 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
The aim of this study was to highlight the clinical association of baseline levels of conjugated dienes in low-density lipoprotein (LDL-BCD) and nitric oxide (NO) with immunoglobulins (Igs) and T helper (Th)1/Th2 ratio in patients with newly diagnosed B-cell non-Hodgkin lymphoma (NHL).
Patients and methods
Thirty-two newly diagnosed patients with aggressive B-cell NHL and 25 age-, sex-, and body-mass-index-matched healthy controls were randomly selected for a cross-sectional case–control study conducted at the Hematology Department of Tlemcen Medical Centre University (northwest of Algeria).
Results
Circulating levels of LDL-BCD and NO and those of IgA and IgM were significantly higher in patients than in controls. The levels of Th1/Th2 ratio and plasma total antioxidant capacity were significantly lower in patients compared with controls, while malondialdehyde and protein carbonyl levels were significantly higher in patients. B-cell NHL was significantly associated with high levels of LDL-BCD from 25th to 75th percentile (25th percentile: relative risk [RR] =2.26, 95% confidence interval [CI] 1.42–3.59, P=0.014; 50th percentile: RR =2.84, 95% CI 1.72–4.68, P<0.001; 75th percentile: RR =5.43, 95% CI 2.58–11.42, P<0.001). Similarly, the disease was significantly associated with high levels of NO production from 25th to 75th percentile (25th percentile: RR =2.07, 95% CI 1.25–3.44, P=0.024; 50th percentile: RR =2.78, 95% CI 1.63–4.72, P<0.001; 75th percentile: RR =4.68, 95% CI 2.21–9.91, P<0.001). Moreover, LDL-BCD levels were positively and significantly correlated with interferon (IFN)-γ, whereas NO levels were inversely and significantly correlated with IFN-γ and Th1/Th2 ratio.
Conclusion
LDL-BCD and NO production seem to be associated with aggressive B-cell NHL and alteration of Th1/Th2 ratio. Our results have to be examined using ex vivo mechanistic studies leading to further investigations of these parameters, with an interest in the link between Epstein–Barr virus infection and NO and immunoglobulins.
Introduction
Non-Hodgkin lymphoma (NHL) is a nonspecific term that includes a spectrum of lymphoproliferative malignant diseases with different clinical and histological appearances.Citation1 Two clinical forms of NHL can be distinguished on the basis of their growth rate: aggressive (fast-growing) or indolent (slow-growing).Citation2 In addition, NHL can be formed not only from an uncontrollable proliferation of either B or T mononuclear lymphoid cells but also from natural killer cells.Citation3
Although the exact causal factors for NHL are not yet well understood, many cancers may develop from chronic irritation and inflammation.Citation4 The strong link between cancer malignancy and chronic inflammation would be due, in part, to the release of many mediators that can induce increased cell proliferation, mutagenesis, oncogenic transformation, and tumor angiogenesis.Citation5 These mediators include especially eicosanoids, cytokines, chemokines, and reactive oxygen species (ROS) and/or radical species derived from nitric oxide (NO). NO and its derivatives may promote oncogenesis through damage to DNA and proteins, inhibition of apoptosis, mutation of DNA, and cellular repair functions such as p53 and also via promotion of angiogenesis.Citation6 It is therefore becoming crucial to fully highlight the link of NO with immune and inflammatory biomarkers in NHL.
Numerous studies have been conducted to show the role of oxidized low-density lipoprotein (ox-LDL) in inflammatory and immune responses. Ox-LDL can induce immune responses by acting as an adjuvant that activates cells of innate immunity.Citation7 They can act as inducers of the expression of adhesion molecules on activated endothelium.Citation8 Ox-LDLs can also display chemotactic activity for monocytes, promote their differentiation into resident macrophages, and inhibit their mobility.Citation9 It has been established that the binding of ox-LDL to the scavenger receptor CD36, lectin-like ox-LDL receptor 1, and CD205 induces the synthesis of proinflammatory cytokines in human dendritic cells (DCs) leading to DC maturation and DC differentiation.Citation10 In addition, it has been recently observed that ox-LDL activates the inflammatory pathway through nuclear factor-kB (NF-kB), leading to cell transformationCitation11 and that high levels of ox-LDL are associated with increased risk of cancer.Citation12 However, to the best of our knowledge, there has been no study that correlates ox-LDL with NHL.
Given that most NHLs are of B-cell origin, the current study aimed to highlight the association of baseline levels of conjugated dienes in low-density lipoprotein (LDL-BCD) and NO with aggressive B-cell NHL risk and to assess their link with T helper (Th)1/Th2 ratio. In this context, a cross-sectional case–control study was conducted at the Department of Hematology of Tlemcen University Hospital Centre (Algeria).
Patients and methods
Thirty-two patients with aggressive B-cell NHL and 25 age-, sex-, and body-mass-index-matched healthy controls were randomly selected for a cross-sectional study conducted at the Hematology Department of Tlemcen Medical Centre University (northwest of Algeria). The mean age of patients (18 men, 14 women) was 52.81 years (range 25–70 years) and that of controls (13 men, 12 women) was 50.5 years (range 25–72 years). The demographic characteristics of patients and controls were recorded through a questionnaire. Histological and immunohistochemical analyses were performed in order to complete the clinical diagnosis and to determine the histologic types of NHL. The main criterion for the inclusion of cases was newly diagnosed aggressive B-cell NHL. The main exclusion criteria were NHL associated with another type of cancer, family history of cancer, indolent lymphoma, a positive serology for HIV, hepatitis C virus, and all diseases that are related to cardiac function. Informed consent was signed by all participants in this study. LDL-BCD were determined to evaluate the levels of LDL oxidation.Citation13 NO production was determined by measuring the levels of nitrate
and nitrite
, with the sensitive colorimetric Griess reaction. Immunoglobulins (Igs) were assessed as biomarkers of humoral immunity.Citation14 Malondialdehyde (MDA) and protein carbonyl (PC) were determined as markers for assessing oxidative stress,Citation15,Citation16 while the concentration of total antioxidant capacity (TAC) and albumin were measured as markers of antioxidative defense.Citation17 This work was approved by the institutional ethics board of University of Tlemcen.
Sample preparation
Venous blood samples were drawn in the morning between 8 am and 9 am after an overnight fast. The blood was collected in sterile tubes without coagulant for Igs, interferon (IFN)-γ, interleukin (IL)-4, protein fractions, NO, β-lipoproteins, and LDL-BCD analyses and with coagulant for TAC, MDA (ethylenediaminetetraacetic acid-containing tube), and PC (heparin-containing tube) assays. The tubes were centrifuged within 20 minutes, and then aliquoted and stored at −20°C. Lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) were collected from the individual’s personal health record.
Immunological and biochemical analyses
Cytokine assay and Th1/Th2 ratio
The Th1/Th2 ratio was estimated from the IFN-γ/IL-4 ratio. The two cytokines were quantified in serum from patients as well as from healthy controls by appropriate human Quantikine sandwich enzyme-linked immunosorbent assay (ELISA) kits according to the instructions of the manufacturer (R&D Systems, Inc., Minneapolis, MN, USA). Limits of detection were 8 pg/mL for IFN-γ and 10 pg/mL for IL-4.
Igs assay
Igs A, M, and G were measured quantitatively by single radial immunodiffusion method using specific antiserums and standard samples from Cypress Diagnostics (Langdorp, Belgium).
Albumin assay
Albumin measurement was carried out by protein zone electrophoresis performed on cellulose acetate plate using a commercial kit (Helena Laboratories, Beaumont, TX, USA). Protein bands were visualized with Ponceau S Stain 5526 (Helena Laboratories). Electrophoretic patterns were analyzed by densitometry using NIH ImageJ software. Albumin content level in grams per liter was determined using serum total protein (TPROT) concentration. TPROT assay was measured by modified Biuret method using Thermo Scientific kit (Thermo Fisher Scientific, Waltham, MA, USA).
NO assay
Serum nitrite and nitrate (NOx,
and
levels were measured as an indirect marker of in vivo NO formation by Griess assay.Citation18 Serum was first deproteinized with trichloroacetic acid. After centrifugation, the clear supernatant was added to the vanadium (III) chloride to reduce nitrate to nitrite. This was followed by addition of the Griess reagent that converts nitrite into a pink-colored azo compound. The absorbance was then measured at 520 nm, and NO concentrations were determined in comparison to the standard curve prepared from sodium nitrate (NaNO3).
TAC measurement
Plasma TAC was measured according to the kit radicaux libres (Spiral/KIRIAL, Dijon, France) biological test based on the hemolysis induced by radical attack.Citation19,Citation20
Protein oxidation analysis
The levels of protein oxidation were determined by measuring PCs using ELISA kit, based on the detection of 2,4-dinitrophenylhydrazine (Biocell carbonyl protein ELISA kit, ALX-850-312-KI01; Axxora Deutschland GmbH, Lorrach, Germany).
Lipid peroxidation assay
The determination of plasma lipid peroxidation as MDA was measured at 535 nm using the thiobarbituric assay as described earlier.Citation21
Serum β-lipoprotein assay
Serum β-lipoprotein (LDL) was measured by Helena lipoprotein electrophoresis on a cellulose acetate plate which had been presoaked in a Tris-barbital buffer at pH 8.8 (Helena Laboratories). The electrophoretic bands were stained using a methanol solution of Oil Red O (Sigma-Aldrich Co., St Louis, MO, USA).
LDL-BCD assay
Serum LDLs were first isolated by precipitation with heparin–trisodium citrate buffer as described earlier.Citation22 The measurement of levels of LDL oxidation products was performed on the insoluble pellet by resuspension of precipitated lipoproteins in 1 mL of 0.1 M Na-phosphate buffer, pH 7.4, containing 0.9% NaCl.Citation23 LDL-BCD samples (100 μL) were measured as an indicator of circulating ox-LDL in vivo as reportedCitation24 and described earlier in detail.Citation23 The absorption spectrum was recorded at room temperature on a spectrophotometer ultraviolet/visible (Perkin-Elmer Lambda 800). The absorbance of each sample was read at 233 nm.
Statistical analysis
Data analyses were carried out using SPSS 16.0 (Statistical Package for the Social Sciences; SPSS Inc., Chicago, IL, USA) or Epi Info 2000 (Version 1.0; Epi Info, Atlanta, GA, USA), appropriately. Depending on the results of a test of normality, the comparison of means was performed by Student’s t-test (for normally distributed variables) or Mann–Whitney U-test (for variables that were not normally distributed).Citation25 The comparison of frequencies was carried out using Yates’s chi-square test. Relative risk (RR) and corresponding 95% confidence interval (CI) were calculated to determine cross-sectional associations among quartiles of 25th, 50th, and 75th percentile values as cutoff points. Bivariate correlation was performed using Pearson’s or Spearman’s correlation coefficients, appropriately, according to the normality of the distribution. The significance level was set at P<0.05.
Results
shows the demographic and clinical characteristics of participants of the current study. The mean age, the sex ratio, and body mass index were similar between patients and controls (for all comparisons, P>0.05). However, the serum levels of LDH and ALP were significantly higher in patients than in controls (P=0.025 and P=0.030, respectively). In addition, the histological types of B-cell lymphoma were diffuse large B-cell lymphoma (DLBCL, 59%), B-cell lymphomas, unclassifiable, with feature intermediate between Burkitt’s lymphoma (BL) and DLBCL (B-UNC/BL/DLBCL, 3%) and BL (38%). B-UNC/BL/DLBCL was diagnosed based on morphologic, molecular genetics, and immunopheno-type features, according to the World Health Organization classification of tumors of hematopoietic and lymphoid tissue (2001 and revised in 2008).Citation26,Citation27
Table 1 Clinical and demographic characteristics of patients with newly diagnosed aggressive B-cell NHL
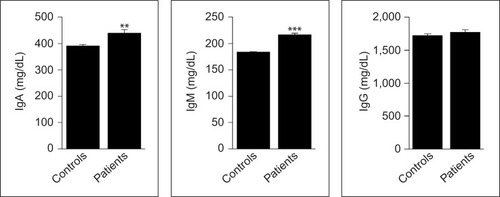
As indicated in , the levels of IFN-γ were significantly lower in patients than in controls (P=0.014), while those of IL-4 were significantly higher (P<0.001). In addition, Th1/Th2 ratio, as estimated from the IFN-γ/IL-4 ratio, was significantly lower in patients compared to controls (P=0.003). Moreover, the serum IgA and IgM levels were significantly higher in patients than in controls (P=0.002 and P<0.001, respectively), whereas IgG levels were similar in the two groups (P=0.385; ).
Figure 1 Serum levels of IFN-γ and IL-4 and Th1/Th2 ratio in newly diagnosed patients with aggressive B-cell NHL and matched control subjects.
Abbreviations: IFN, interferon; IL, interleukin; NHL, non-Hodgkin lymphoma; Th, T helper.
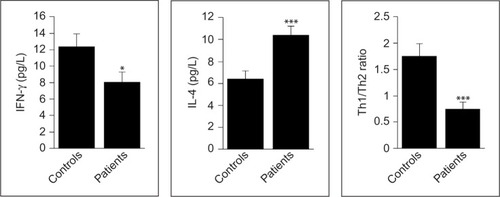
Figure 2 Serum levels of Igs A, M, and G in newly diagnosed patients with aggressive B-cell NHL and matched control subjects.
Abbreviations: Ig, immunoglobulin; NHL, non-Hodgkin lymphoma.
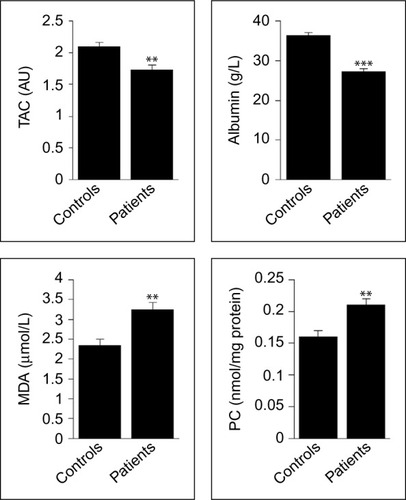
As indicated in , the plasma levels of total anti-oxidative defense capacity and the concentration of albumin were significantly decreased in patients when compared to controls (P=0.001 and P<0.001, respectively). However, the circulating levels of MDA and PC were significantly increased in patients compared to controls (for the two comparisons, P=0.001).
Figure 3 Oxidative stress biomarkers in patients with newly diagnosed B-cell NHL and healthy controls.
Abbreviations: MDA, malondialdehyde; NHL, non-Hodgkin lymphoma; PC, protein carbonyl; TAC, total antioxidant capacity.
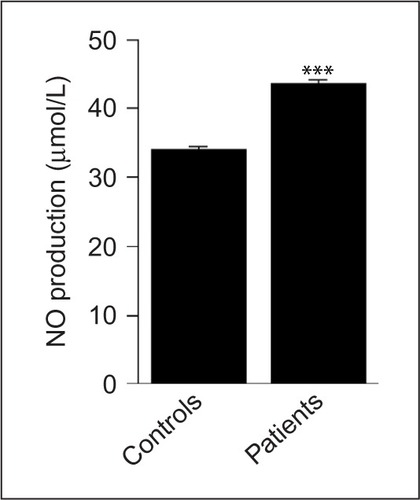
Although the levels of LDL are similar in both groups (P=0.853), those of LDL-BCD were significantly increased in patients compared to controls (P<0.001; ). Similarly, the serum levels of NO were significantly higher in patients when compared to controls (P<0.001; ).
Figure 4 β-Lipoprotein and LDL-BCD levels in newly diagnosed patients with aggressive B-cell NHL and healthy controls.
Abbreviations: NHL, non-Hodgkin lymphoma; LDL-BCD, baseline levels of conjugated dienes in low-density lipoprotein; β-LP, β-lipoprotein (LDL).
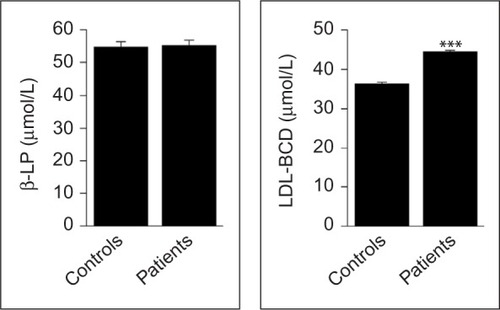
Figure 5 NO production levels in newly diagnosed patients with aggressive B-cell NHL and healthy controls.
Abbreviations: NHL, non-Hodgkin lymphoma; NO, nitric oxide.
The association analysis of LDL-BCD and NO with aggressive B-cell NHL among quartiles of 25th, 50th, and 75th percentile is highlighted in .
Table 2 Association of LDL-BCD and NO production with newly diagnosed aggressive B-cell NHL among quartiles of 25th, 50th, and 75th percentile
As indicated in , aggressive B-cell NHL was significantly associated with high levels of LDL-BCD from 25th to 75th percentile (25th percentile: RR =2.26, 95% CI 1.42–3.59, P=0.014; 50th percentile: RR =2.84, 95% CI 1.72–4.68, P<0.001; 75th percentile: RR =5.43, 95% CI 2.58–11.42, P<0.001). Similarly, the disease was significantly associated with high levels of NO production from 25th to 75th percentile (25th percentile: RR =2.07, 95% CI 1.25–3.44, P=0.024; 50th percentile: RR =2.78, 95% CI 1.63–4.72, P<0.001; 75th percentile: RR =4.68, 95% CI 2.21–9.91, P<0.001).
The correlation coefficients and their respective P-values are shown in . The levels of LDL-BCD were positively and significantly correlated with IFN-γ (r=0.377, P=0.033), and IgA (r=0.422, P=0.016), IgM (r=0.519, P=0.002). In addition, NO levels were positively and significantly correlated with IgM (r=0.461, P=0.008), but inversely and significantly correlated with IFN-γ (r=-0.528, P=0.002) and Th1/Th2 ratio (r=-0.354, P=0.047).
Table 3 Relationship of LDL-BCD and NO production with newly diagnosed aggressive B-cell NHL
Discussion
An increase in ALP levels is frequently associated with a variety of cancers,Citation28 including NHL.Citation29,Citation30 ALP can also be suggested as a valuable prognostic biomarker for malignant tumors.Citation31 Similar to ALP, we observed an increase in the levels of LDH in patients with B-cell NHL. Also of note, increased enzymatic activity of LDH leads to the release of huge amounts of lactate from pyruvic acid and therefore to the activation of proinflammatory pathways and angiogenesis. This phenomenon has been reported as Warburg effect.Citation32 In addition, increased LDH levels have been associated with poor prognosis in patients with cancer.Citation33
Alteration of Th1/Th2 ratio was usually highlighted in NHL.Citation29 Low plasma concentrations of Th1-related cytokines IFN-γ have been recently shown in patients with NHL enrolled in a prospective case–control study; among them 92% were diagnosed with B-cell NHL.Citation34 Therefore, the deletion or inhibition of Th1 cells may reduce the capacity of cancer cell elimination by cytotoxic CD8+ T-cells.Citation35
Serum concentrations of IgA and IgM levels were significantly higher in patients than in controls. Generation of Igs have been implicated in contributing to the disease course of multiple cancers,Citation36 and may serve as useful biomarker of disease activity and/or prognostic. Our results corroborate those of the previous studyCitation37 regarding the significant increase in IgA as well as IgM levels. Both IgA and IgM could be increased early in response to invasion by bacteria and viruses, such as Epstein–Barr virus (EBV).Citation38 Hence, numerous recent epidemiological and experimental studies have revealed that EBV is highly associated with several different types of aggressive NHL.Citation39 Therefore, our results indicate a higher concordance with inflammatory conditions that could be immunologically mediated.
Excessive ROS production can cause persistent oxidative stress that may contribute to promote genetic instability and malignant transformation of cells.Citation40 In the current study, increased levels of MDA and PC and decreased levels of TAC and albumin in patients point toward an oxidative stress.
MDA is one of the lipid peroxidation end products, whereas PC is a product of irreversible nonenzymatic oxidation or carbonylation of protein.Citation41 Their excessive levels have been described in inflammatory conditions, and in various types of cancers including hematological malignancies.Citation42 MDA is able to contribute to the development of human cancer by acting as a mutagen and genotoxic agent.Citation43 Of note, it has been suggested that its increased levels may have an important role in the pathogenesis of aggressive B-cell NHL.Citation44
TAC is considered as useful and one of the best biomarkers of overall plasma antioxidant status. Only one study on total radical scavenging ability and the risk of NHL, based on a food consumption survey, has recently reported that higher antioxidant intake as estimated by the food-frequency questionnaire-based oxygen radical absorbance capacity (ORAC) values is associated with a lower risk of NHL.Citation45 Albumin is described as the predominant antioxidant molecule in plasma.Citation46 Our findings are similar with recent results reporting lower levels of ORAC and/or albumin in patients with NHL.Citation29 In addition, several clinical trials have shown that serum albumin is a significant prognostic marker for the disease.Citation47
It is now well established that oxidative stress leads to LDL oxidation and vice versa.Citation48,Citation49 Our study demonstrates, for the first time, that circulating levels of LDL-BCD are increased in newly diagnosed aggressive B-cell NHL and significantly associated with the disease. In addition, LDL-BCD was significantly and positively correlated with IFN-γ, IgA, and IgM. Although the correlation does not imply causation, our results corroborate with those showing that LDL-BCD induces B-cell activation and production of antibodies and proinflammatory Th1 cytokines, such as IFN-γ.Citation50 In addition, it has been observed that the majority of CD5+/B-1 B-cells produce natural IgM antibodies that recognize altered self-molecules such as ox-LDL.Citation51 This observation is consistent with our findings regarding the correlation between LDL-BCD and IgM; nevertheless, the determination of the level of IgM anti-ox-LDL antibodies would still be interesting.
NO plays very diverse roles in physiological, neurological, and immunological functions. It may lead to different effects, which are sometimes opposed. The protective and toxic effects of NO are frequently seen in parallel.Citation52 Thus, depending upon the specific situation, it can act as a mediator of apoptosis and cell death,Citation53 or otherwise opposes apoptosis and contributes to mutagenesis and carcinogenesis.Citation54,Citation55 To date, there are no data on circulating levels of NO production in patients with B-cell NHL. In this study, the levels of NO production were significantly higher in patients with newly diagnosed aggressive B-cell NHL, and strongly associated with the disease. Increased circulating levels of NO could be attributed to its role in tumor-associated inflammatory and antiviral responses.Citation56 Indeed, it has previously been shown that NO may be generated to counteract the reactivity of EBV.Citation57 Of note, during rapid cell proliferation, there is an overproduction of ROS and reactive nitrogen species resulting in oxidative stress. Protection against oxidative stress could be provided through the Warburg effect. The carcinogenic effects of excessive reactive nitrogen species and ROS can be attributed to their ability to cause DNA damage.Citation58 It has previously been reported that the NO activity is strongly influenced by its concentration.Citation52 At high concentrations, NO is rapidly oxidized to reactive nitrogen species,Citation59 following its interaction with O2 or
.Citation55 The harmful effects of NO are related to its ability to form the potent cytotoxic oxidants peroxynitrite (ONOO–) and its conjugate acid ONOOH after interaction with the superoxide anion
. Interestingly, peroxynitrite was reported to play key roles in a significant proportion of various diseases, including cancers.Citation60,Citation61
Conclusion and future prospects
In light of our results, it seems that the newly diagnosed aggressive B-cell NHL is associated not only with a marked increase in LDL-BCD and NO production but also with immune and physiological disorders, including a shift of Th1/Th2 balance toward Th2 dominance, high circulating Igs, particularly IgA and IgM, and an excessive oxidative stress. Nevertheless, our results have to be examined using ex vivo mechanistic studies leading to further investigations of these parameters, with an interest in the link between EBV infection and NO and Igs.
Acknowledgments
The authors are grateful to patients and subjects for their participation. They would also like to address special thanks to all the staff of the Haematology Department at Tlemcen Medical Centre University for their help during this study. The authors also thank Ms Maliha Meziane for proofreading the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- Hadzi-PecovaLPetrusevskaGStojanovicANon-Hodgkin’s lymphomas: immunologic prognostic studiesPrilozi2007281395517921917
- RogersBBOverview of non-Hodgkin’s lymphomaSemin Oncol Nurs2006222677216720228
- CivalleroMCosenzaMPozziSBariAFerriPSacchiSActivity of BKM120 and BEZ235 against lymphoma cellsBiomed Res Int2015201587091826557706
- CoussensLMWerbZInflammation and cancerNature2002420691786086712490959
- FergusonLRChronic inflammation and mutagenesisMutat Res20106901-231120223251
- JaiswalMLaRussoNFGoresGJNitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesisAm J Physiol Gastrointest Liver Physiol20012813626634
- PalmNWMedzhitovRPattern recognition receptors and control of adaptive immunityImmunol Rev2009227122123319120487
- RobbesynFSalvayreRNegre-SalvayreADual role of oxidized LDL on the NF-kappaB signaling pathwayFree Radic Res200438654155115346645
- QuinnMTParthasarathySSteinbergDLysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesisProc Natl Acad Sci U S A1988858280528093357891
- NickelTSchmaussDHanssenHoxLDL uptake by dendritic cells induces upregulation of scavenger-receptors, maturation and differentiationAtherosclerosis2009205244245019203752
- LuJMitraSWangXKhaidakovMMehtaJLOxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesisAntioxid Redox Signal20111582301233321338316
- ZabirnykOLiuWKhalilSSharmaAPhangJMOxidized low-density lipoproteins upregulate proline oxidase to initiate ROS-dependent autophagyCarcinogenesis201031344645419942609
- AhotupaMAsankariTJBaseline diene conjugation in LDL lipids: an indicator of circulating oxidized LDLFree Radic Biol Med19992711–121141115010641705
- SteadADouglasJGBroadfootCJKaminskiERHerriotRHumoral immunity and bronchiectasisClin Exp Immunol2002130232533012390323
- Dalle-DonneIRossiRGiustariniDMilzaniAColomboRProtein carbonyl groups as biomarkers of oxidative stressClin Chim Acta20033291–2233812589963
- ThérondPBonnefont-RousselotDDavit-SpraulAContiMLegrandABiomarkers of oxidative stress: an analytical approachCurr Opin Clin Nutr Metab Care20003537338411151083
- HaddoucheMAribiMMoulessehoulSSmahiMCLammaniMBenyoucefMAlteration of antioxidant defense status precedes humoral immune response abnormalities in macrosomiaMed Sci Monit20111711CR650CR65622037745
- GuevaraIIwanejkoJDembińska-KiećADetermination of nitrite/nitrate in human biological material by the simple Griess reactionClin Chim Acta199827421771889694586
- LesgardsJFDurandPLassarreMAssessment of lifestyle effects on the overall antioxidant capacity of healthy subjectsEnviron Health Perspect2002110547948612003751
- BlacheDDurandPProstMLoreauN(+)-Catechin inhibits platelet hyperactivity induced by an acute iron load in vivoFree Radic Biol Med200233121670168012488135
- Nourooz-ZadehJTajaddini-SarmadiJLingKLWolffSPLow-density lipoprotein is the major carrier of lipid hydroperoxides in plasma. Relevance to determination of total plasma lipid hydroperoxide concentrationsBiochem J1996313Pt 37817868611155
- WielandHSeidelDA simple specific method for precipitation of low density lipoproteinsJ Lipid Res19832479049096631224
- AhotupaMRuutuMMäntyläESimple methods of quantifying oxidation products and antioxidant potential of low density lipoproteinsClin Biochem19962921391448601322
- VasankariTAhotupaMViikariJEffects of statin therapy on circulating conjugated dienes, a measure of LDL oxidationAtherosclerosis2005179120720915721029
- FayMPProschanMAWilcoxon-Mann-Whitney or t-test? On assumptions for hypothesis tests and multiple interpretations of decision rulesStat Surv2010413920414472
- JaffeESHarrisNLSteinHVardimanJWorld Health Organization Classification of Tumours Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues3th edLyon, FranceIARC Press2001
- SwerdlowSHCampoEHarrisNLWHO Classification of Tumours of Haematopoietic and Lymphoid Tissues4th edLyon, FranceIARC Press2008
- SaifMWAlexanderDWicoxCMSerum alkaline phosphatase level as a prognostic tool in colorectal cancer: a study of 105 patientsJ Appl Res200551889519750205
- ZahzehMRLoukidiBMezianeWRelationship between NADPH and Th1/Th2 ratio in patients with non-Hodgkin lymphoma who have been exposed to pesticidesJ Blood Med201569910725878515
- KittivorapartJChinthammitrYIncidence and risk factors of bone marrow involvement by non-Hodgkin lymphomaJ Med Assoc Thai201194suppl 1S239S24521721453
- YuMCChanKMLeeCFAlkaline phosphatase: does it have a role in predicting hepatocellular carcinoma recurrence?J Gastrointest Surg20111581440144921541770
- ColenCBShenYGhoddoussiFMetabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo studyNeoplasia201113762063221750656
- ArmstrongAJGeorgeDJHalabiSSerum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycinJ Clin Oncol201230273402340722891270
- Saberi HosnijehFKropEJScocciantiCPlasma cytokines and future risk of non-Hodgkin lymphoma (NHL): a case-control study nested in the Italian European Prospective Investigation into Cancer and NutritionCancer Epidemiol Biomarkers Prev20101961577158420501772
- ChanCJAndrewsDMSmythMJReceptors that interact with nectin and nectin-like proteins in the immunosurveillance and immunotherapy of cancerCurr Opin Immunol201224224625122285893
- SmithLHYinABieberMTengNNHGeneration of human monoclonal antibodies to cancer associated antigens using limited numbers of patient lymphocytesJ Immunol Methods198710522632732826600
- Planinc-PeraicaAKolonićSORadić-KristoDDominisMJaksićBSerum immunoglobulins in non-Hodgkin’s lymphoma patientsColl Antropol201034240741120698110
- MalbranABelmonteLRuibal-AresBLoss of circulating CD27+ memory B cells and CCR4+ T cells occurring in association with elevated EBV loads in XLP patients surviving primary EBV infectionBlood200410351625163114604960
- De RoosAJMartínez-MazaOJeromeKRInvestigation of epsteinbarr virus as a potential cause of B-cell non-Hodgkin lymphoma in a prospective cohortCancer Epidemiol Biomarkers Prev201322101747175523885038
- NakanomeABrydunAMatsumotoMBach1 is critical for the transformation of mouse embryonic fibroblasts by Ras(V12) and maintains ERK signalingOncogene201332273231324522847612
- Dalle-DonneIRossiRColomboRGiustariniDMilazaniABiomarkers of oxidative stress in human diseaseClin Chem200652460162316484333
- AhmadRTripathiAKTripathiPSinghSSinghRSinghRKMalondialdehyde and protein carbonyl as biomarkers for oxidative stress and disease progression in patients with chronic myeloid leukemiaIn Vivo200822452552818712183
- MarnettLJLipid peroxidation-DNA damage by malondialdehydeMutat Res19994241–2839510064852
- EissaLAEsmaeelMIRelevance of some serum biomarkers (E cadherin, GAGs and MDA in patients with diffuse large B-cell lymphomaPak J Pharm Sci2008211293518166516
- HoltanSGO’ConnorHMFredericksenZSFood-frequency questionnaire-based estimates of total antioxidant capacity and risk of non-Hodgkin lymphomaInt J Cancer201213151158116822038870
- RocheMRondeauPSinghNRTarnusEBourdonEThe antioxidant properties of serum albuminFEBS Lett2008582131783178718474236
- AliciSBavbekSEKaytanEEralpYOnatHPrognostic factors in localized aggressive non-Hodgkin’s lymphomaAm J Clin Oncol20032611512576915
- SemlitschMShackelfordREZirklSSattlerWMalleEATM protects against oxidative stress induced by oxidized low-density lipoproteinDNA Repair (Amst)201110884886021669554
- VogiatziGTousoulisDStefanadisCThe role of oxidative stress in atherosclerosisHellenic J Cardiol200950540240919767282
- HuangYHRönnelidJFrostegårdJOxidized LDL induces enhanced antibody formation and MHC class II-dependent IFN-gamma production in lymphocytes from healthy individualsArterioscler Thromb Vasc Biol19951510157715837583530
- GetzGSThematic review series: the immune system and atherogenesis. Bridging the innate and adaptive immune systemsJ Lipid Res200546461962215722562
- BogdanCNitric oxide and the immune responseNat Immunol200121090791611577346
- BrüneBSchneiderhanNNitric oxide evoked p53-accumulation and apoptosisToxicol Lett20031392–311912312628747
- GalAWoganGNMutagenesis associated with nitric oxide production in transgenic SJL miceProc Natl Acad Sci U S A1996932615102151078986771
- ChoudhariSKChaudharyMBagdeSGadbailARJoshiVNitric oxide and cancer: a reviewWorld J Surg Oncol20131111823718886
- NiedbalaWCaiBLiewFYRole of nitric oxide in the regulation of T cell functionsAnn Rheum Dis200665Suppl 3iii37iii4017038470
- AgawaHIkutaKMinamiyamaYInoueMSairenjiTDown-regulation of spontaneous Epstein-Barr virus reactivation in the P3HR-1 cell line by L-arginineVirology2002304111412412490409
- MaNKawanishiMHirakuYReactive nitrogen species-dependent DNA damage in EBV-associated nasopharyngeal carcinoma: the relation to STAT3 activation and EGFR expressionInt J Cancer2008122112517252518307254
- ColemanJWNitric oxide in immunity and inflammationInt Immunopharmacol2001181397140611515807
- StrzelczykJKWiczkowskiAOxidative damage and carcinogenesisContemp Oncol (Pozn)201216323023323788885
- HirakuYFormation of 8-nitroguanine, a nitrative DNA lesion, in inflammation-related carcinogenesis and its significanceEnviron Health Prev Med2010152637219921494
