of minors, it was obtained from next of kin, caregivers, or guardians. For each subject, the anonymized case report form was returned to the clinical research organization (Delta Consultants, Eybens, France) for analysis. All statistical analyses were performed using SAS® (version 9.2; SAS Institute Inc., Cary, NY, USA). Mean, standard deviation, median, range values, and 95% confidence intervals were reported as continuous variables, and absolute (number of subjects) and relative frequencies (%) were reported as discrete variables. Wilcoxon signed rank sum test was used to compare the quantitative values between two time points for the same subject. For all analyses, statistical significance was set at P<0.05.
Results
Subject demographics at baseline prior to SLIT initiation
Eighty-six subjects with proven AR associated with olive pollen and with 2 years of completely documented treatment with the 300 IR SLIT solution were included in the full analysis set. provides the characteristics of the study population. Approximately equal number of male (51.2%) and female (48.8%) subjects were represented with a mean age of 29.2±12.0 years (ranging between 4 and 71 years). Most subjects (88.4%) were ≥15 years of age, with ten children <15 years of age being included. The majority of the patients (57.0%) had AR symptoms lasting >2 months in each season, with most patients having symptoms graded as moderate (52.3%) or severe (26.7%). Coexistent asthma was reported in 52.3% of the subjects.
Table 1 Subject demographics at treatment initiation (V1)
provides the clinical symptoms during the previous pollen season (and their evolution across the study). The most common rhinitis symptoms were rhinorrhea, sneezing, and nasal congestion, each occurring in >90% of the subjects. Olfactory impairment was also common as were nasal and ocular itching, each occurring in >80% of the subjects. Respiratory symptoms included wheezing (during daytime or at night) and tightness of the chest, each of which was reported in approximately half of the overall study population, in line with the proportion of subjects with coexistent asthma. Majority of the subjects also reported sleep disturbance, overt insomnia, and headache, and >75% reported some impact on daily activities (eg, work, sports, or study).
Table 2 Clinical symptoms at study inclusion (n=86) and across study
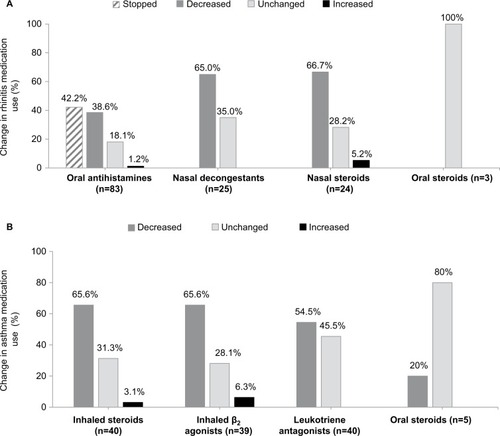
Prior to initiation of an SLIT, all subjects received oral antihistamines as a symptomatic treatment for rhinitis, with 72.1% receiving intranasal steroids and 29.1% nasal decongestants (). Just less than half of the overall study population received asthma medications: inhaled steroids (46.5%), β2 agonists (45.3%), and leukotriene antagonists such as montelukast (46.5%); a small minority received theophylline (2.3%). Few subjects received oral steroids for either AR (3.5%) or asthma (5.8%).
Table 3 Concomitant medication use in previous season at treatment initiation (V1)
Treatment effectiveness
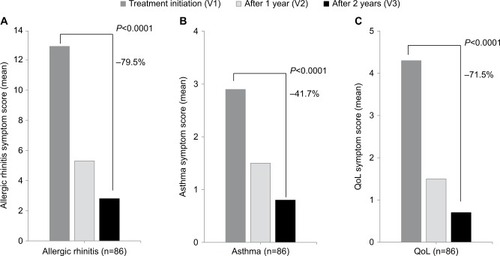
After initiation of treatment with SLIT, a reduction in rhinitis, asthma, and QoL symptom scores was noted at the end of each subsequent season. After 2 years of discontinuous pre- and coseasonal treatment, mean rhinitis scores decreased from 12.9 to 2.8, mean asthma scores from 2.9 to 0.8, and QoL scores from 4.3 to 0.7 (P<0.0001 for all; ).
Figure 1 Change in rhinitis, asthma, and QoL symptom scores across study.
Abbreviation: QoL, quality of life.
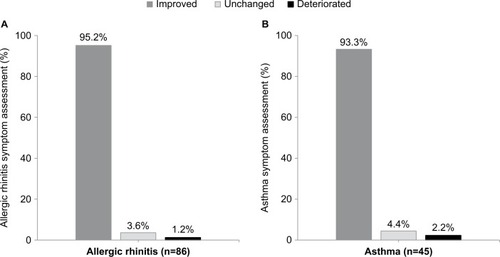
Physician assessment of rhinitis and asthma symptom improvement, was performed, based upon whether they felt the condition was improved, or unchanged, or deteriorated, in comparison to previous seasons. At the end of the second year of treatment (V3), 95.2% of the subjects were considered to have improvement in rhinitis symptoms, and 93.3% had improvement in asthma symptoms, compared with the previous season (). Similar improvements after the first year of treatment compared with baseline were also found (data not shown). Treatment compliance to the pre- and coseasonal SLIT therapy was considered to be good by the physician in 98.8% of the subjects for both the seasons.
Concomitant medication use during treatment with SLIT
Symptomatic medication use for rhinitis decreased after initiation of SLIT. At the end of the study (V3), after 2 years of SLIT, reductions in the use of intranasal steroids (66%) and nasal decongestants (65%) compared with the use prior to treatment initiation were found. Oral antihistamines were stopped by 42.2% of the subjects, with a further 38.6% using these less frequently. Reduction in the use of asthma medication was also found, with 65.6% of the subjects reporting decreased use of inhaled steroids, 65.6% reporting decreased use of β2 agonists, and 54.5% reporting decreased use of leukotriene antagonists. Use of oral steroids for either condition was relatively unchanged (). Reductions in medication use after the first year of treatment (V2) compared with baseline were also found; however, these were of lower magnitude (data not shown).
Safety and adverse events
SLIT was well tolerated. Across the study period, no treatment-emergent adverse events were recorded.
Discussion
This study aimed at characterizing the clinical burden of rhinitis and asthma due to olive pollen sensitivity in Jordan in a real-life clinical setting. The use of a daily dose of a 300 IR SLIT solution containing a standardized allergen extract derived from O. europaea, administered in a pre- and coseasonal scheme across two consecutive pollen seasons, was also evaluated. In this study, the clinical characteristics of olive-induced allergy in the season prior to SLIT initiation and their evolution in terms of disease and QoL symptom scores, physician assessments of clinical improvement, changes in medication use, and treatment compliance were documented.
To our knowledge, this is the largest cohort of patients (n=86) with olive-associated AR (with or without asthma) treated by SLIT reported so far. Although a previous randomized controlled study evaluated the use of this sublingual solution in 66 patients with olive-induced allergy, it included only pediatric patients.Citation15 In our cohort, both children and adults were included, and 52.3% had coexistent asthma. This prevalence, although greater than that seen in randomized studies on AR, is comparable with that seen in other real-life observational studies.Citation17,Citation21–Citation23 Prior to treatment initiation, we found that majority of the patients suffered a wide range of symptoms during the previous olive pollen season, with most reporting sleep disturbance and headache, which negatively impacted their daily activities.
Treatment was scheduled as a pre- and coseasonal treatment scheme, in line with the recommended use of sublingual solution, administered as 300 IR dosing taken once daily. This schedule has been used and reported in a number of observational studies of this agent for allergic disease caused by other allergens.Citation17,Citation21–Citation23 Treatment was well tolerated, and the high level of compliance across 2 years (98.8%) indicates that treatment was acceptable to both patients and (in the case of children) their parents. This finding is accepted, as compliance is a critical aspect of SLIT, with data from clinical trials showing that continued therapy with allergen immunotherapy provides a sustained benefit in terms of symptom control and QoL.Citation7,Citation12 This finding aligns with our findings in the present study. While benefits in all outcomes were seen after 1 year of pre- and coseasonal treatment, the benefits were greater after 2 years. At this point, treatment was associated with significant reductions in both rhinitis (79.6%) and asthma symptom scores (by 79.6% and 41.7%, respectively; P<0.0001 for both), accompanied by physician-assessed symptom improvement ( and ).
Reduction in symptom scores was accompanied by improvement in QoL as shown by a 71.6% reduction in QoL scores. This is reassuring, as some data suggest that olive-induced allergy may have a greater impact on QoL than other allergens.Citation6 Whether the improvements that were found in this study reflect a global improvement in disease symptoms or are they a result of specific aspects is uncertain. While several factors may predict QoL outcomes in patients with AR following therapy, it has been reported that improvements in olfactory function and reduction in asthma symptoms may be of particular importance,Citation24,Citation25 both of which were seen in our cohort across the study period (). These were matched by the reduction in medication use by patients both with and without asthma (). Also, reduction in medication use following the use of this sublingual solution is consistently reported in previous studies, as recently reviewed.Citation13
Strengths and limitations
The present study has some limitations, as is common in observational real-life studies. The retrospective study design and the nature of physician/patient recruitment may have contributed to selection bias. As this was an observational study, there was no active or placebo-controlled group. Heterogeneity in the study population in terms of age, symptoms and medication use at SLIT initiation is another consideration, and we did not perform co-variate analyses to assess this aspect, nor did we account for seasonal variation in pollen activity were accounted. As seasonal pollen counts were not monitored during the study period, the possibility that lower levels of pollen exposure could have contributed to the reduction in symptoms and medication use for the seasons evaluated should be considered. Other limitations include not having patient data on allergen sensitization and changes in sensitization status during the study period. As a result we cannot comment upon the impact polysensitization to other allergens may have had, nor on the impact of the olive SLIT upon sensitization to olive allergens. No AEs were reported in our cohort. In this respect, it should be understood that monitoring and reporting on treatment-emergent adverse events was not an aim of this study. Furthermore, as it is well recognized that AEs are an important reason for treatment discontinuation, the nature of this study reports only on those patients who had completed 2 years of treatment would understandably limit the number of AEs reported by patients. However, as SLIT was well tolerated, based on this limited data we cannot comment further on the safety of this therapy in these patients. Finally, our study was not designed to evaluate and compare the efficacy in asthmatic and nonasthmatic rhinitis populations. As such, specific analysis on the nonasthmatic (ie, rhinitis without asthma) population was not performed, and therefore, the role of asthmatic medications on reducing rhinitis symptoms cannot be examined directly.
Nevertheless, this study has considerable strengths. The study population specifically included only patients with confirmed olive-associated AR (with or without asthma), and so the study provides valuable data on respiratory allergy associated with this allergen. Since the study was performed within a real-life setting, with outcomes assessed and documented by the treating physicians at clinical follow-up, follow-up data for SLIT across two seasons have been provided and clinical benefits in terms of reduction in symptom scores, medication use, and improvements in QoL compared with those seen prior to treatment initiation have been shown.
Conclusion
The results of this observational real-life study show that the use of a daily dose of a 300 IR sublingual solution with a standardized allergen extract derived from O. europaea in subjects suffering from AR (with or without asthma) due to olive pollen sensitization is associated with clinically meaningful benefits. These include reduction in disease symptom scores, medication use, and improved QoL. The therapy was well tolerated with a high treatment compliance.
Acknowledgments
The authors wish to thank Delta Consultants for assistance with the study data and Newmed Publishing Services for editorial support in manuscript preparation. The authors also wish to specially thank all the physicians and patients who participated in this study. Financial support for this study and editorial support in formatting the manuscript were provided by Stallergenes.
Disclosure
The authors report no conflicts of interest in this work.
References
- D’AmatoGCecchiLBoniniSAllergenic pollen and pollen allergy in EuropeAllergy200762997699017521313
- MorenoCJusticiaJLQuiralteJOlive, grass or both? Molecular diagnosis for the allergen immunotherapy selection in polysensitized pollinic patientsAllergy201469101357136324988991
- PalomaresOSwobodaIVillalbaMThe major allergen of olive pollen Ole e 1 is a diagnostic marker of sensitization to OleaceaeInt Arch Allergy Immunol2006141211011816864978
- QuiralteJPalaciosLRodríguezRModelling diseases: the allergens of Olea europaea pollenJ Investig Allergol Clin Immunol200717Suppl 12430
- MilaniMPecoraSRainbow Study Investigator GroupClinical relevance of non-grass pollens respiratory allergies in Italy and effects of specific sublingual immunotherapy: The Rainbow Trial, a multicentre 3-year prospective observational studyEur Ann Allergy Clin Immunol201143411111621980798
- DelgadoJDávilaIDDomínguez-OrtegaJQuirceSMartí-GuadañoEValeroAQuality of life in patients with respiratory allergy is influenced by the causative allergenJ Investig Allergol Clin Immunol2013235309314
- BurksACalderonMCasaleTUpdate on allergy immunotherapy: American Academy of Allergy, Asthma & Immunology/European Academy of Allergy and Clinical Immunology/PRACTALL consensus reportJ Allergy Clin Immunol201313151288129623498595
- CanonicaGWCoxLPawankarRSublingual immunotherapy: World Allergy Organization position paper 2013 updateWorld Allergy Organ J201471624679069
- RadulovicSCalderonMAWilsonDDurhamSSublingual immunotherapy for allergic rhinitisCochrane Database Syst Rev201012CD00289321154351
- DevillierPDreyfusJFDemolyPCalderonMAA meta-analysis of sublingual allergen immunotherapy and pharmacotherapy in pollen-induced seasonal allergic rhinoconjunctivitisBMC Med201412724433458
- LinSYErekosimaNKimJMSublingual immunotherapy for the treatment of allergic rhinoconjunctivitis and asthma: a systematic reviewJAMA2013309121278128823532243
- DretzkeJMeadowsANovielliNHuissoonAFry-SmithAMeadsCSubcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparisonAllergy Clin Immunol2013131513611366
- Lyseng-WilliamsonKAStandardized sublingual allergen extract solution (Staloral®): a guide to its use as allergen-specific immunotherapyDrug Ther Perspect20143012401410
- FratiFScuratiSPuccinelliPDevelopment of an allergen extract for sublingual immunotherapy – evaluation of StaloralExpert Opin Biol Ther2009991207121519601728
- VourdasDSyrigouEPotamianouPCaratFBatardTAndréCPapageorgiouPSDouble-blind, placebo-controlled evaluation of sublingual immunotherapy with standardized olive pollen extract in pediatric patients with allergic rhinoconjunctivitis and mild asthma due to olive pollen sensitizationAllergy19985376626729700035
- LeonardiSArenaABrunoMEOlea sublingual allergoid immunotherapy administered with two different treatment regimensAllergy Asthma Proc2010312e25e2920406589
- IraniCSalehRAJammalMHaddadFHigh-dose sublingual immunotherapy in patients with uncontrolled allergic rhinitis sensitized to pollen: a real-life clinical studyInt Forum Allergy Rhinol201441080280725224283
- BatardTNonyEHrabinaMChabreHFratiFMoingeonPAdvances in the quantification of relevant allergens in allergenic extractsEur Ann Allergy Clin Immunol201345Suppl 2333724129086
- World Medical AssociationWorld Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjectsJAMA2013310202191219424141714
- ICH Harmonised Tripartite Guideline E6 S5Guideline for Good Clinical Practice1996 Available from: http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/good-clinical-practice.htmlAccessed August 12, 2016
- FerrésJJusticiaJLGarcíaMPMuñoz-TuduríMAlvàVEfficacy of high-dose sublingual immunotherapy in children allergic to house dust mites in real-life clinical practiceAllergol Immunopathol (Madr)201139312212720570032
- TrebuchonFDavidMDemolyPMedical management and sublingual immunotherapy practices in patients with house dust mite-induced respiratory allergy: a retrospective, observational studyInt J Immunopathol Pharmacol201225119320622507332
- TrebuchonFLhéritier-BarrandMDavidMDemolyPCharacteristics and management of sublingual allergen immunotherapy in children with allergic rhinitis and asthma induced by house dust mite allergensClin Transl Allergy201441524910771
- KatotomichelakisMSimopoulosEZhangNOlfactory dysfunction and asthma as risk factors for poor quality of life in upper airway diseasesAm J Rhinol Allergy201327429329823883811
- KatotomichelakisMRigaMTripsianisGPredictors of quality of life improvement in allergic rhinitis patients after sublingual immunotherapyAnn Otol Rhinol Laryngol2015124643043625539660