Abstract
Classical Hodgkin’s lymphoma (cHL) is a B-cell malignancy comprised of pathologic Reed Sternberg cells with a surrounding immune-tolerant inflammatory milieu. RS cells evade immune recognition in part through programmed death ligand 1 (PD-L1) overexpression, which is genetically programmed through copy number alterations, polysomy, and amplification of the 9p24.1 locus encoding PD-L1. By engaging with PD-1+ T-cells, PD-L1 delivers a potent immune suppressive signal promoting immunologic escape of the tumor cell. Enhancing antitumor immune response by targeting PD-1 with the monoclonal antibody nivolumab has proved to be effective in multiple solid tumors, but the highest response rates to date have been reported in patients with cHL, with over 65% of treated patients achieving an objective clinical response. In this review, we will summarize the published evidence regarding the activity of nivolumab in cHL as well as its current place in therapy. We will review the pharmacology, mechanism of action, and side effects of nivolumab as well as the emerging data indicating possible increased risk of graft versus host disease in patients treated with PD-1 inhibitors either pre- or post-allogeneic stem cell transplant. Given the remarkable single-agent activity and safety profile of PD-1 inhibitors in heavily pretreated patients with cHL, the possibility of employing nivolumab in combination with other active agents and earlier in therapy is a promising area of active investigation, and we will briefly summarize current clinical trials.
Relapsed/refractory classical Hodgkin’s lymphoma
Classical Hodgkin’s lymphoma (cHL) is a lymphoid malignancy characterized by a small percentage of pathologic Reed Sternberg (RS) cells within a robust nodal inflammatory environment. Standard options for frontline therapy for patients with cHL depend upon stage and risk factors at diagnosis, and consist of combination chemotherapy with or without radiation therapy.Citation1,Citation2 Acceptable frontline chemotherapy regimens for cHL include adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD), Stanford V, as well as escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) for patients with advanced stage disease and higher risk international prognostic score.Citation3–Citation9 For patients with favorable risk early-stage cHL, treatment with ABVD with or without radiation therapy is associated with 5-year progression-free survival (PFS) of ≥90%.Citation10,Citation11 For unfavorable risk or bulky early-stage disease, 5 year failure-free survival rates of 85% and 79% are reported with ABVD + radiation therapy and Stanford V, respectively.Citation12 In patients with high-risk stage III/IV disease, frontline treatment with 6–8 cycles of ABVD leads to 5 years PFS rates ranging from 68% to 73%, while escalated BEACOPP has been associated with 5 year PFS rates from 81%–85% but also with significantly increased hematologic and gonadal toxicity.Citation13–Citation16 While the initial management of patients with advanced-stage Hodgkin’s lymphoma is beyond the scope of this review, we would refer the reader to a recent review by Vassilakopoulos and JohnsonCitation17 for an updated and comprehensive summary of published evidence to date.
For patients with relapsed/refractory disease after initial therapy, standard treatment includes salvage chemotherapy followed by autologous stem cell transplant (ASCT).Citation18–Citation23 With ASCT, 5-year PFS rates of 50%–60% can be achieved in patients with relapsed chemosensitive disease, compared with 5-year PFS rates of 40%–45% for patients with primary refractory disease.Citation18–Citation20,Citation23,Citation24 For patients with relapsed/refractory disease following ASCT, the anti-CD30 monoclonal antibody drug conjugate brentuximab vedotin (BV) (Adcetris, Seattle Genetics, Bothell, WA, USA) demonstrated an overall response rate (ORR) of 75%, including complete response (CR) rate of 36% with a median PFS of 9.3 months in a pivotal phase II trial, leading to US Food and Drug Administraction (FDA) approval in this setting.Citation25,Citation26 In the phase III AETHERA trial, BV consolidation after ASCT resulted in an improvement in PFS (43 vs 24 months) compared with placebo in patients at high risk for relapse.Citation27
Although there are multiple treatment options available for patients who fail ASCT and BV, historical median overall survival (OS) for these patients is about 2 years.Citation28 Allogeneic stem cell transplant (SCT) remains the only known curative option for this patient population, with the presence of a graft versus lymphoma effect suggested by indirect evidence such as lower relapse rates in patients that develop chronic graft-versus-host disease (GVHD).Citation29–Citation31 However, despite potential for durable remissions, the historical 5-year PFS has been about 20% with a 5-year OS of 30%.Citation29,Citation32 For patients who are candidates for allogeneic SCT and desire this approach, combination chemotherapy may be used for maximal disease reduction prior to transplant, albeit at the cost of significant treatment-associated toxicities.Citation33–Citation35 Asymptomatic patients may be observed for a period of time or treated with radiation therapy in case of localized relapse. Palliative single-agent chemotherapy options include gemcitabine, vinorelbine, vinblastine, bendamustine, liposomal doxorubicin, or biological agents such as lenalidomide, the histone deacetylase inhibitors vorinostat and panobinostat, or the mammalian target of rapamycin inhibitor everolimus.Citation36–Citation46 While there are many available options for therapy, responses are not durable, and new treatments are needed for patients with relapse following ASCT and BV.
RS cells avoid antitumor immune response by release of immunosuppressive cytokines such as interferon-gamma, TGF β, chemokine ligands 17 (CCL17) and 22 (CCL22), and interleukin 10 (IL-10) as well as expression of immunetolerance-inducing surface molecules.Citation47 The identification that two of these immunomodulatory surface proteins, PD-L1 (B7H1) and PD-L2 (B7DC), that are expressed by RS cells provided the rationale for therapeutic targeting of their corresponding T-cell target, the receptor programmed death 1 (PD-1, CD279).Citation48 As will be discussed in this review, PD-1 inhibition with the monoclonal antibodies nivolumab and pembrolizumab have emerged as a viable treatment option for patients with cHL after ASCT failure. In this review, we will summarize the mechanism of action, pharmacology, and side effects of nivolumab, the role of PD-1 signaling in cHL, published results to date regarding treatment of cHL with nivolumab, the current role for nivolumab in the treatment of cHL, and future areas of research including ongoing trials in cHL with nivolumab and other PD-1 inhibitors.
PD-1/PD-L signaling
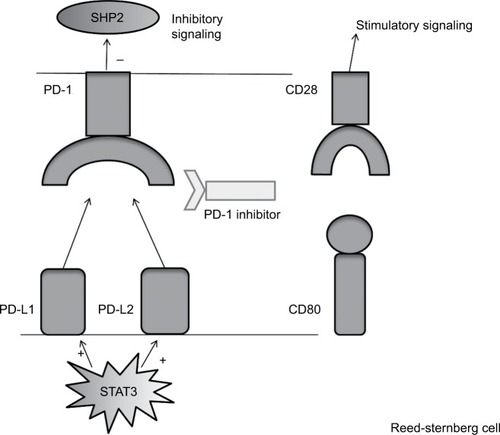
PD-1 is a coinhibitory receptor of the CD28 superfamily expressed on T-cells. By interacting with its corresponding ligands (PD-L1 and PD-L2) on antigen-presenting cells (APCs), PD-1 attenuates T-cell response and promotes T-cell tolerance by inhibiting cytokine production and T-cell proliferation via suppression of Src Homology Phosphatase 2 (SHP-2) signaling within the T-cell ().Citation49–Citation52 In a healthy host, PD-1 expression is increased in activated T-cells to counter-regulate immune response to infection to prevent autoimmunity. In addition to PD-1, PD-L1 has also been shown to competitively engage CD80 (CD28 ligand), decreasing the stimulatory signal mediated by the CD80/CD28 interaction and further inhibiting T-cell proliferation and function.Citation53 The identification of these ligands was followed by the discovery that PD-L1 can also be expressed on tumor cells to evade antitumor immune response.Citation54 PD-L2 expression by tumors cells is less extensively reported, but has relevance for cHL as well as the closely related disease primary mediastinal B cell lymphoma, where it appears to be overexpressed by tumor cells due to gene amplification.Citation55–Citation57
Figure 1 This figure depicts PD-L1 and PD-L2 signaling between an RS cell and a T-cell within the tumor microenvironment.
Abbreviations: PD-1, programmed death 1; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; RS, Reed Sternberg cell; SHP-2, Src homology phosphotase 2.
Gain in chromosome 9p was first observed in primary mediastinal B-cell lymphoma specimens and later discovered in RS cells, distinguishing these diseases genetically from other B cell lymphomas.Citation55,Citation58–Citation61 Via high-density single- nucleotide polymorphism arrays, the 9p24.1 amplicon was shown to contain CD274, which encodes PD-L1, and PDCD1LG2, which encodes PD-L2, as well as Janus Kinase 2 (JAK2), which has been shown to upregulate PD-L1 and PD-L2 expression via the STAT signaling pathway.Citation57 This finding provided a genetic basis for the increased PD-L1 and PD-L2 expression in RS cells and suggested the importance of PD-1 signaling leading to immune evasion in this disease.Citation48,Citation57 Increased PD-L1 and PD-L2 expression in RS cells has also been shown to be mediated by the CTIIA gene fusionCitation62 as well as by Epstein–Barr virus (EBV) infection.Citation63 Subsequent analyses of RS cells isolated from biopsy specimens in cohorts of patients with both newly diagnosed and relapsed/refractory cHL have shown almost universal genetic modification of the PD-L1 and PD-L2 loci via either polysomy of 9p or copy gain or amplification of 9p24.1.Citation56,Citation64,Citation65 In a cohort of 108 patients with newly diagnosed cHL treated with the Stanford V regimen, amplification of 9p24.1 was associated with advanced-stage disease and shorter PFS compared with polysomy or copy gain, suggesting that increased amplification of PD-L1 and PD-L2 may mediate a more aggressive clinical course.Citation56
cHL is characterized by a small percentage of PD-L1+ RS cells within a robust but ineffective inflammatory and immune environment that includes PD-1 expressing T-cells.Citation47,Citation66 This body of evidence, suggesting the importance of PD-L1 and PD-L2 signaling as a common pathway for immune evasion in cHL, provides the rationale for PD-1 targeting in cHL.
Introduction to nivolumab pharmacology, mode of action, and pharmacokinetics
Nivolumab (Opdivo, formerly BMS-936558 and MDX-1106; Bristol-Myers Squibb, New York, NY, USA) is a fully human monoclonal IgG4 antibody targeting PD-1. Initial studies of the safety and activity of nivolumab were performed in patients with advanced melanoma, castration resistant prostate cancer, non-small-cell lung cancer (NSCLC), renal-cell cancer (RCC), and colorectal cancer, demonstrating an acceptable safety profile and response rates ranging from 18%–28% in melanoma, NSCLC, and RCC, including durable response in a significant proportion of responders.Citation67,Citation68 In these initial studies, nivolumab was given at 14 day-intervals with doses escalated from 0.1 mg/kg, 1 mg/kg to 3 mg/kg, and 10 mg/kg with no maximum tolerated dose determined.Citation68 A peak concentration of antibody was seen 1–4 hours after infusion, and while there was a linear correlation between dose, serum concentration, and area under the curve at doses ranging from 0.1 to 10 mg/kg, the PD-1 receptor occupancy of peripheral blood mononuclear cells was similar at all dose levels in 65 melanoma patients (median of 64% at 0.1 mg/kg, median of 70% at 10 mg/kg).Citation68 Objective response rates were numerically similar at all dose levels for patients with melanoma and RCC. However, in NSCLC, all responses in the Phase I study were seen at a dose level of ≥3 mg/kg, with none of the 17 patients treated with the 1 mg/kg dose level achieving objective response. A subset of tumor samples was examined for PD-L1 expression by immunohistochemistry, and preliminary data suggested a correlation between PD-L1 expression on tumor cells and response to PD-1 blockade.Citation68
Subsequent studies in melanoma, RCC, and NSCLC utilizing nivolumab at a dose of 3 mg/kg every 2 weeks validated its clinical activity in these diseases, leading to respective FDA approvals as second-line therapy.Citation69–Citation72 In studies to date, the dose response rate and adverse event (AE) rate for nivolumab appears relatively flat through a wide range of doses, and the FDA cited this lack of apparent dose–response relation when changing the approved dose of nivolumab monotherapy for NSCLC, RCC, and melanoma to a non-weight-based dose of 240 mg every 2 weeks.Citation73 Studies to date of nivolumab as monotherapy for cHL, discussed in greater detail later, have utilized a dose of 3 mg/kg given every 2 weeks, which remains the FDA approved dose for this disease.Citation64,Citation65
Pharmacokinetic studies of 909 patients with different types of solid tumors and hematologic malignancies treated with nivolumab showed an elimination half-life of 26.7 days, mean time to steady state concentration of 12 weeks, and volume of distribution at steady state of 8.0 L.Citation74 Among 1,086 patients treated on 4 clinical trials of nivolumab for multiple solid tumor types, the presence of antidrug antibodies was detected in a minority of patients. This did not appear to lead to clinically meaningful loss of response, hypersensitivity reactions, or accelerated drug clearance.Citation75
Activity of nivolumab in cHL
The initial evidence for the activity of nivolumab in patients with cHL comes from a phase I study of 23 patients with relapsed/refractory cHL and a phase II study of 80 patients with relapsed and refractory disease and prior treatment with both BV and ASCT ().Citation64,Citation65 The genetic basis of PD-L1 expression in cHL with consequent T-cell exhaustion provided the rationale to include 23 patients with relapsed/refractory cHL as a cohort-expansion group in a phase I dose-escalation trial of single-agent nivolumab in patients with relapsed/refractory hematologic malignancies.Citation64 Patients were treated with nivolumab 3 mg/kg every 2 weeks until disease progression, unacceptable toxicity, or complete response.Citation64 Of the cHL patients included, 15/23 had received at least 4 prior lines of therapy, 78% had been previously treated with BV, and 78% had previously undergone ASCT. ORR was 87%; CRs were reported in 4 patients at time of initial publication, and 2 additional patients achieved a CR when extended follow-up was later reported at the 2015 American Society of Hematology (ASH) annual meeting.Citation64,Citation76 With extended follow-up, while 10 patients were found to have durable responses to treatment, 4 of the 20 responding patients eventually developed progressive disease, 5 discontinued nivolumab while in response in order to undergo SCT (allogeneic in 4, ASCT in 1), and 1 patient discontinued nivolumab due to toxicity with response maintained off of nivolumab at 120 days follow-up.Citation76 In 2 of the responding patients, CR was maintained at 40 weeks after discontinuation of treatment. One patient with CR relapsed 43 weeks after cessation of nivolumab, but was able to again achieve CR when retreated with nivolumab. At the time of extended follow-up, 5 patients continued to receive nivolumab at ≥82 weeks of therapy.
Table 1 Summary of published trials of PD-1 inhibitors for cHL
Recently, the results of a multicenter, multicohort, phase II trial of nivolumab for cHL patients after failure of both ASCT and BV were reported.Citation65 Eighty patients enrolled across 34 medical centers in North America and Europe were treated with nivolumab every 2 weeks at a dose of 3 mg/kg and continued until unacceptable toxicity, progression, death, or withdrawal from the study. Eligible patients were required to have previously undergone ASCT followed by BV but were not required to be BV refractory. The median patient age was 37, 64% were males, and about half (49%) had received at least five prior lines of therapy. Forty three (54%) patients were refractory to BV, and 6 (8%) had received more than one prior line of BV. The ORR was 66% after review by independent radiological review committee, with 9% of patients achieving CR as defined by independent radiological review committee.Citation65 The investigator-assessed ORR was similar (58%), with a higher assessment (28%) of patients deemed to have achieved CR. Of the 43 patients refractory to most recent treatment with BV, 31 (72%) responded to nivolumab. Responses were seen at a median of 2.1 months from the start of treatment, although 22 of the 58 patients responding to treatment did not demonstrate response on initial followup scans 9 weeks after the start of treatment. At 6 months, the PFS was 77%. Updated results with a minimum of 12 months of follow-up (median follow-up 15.4 months) were presented at the 2016 ASH annual meeting with a median PFS of 14.8 months, median duration of partial response (PR) of 13.1 months, and median duration of CR not reached.Citation77 At updated follow-up, 37 (46%) patients had discontinued therapy, including 19 (24%) due to disease progression, 7 (9%) who proceeded to allogeneic SCT, and 5 (6%) who discontinued therapy due to AEs.Citation77
Consistent with the immune-mediated mechanism of action of nivolumab, delayed responses were seen, including 1 patient with appearance of a new lesion at week 9 of therapy who went on to have 2 subsequent negative positron emission tomography scans at weeks 25 and 33.Citation65 By protocol definition, this patient’s best response was defined as progressive disease despite the subsequent response to treatment. Given the pattern of late response and benefit beyond traditionally defined progression, the trial was amended to allow patients to continue nivolumab beyond progression at the investigator’s discretion. Of 9 patients who continued nivolumab beyond progression, 5 maintained reduction in total tumor volume.Citation65 In an exploratory post hoc analysis, higher PD-L1 expression was associated with improved best overall response, but the majority of patients achieved at least PR even in the lowest quartile of PD-L1 expression.Citation65 Based on the results of this trial, on May 17, 2016, the FDA granted accelerated approval to nivolumab for the treatment of patients with cHL that has relapsed or progressed after ASCT and posttransplantation BV.
Safety and tolerability
To date, experience with nivolumab in both cHL and other malignancies shows a unique but acceptable toxicity profile when compared with conventional chemotherapy (). PD-1 functions to attenuate immune response in order to prevent autoimmunity and, as could be anticipated, patients treated with nivolumab have demonstrated autoimmune reactions following treatment, termed immune-related AEs (IRAEs).Citation78 These IRAEs include acute hepatitis, colitis, dermatitis, pneumonitis, pancreatitis, and autoimmune endocrine disorders, including hypophysitis and immune-related thyroid disease.Citation74,Citation79,Citation80 The combination of nivolumab with other immune checkpoint inhibitors such as the anti-cytotoxic T-lymphocyte associated protein 4 (CTLA-4) monoclonal antibody ipilumumab has been associated with more frequent incidence of high grade IRAE, but serious adverse effects are also seen in a minority of patients treated with single-agent nivolumab, leading to drug discontinuation in about 5% of cases.Citation67,Citation81 Grade 3–4 IRAEs are most often treated initially with corticosteroid therapy; TNF inhibitors or other immunosuppressant therapy appears to be effective in some steroid refractory cases.Citation74 The management of these autoimmune toxicities is reviewed in detail separately.Citation74,Citation78,Citation82 While the side effects seen with PD-1 inhibitors have been similar across disease groups, there has been a suggestion of a higher incidence of hematologic toxicities in patients with lymphoma compared to those with solid organ malignancies.Citation83 The reason for this disparity is unclear, but may be in part due to the heavy pretreatment of the patients with lymphoma included in early studies.
Table 2 Summary of common and serious AEs reported with nivolumab
In the first published study of nivolumab for the treatment of cHL, AEs of any grade were observed in 22 of 23 patients, with grade 3 or 4 events seen in 12 (52%) patients.Citation64 The most common adverse effects attributed to treatment were rash (22%), thrombocytopenia (17%), pyrexia (13%), diarrhea (13%), nausea (13%), pruritus (13%), and fatigue (13%).Citation64 Two patients experienced grade 3 AEs were believed to be drug related, acute pancreatitis and myelodysplastic syndrome in 1 patient each.Citation64 The patient with myelodysplastic syndrome was heavily pretreated with 6 lines of prior chemotherapy, radiation therapy, and ASCT. Newly diagnosed hypothyroidism was reported in 2 (9%) patients, and no other autoimmune endocrine toxicities were noted. Grade 1 hypersensitivity infusion reactions were noted in 2 patients (9%) not requiring treatment discontinuation. There were no deaths due to toxicity and no grade 4 AEs attributed to nivolumab.Citation64
In the landmark phase II study of 80 patients with relapsed or refractory cHL following ASCT and BV treated with nivolumab, 71 (89%) patients had a reported treatment-related AE of any grade.Citation65 The most commonly reported treatment-related AEs were fatigue (25%), infusion-related reaction (20%), rash (15%), pyrexia (14%), arthralgia (14%), and diarrhea (10%).Citation65 Grade 3 AEs were reported in 17 (21%) patients, and 3 patients had reported grade 4 events (4%). Drug-related grade 3 AEs included neutropenia (5%), increased lipase (3%), increased AST/ALT (3%), abdominal pain (3%), and rash (1%).Citation65 Two patients discontinued treatment due to AEs believed to be drug related; 1 patient with newly diagnosed autoimmune hepatitis, and 1 patient with increased AST/ALT. One patient died from multiorgan failure and was found on autopsy to have a new diagnosis of EBV-positive T-cell lymphoma, believed to be unrelated to nivolumab therapy.Citation65 Pneumonitis was seen in 2 patients (1 with grade 2 and the other with grade 3), which was responsive to corticosteroids in both cases. The patient with grade 3 pneumonitis had already discontinued nivolumab due to grade 3 autoimmune hepatitis diagnosed prior to the onset of pneumonitis.Citation65 Most cHL patients are treated with agents with potential lung toxicity including bleomycin, radiation therapy, and BV, and therefore may be at an increased risk for pulmonary toxicity or pneumonitis. Despite this potential increased risk, the incidence of pneumonitis in published cHL trials with nivolumab has been rare and is similar to that reported in other malignancies.Citation64,Citation65,Citation78 Hypothyroidism has been seen in 5%–10% of patients treated with nivolumab to date, and clinicians should monitor thyroid function through routine thyroid-stimulating hormone measurement during treatment.Citation64,Citation68–Citation70 Although less commonly observed with PD-1 inhibitors than with CTLA-4 inhibitors, providers should be aware of the potential for autoimmune hypophysitis with checkpoint inhibition and be alert to signs and symptoms of adrenal insufficiency or central hypothyroidism.Citation78 New-onset type I diabetes mellitus has been reported in at least 1 instance in a patient with cHL treated with nivolumab.Citation84 While rare and not yet reported in patients with cHL treated with nivolumab, it is worthwhile for the clinician to be aware of the potential for life-threatening myocarditis, rhabdomyolysis, neurologic disorders including myasthenia gravis and Guillain-Barré syndrome, and skin reactions including Stevens–Johnson syndrome, bullous pemphigoid, and toxic epidermal necrolysis, which have been seen in patients following checkpoint blockade.Citation80,Citation82,Citation85–Citation88 Despite the side effects associated with PD-1 blockade, nivolumab is relatively well tolerated overall and generally associated with fewer high-grade side effects when compared with conventional chemotherapy.Citation89
Other PD-1 blocking agents, pembrolizumab
Pembrolizumab (Keytruda, formerly lambrolizumab and MK-3475, Merck Oncology, Kenilworth, NJ, USA) is a humanized monoclonal IgG4 antibody also targeting PD-1. Pembrolizumab has a higher reported affinity for PD-1, but whether this is clinically meaningful remains unclear given the wide therapeutic range seen with PD-1 inhibitors.Citation90
In the Phase 1b KEYNOTE 013 trial, 31 patients with relapsed/refractory cHL were treated with pembrolizumab at a dose of 10 mg/kg every 2 weeks.Citation91 The patients enrolled were heavily pretreated, with over half (55%) having received at least 5 prior lines of therapy. The majority (77%) of patients had previously undergone ASCT and, by design, all patients had failed BV, including 18 (58%) patients with BV-refractory disease. Of the 31 patients, 5 (16%) patients achieved a CR and 15 (48%) patients attained a PR for an ORR of 65%. The responses achieved appeared to be durable in many patients, with a PFS rate of 46% at 1 year. Treatment-related AEs were reported in 68% of patients including hypothyroidism (16%), diarrhea (16%), and pneumonitis (10%). Two patients were taken off therapy due to AEs; 1 with grade 2 pneumonitis and 1 with grade 3 nephrotic syndrome, both of whom responded to treatment with corticosteroids after discontinuation of pembrolizumab.Citation91 Grade 3 AEs not requiring treatment discontinuation were reported in 4 patients including colitis, joint swelling, back pain, axillary pain, and elevated liver aminotransferase levels.
In the phase 2 KEYNOTE-087 trial (NCT 02453594), 210 patients with relapsed/refractory cHL were enrolled across 3 cohorts, including patients with relapsed/refractory disease after ASCT and subsequent treatment with BV (cohort 1), patients ineligible for ASCT due chemo-resistant disease and BV therapy failure (cohort 2), and patients with prior ASCT without subsequent BV therapy (patients with and without exposure to BV prior to ASCT allowed) (cohort 3). Patients were treated with pembrolizumab 200 mg every 3 weeks, and response was assessed every 12 weeks. Preliminary results were reported at the 2016 ASH annual meeting: ORR was 67% (29% CR) in cohort 1, 65% (25% CR) in cohort 2, and 68% (22% CR) in cohort 3, with any degree of reduction in tumor volume from baseline seen in 94% of patients.Citation92 At the time of data presentation, 115 patients had an ongoing response. Reported treatment-related toxicities included pyrexia (11%), hypothyroidism (11%), diarrhea (7%), rash (6%), and nausea (6%).Citation92 Grade 3 treatment-related toxicities included neutropenia (1%), thrombocytopenia (1%), and diarrhea (1%), with no reported treatment-related deaths.Citation92 While these results both in terms of activity and safety are comparable to those reported with nivolumab in the treatment of cHL, it is unknown whether 1 agent has a superior efficacy or safety profile over the other for the treatment of cHL in the absence of a head-to-head clinical trial. On the basis of KEYNOTE 013 and 087 trials discussed above, on March 15, 2017 the US FDA granted accelerated approval to pembrolizumab for the treatment of patients with refractory cHL or patients with cHL who have relapsed after 3 or more prior lines of therapy, making it the second PD-1 inhibitor approved for the treatment of cHL.
Risk in pre- and post- allogeneic SCT
In selected cHL patients with relapsed or refractory disease after ASCT, allogeneic SCT can provide potential durable disease control with similar PFS and OS seen with either myeloablative or reduced intensity conditioning regimens.Citation29,Citation93 PD-1 inhibition has been shown in preclinical models to augment acute GVHD due to T-cell disinhibition, raising concern regarding the safety of checkpoint inhibition in patients who are either being considered for allogeneic SCT or who have relapsed after allogeneic transplant.Citation94,Citation95 Outcomes for 17 cHL patients treated in the phase I study (Checkmate 039) and multicohort phase II (CheckMate 205) trials of nivolumab who underwent subsequent allogeneic SCT were reported at the 2016 ASH annual meeting.Citation96 Of the 17 patients treated, there were 6 deaths, all due to non-relapse mortality. Five of the 6 patients died from acute GVHD after undergoing reduced-intensity transplant. Acute GVHD was seen in 82% of patients, most commonly occurring in the skin (12 patients including 4 with grade 4), GI tract (4 with grade 4), and liver (4 with grade 4). Hyper-acute GVHD occurring within 14 days of transplant was seen in 2 patients, and 2 patients developed encephalitis, including 1 patient with no infectious cause identified who recovered after treatment with corticosteroids. Hepatic sinusoidal obstruction syndrome was reported in 1 patient who died from multiorgan refractory acute GVHD. The proportion of patients experiencing acute GVHD was higher than expected following allogeneic SCT, including atypical manifestations of GVHD such as apparent autoimmune encephalitis and hyper-acute GVHD.Citation96
The largest published experience to date regarding allogeneic SCT following treatment with PD-1-targeting monoclonal antibodies come from a retrospective study of 39 patients with lymphoma, including 31 patients with cHL, treated with either nivolumab (72%) or pembrolizumab (28%) across multiple studies who went on to receive allogeneic SCT.Citation97 Four of these patients were treated with a combination of a PD-1 inhibitor with ipilumumab, and the remainder received PD-1 inhibitor monotherapy; 49% underwent salvage therapy after treatment with PD-1 inhibitor and before SCT. Of the patients included in the analysis, 38 of 39 underwent reduced intensity conditioning, all underwent T-cell replete transplant, with a median time from last treatment with PD-1 inhibitor to SCT of 62 days.Citation97 Cumulative incidence of grade 3–4 acute GVHD was 23%, including 13% of patients with grade 4 acute GVHD with a median onset of 27 days. Four treatment-related deaths were reported, including 3 patients who died from acute GVHD with onset within 14 days of SCT and 1 patient who died due to sinusoidal obstruction syndrome.Citation97 Seven (18%) patients experienced a prolonged noninfectious febrile syndrome, which was treated with corticosteroids in all cases. Despite these toxicities, the 1-year PFS and OS rates were 89% and 76%, respectively, with a cumulative incidence of relapse of 14% and nonrelapse mortality of 11%. A higher incidence of acute GVHD (100%) including 1 case of fatal GVHD was seen in patients treated with both ipilumumab and PD-1 inhibition in comparison to those receiving PD-1 inhibitors alone, suggesting a potential increased risk of acute GVHD with combined checkpoint inhibition prior to SCT. Correlative studies showed that, when compared to a matched control cohort, patients treated with PD-1 inhibition prior to SCT had a decreased ratio of CD4+ T-regulatory cells (T-regs) to CD4+ T-cells up to 1 month after transplant, as well as decreased PD-1 expression on T-cells seen up to 6 months after transplant.Citation97 A lower proportion of T-regs has been associated with increased incidence of acute GVHD, but may also result in enhanced graft-versus-lymphoma effect.Citation98 These findings suggest that the immune effects of PD-1 inhibition remain present months after cessation of PD-1 inhibitor therapy, well beyond what would be expected by pharmacokinetic models of drug clearance.Citation97 Clinicians should be aware of the potential risks of the combination of PD-1 blockade followed by allogeneic SCT, but based on experience to date this approach should not be considered contraindicated. More mature data from ongoing and completed trials as well as validation of these findings in a larger cohort of patients will better define the magnitude of the risk for GVHD and the risks and benefits of allogeneic SCT in patients responding to checkpoint inhibition.
Several case reports have demonstrated clinical responses with nivolumab in patients with relapsed cHL following allogeneic SCT, but there has also been 1 case reported of fatal GVHD in a patient treated for relapsed cHL with pembrolizumab who was over 18 months out from transplant.Citation99–Citation105 The results from a retrospective series of 27 patients (26 with cHL) treated at 8 institutions for relapsed lymphoma following allogeneic SCT with either nivolumab or pembrolizumab were reported at the 2016 ASH annual meeting with 10 patients experiencing acute GVHD after treatment, including 3 patients with fatal GVHD.Citation106 The ORR in this series was 79%, including 13 patients with CR. More recently, Herbaux et alCitation107 reported outcomes of 20 patients with relapsed cHL following allo-SCT treated with nivolumab at the standard dose of 3 mg/kg every 2 weeks at medical centers across France. The median patient age was 33; all patients had received prior ASCT and BV. Ten (50%) patients had a history of prior acute GVHD (grade I or II) and 3 (15%) had a history of limited chronic GVHD. All patients were off of immunosuppression for ≥4 weeks prior to treatment initiation. Acute GVHD was seen during the first cycle of treatment in 6 (30%) patients, including 1 patient with febrile multiorgan dysfunction who died within 3 weeks of his first treatment and 3 patients with steroid refractory GVHD which was fatal in 1 of the 3 cases.Citation107 All of these 6 patients had a prior history of acute GVHD. Flare of chronic GVHD was not observed following nivolumab treatment in this cohort of patients. Other toxicities included grade 4 neutropenia and grade 3 thrombocytopenia in 1 patient and grade 2 cerebellar ataxia in a second patient, which required therapy discontinuation in both cases. The ORR was 95%, including a CR rate of 42%. After a median follow-up of 370 days, the 1-year PFS and OS rates were 58.2% and 78.7%, respectively, with 5 relapses reported at last follow-up.Citation107
Interestingly, Raiola et alCitation108 reported an encouraging 3-year PFS and OS rate of 63% and 77%, respectively, in 26 cHL patients treated with nonmyeloablative conditioning regiment followed by a haploidentical SCT and posttransplant high-dose cyclophosphamide for GVHD prophylaxis. The incidence of grade II-IV acute GVHD and of chronic GVHD was 24% and 8%, respectively, suggesting exploration of the potential role of cyclophosphamide in reducing the incidence and the severity of checkpoint-inhibitor induced GVHD through the elimination of allo-reactive T-cells.
Although the high ORR seen in these retrospective series is promising, the risk of severe and treatment-refractory acute GVHD in this setting is a concern warranting further evaluation and prospective study to better quantify the magnitude of this risk and to evaluate treatment options for GVHD in this setting. For patients with cHL relapsing after allogeneic SCT, given the limited options for inducing durable remission, checkpoint blockade is still a consideration with the awareness of the potential for life-threatening GVHD.
Nivolumab in combination
Although ORR to nivolumab in cHL has been >60%, the majority of patients achieve PR as best response and a subset of patients progress while on therapy. Whether improved response rates can be achieved with combination therapy is an area of active investigation. At the time of writing, there are 14 clinical trials of nivolumab that include patients with cHL listed on clinicaltrials.gov, 9 of which are open to enrollment (). We will briefly discuss potential rational combinations of treatment with PD-1 inhibitors including combination with alternate checkpoint inhibitors, combination with cytotoxic chemotherapy, and combination with biologic agents.
Table 3 Summary of current clinical trials of nivolumab combination therapy in cHL
Combining multiple checkpoint inhibitors has been shown to be effective in melanoma, where the combination of nivolumab with the CTLA-4 inhibitor ipilimumab lead to increased response rates, albeit with associated increased toxicity.Citation81 Preliminary results from the CheckMate 039 study of ipilumumab in combination with nivolumab for patients with relapsed/refractory hematologic malignancies were recently presented, with 5 of 65 (8%) patients discontinuing treatment due to AEs at a median follow-up of 11.4 months; no treatment-related deaths were reported.Citation109 A total of 19 (29%) patients experienced grade 3 toxicity, with the most commonly reported toxicities of any grade being pyrexia (23%), fatigue (23%), and diarrhea (18%).Citation109 The ORR for the 31 patients with cHL treated with ipilimumab and nivolumab was 74%, and the rate of CR was 19%.Citation109 Although the relatively small number of cHL patients limits interpretation of results, the response rates seen in this trial are similar to those seen with nivolumab monotherapy. The hypothesis that the combination of checkpoint inhibitors will improve response rates over a single-agent approach should be tested in future prospective clinical trials, but at the present time there is no evidence to support this approach in cHL.
Combining checkpoint inhibition with conventional chemotherapy is based on the rationale of increasing neo-antigen expression on tumor cells as a consequence of chemotherapy treatment to stimulate a T-cell-mediated antitumor response, which would be further enhanced by the addition of a checkpoint inhibitor.Citation110 However, the potential benefit of this approach may be limited by the immune-suppressive effects of cytotoxic agents. A phase II trial of nivolumab combined with doxorubicin, vinblastine, and dacarbazine (AVD) for previously untreated patients with cHL (NCT02181738, CheckMate205 Arm D) is currently closed to accrual, with results not yet reported as of the time of writing. Future trials are planned examining the safety and efficacy of the combination of nivolumab with cytotoxic chemotherapy in both the frontline setting and for relapsed or refractory disease (NCT03016871 and NCT03004833) that will shed further light on the feasibility and efficacy of this approach.
The combination of BV with nivolumab is of particular interest given the high response rates with both drugs as monotherapy.Citation25,Citation65 Preliminary results from 2 early-phase studies of the combination of BV and nivolumab were recently reported at the 2016 ASH annual meeting.Citation111,Citation112 The first is a phase 1/2 study (NCT02572167) of 25 cHL patients treated with BV dosed at 1.8 mg/kg in combination with nivolumab dosed at 3 mg/kg, both given on day 1, except for cycle 1 where nivolumab was given on day 8, of a 21-day cycle for up to 4 cycles after failure of frontline therapy and prior to ASCT.Citation111 Of the 25 patients enrolled, 16 (64%) had relapsed and 9 (36%) had refractory disease after frontline treatment. Toxicity was acceptable, with 3 (15%) patients experiencing grade 3 toxicity, but no grade 4 toxicity or toxicity leading to discontinuation of treatment reported.Citation111 An increased incidence of infusion reactions were noted with BV and nivolumab when the 2 drugs were administered together, but this was improved with the addition of hydro-cortisone and antihistamine premedication. Six patients had completed treatment at the time of the presentation with an ORR of 100%, including 3 patients with complete metabolic response.Citation111 Results from the phase I ECOG-ACRIN Cancer Research Group E4412 trial with 10 relapsed/refractory cHL patients treated with BV at a dose of 1.2 mg/kg or 1.8 mg/kg combined with nivolumab at a dose of 3 mg/kg given every 21 days for 16 cycles were also reported at the same meeting.Citation112 Grade 3 toxicity was observed in 2 of 10 patients including 1 patient treated with BV at a dose of 1.8 mg/kg who discontinued treatment due to grade 3 pneumonitis and grade 3 typhlitis.Citation112 No grade 4 toxicities were observed, and the remaining 9 patients were able to complete treatment. Of 8 patients who were evaluable for response at the time of presentation, 5/8 achieved CR and the remaining 3 achieved PR.
Finally, the use of biological agents in combination with PD-1 inhibition is another potential future approach to combination therapy. DNA methyltransferase inhibitors were shown in a preclinical ovarian cancer model to upregulate PD-L1 expression, suggesting a potential role for DNA methyltransferase inhibitors in priming patients for sensitization to checkpoint inhibitor therapy.Citation113 In support of this role for epigenetic therapy, in a small single-institution case series of patients treated with either pembrolizumab or nivolumab, 5 cHL patients who were previously treated with azacitidine in combination with romidepsin on a prior clinical trial all achieved a CR to PD-1 inhibitor therapy.Citation114 While this higher-than-expected rate of CR is provocative, results from future prospective clinical trials are needed to further evaluate this potential combination therapy.
The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib (Imbruvica, Phamacyclics, and Janssen) has been successfully employed in the treatment of chronic lymphocytic leukemia as well as subtypes of non-Hodgkin’s lymphoma, including mantle cell lymphoma and Waldenström’s macroglobulinemia.Citation115–Citation121 While B-cell non-Hodgkin lymphoma cells exhibit nearly universal BTK expression, BTK expression by RS cells has been reported in only a minority (22%) of cHL biopsy specimens.Citation122 Moreover, RS cells lack several components of the B cell receptor signaling pathway, and, to our knowledge, there is no published evidence that this pathway is constitutively activated in cHL. However, in addition to targeting BTK signaling, ibrutinib has been shown to irreversibly inhibit interleukin-2-inducible kinase (ITK), promoting a shift to Th-1-dominant signaling, thereby enhancing T-cell response to antigenic stimuli.Citation123 In a published case report, 2 patients with cHL with relapsed disease following allogeneic SCT were treated with ibrutinib. One achieved a PR with subsequent progression 4 months later, and 1 achieved CR and remained in CR at 6 months follow-up.Citation124 In 1 of these patients, expression of interferon-inducible-protein 10 was reported in serum samples after treatment, suggesting shift to Th-1 signaling and a potential immune modulatory effect of ibrutinib mediated by its activity on ITK.Citation124 The hypothesis that ibrutinib may enhance the immune effect of checkpoint inhibition in cHL warrants exploration in clinical trials, with a current phase II trial open to enrollment (NCT 02940301).
At this time, until results from these and future studies are available, the use of nivolumab in combination with other agents for the treatment of cHL remains investigational.
Patient-focused perspectives
As discussed previously, the side effects seen to this point with nivolumab in clinical practice are distinct from the side effect profile seen with cytotoxic agents that remain the mainstay of treatment for patients with cHL in the frontline setting and at first disease relapse. The long-term effects of PD-1 blockade are unknown, and this as well as the durability of response will be an important question for patients as outcomes mature.
Given the wide therapeutic index seen in trials utilizing nivolumab in other disease groups, 1 question for patients is the necessity of an every 2 week dosing schedule. As discussed previously, the elimination half-life of nivolumab is 26.7 days, and ongoing prospective trials of combination therapy with nivolumab in cHL and other diseases are utilizing an every 3-week dosing schedule. As patients with clinical response to nivolumab may remain on therapy for months and even years, the question of whether the dosing schedule can be switched to an every 3-week schedule and whether nivolumab can be safely stopped at any point is relevant to the quality of life for patients benefitting from this therapy. Results from ongoing prospective trials with nivolumab will help in answering this important question in the coming years.
Conclusion and future directions
Nivolumab has demonstrated impressive response rates and in some cases durable remissions in patients with cHL. Given the excellent response rates and acceptable side effect profile, nivolumab received FDA approval for cHL patients who have progressed following both prior ASCT and BV therapy. The long-term durability of response and the magnitude of benefit and risk of allogeneic SCT in patients who have responded to nivolumab will be better elucidated with long-term follow-up from completed trials of nivolumab monotherapy and potential future prospective investigation. Preliminary results suggest that administration of pembrolizumab results in similarly impressive responses in this patient population; whether a clinical meaningful difference between these 2 PD-1 inhibitors exists would be best answered in a phase III, head-to-head clinical trial.
Given the high response rates seen with nivolumab, whether it can be incorporated into earlier lines of therapy remains to be seen in future trials, including ongoing trials of the combination of nivolumab with AVD chemotherapy in untreated patients (NCT 02181738) and the combination of nivolumab with BV in patients aged ≥60 years (NCT 27758717). Incorporating nivolumab into earlier lines of therapy offers the potential to reduce exposure to cytotoxic therapy and the associated risks and toxicities and abrogates the potential immunosuppressive effect of prior lines of therapy. While the ultimate role of nivolumab in the treatment of cHL remains to be seen, it is already an established part of treatment in the relapsed and refractory setting and represents a success story in the rational application of genomic-guided cancer therapy.
Author contributions
DAB and LA contributed toward literature review, analysis, drafting, and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- AnsellSMHodgkin lymphoma: MOPP chemotherapy to PD-1 blockade and beyondAm J Hematol201691110911226505486
- ArmitageJOEarly-stage Hodgkin’s lymphomaN Engl J Med2010363765366220818856
- JohnsonPWRadfordJACullenMHComparison of ABVD and alternating or hybrid multidrug regimens for the treatment of advanced Hodgkin’s lymphoma: results of the United Kingdom Lymphoma Group LY09 Trial (ISRCTN97144519)J Clin Oncol200523369208921816314615
- HoskinPJLowryLHorwichARandomized comparison of the stanford V regimen and ABVD in the treatment of advanced Hodgkin’s Lymphoma: United Kingdom National Cancer Research Institute Lymphoma Group Study ISRCTN 64141244J Clin Oncol200927325390539619738111
- GordonLIHongFFisherRIRandomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496)J Clin Oncol201331668469123182987
- EngertADiehlVFranklinJEscalated-dose BEACOPP in the treatment of patients with advanced-stage Hodgkin’s lymphoma: 10 years of follow-up of the GHSG HD9 studyJ Clin Oncol200927274548455419704068
- EngertASchillerPJostingAInvolved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study GroupJ Clin Oncol200321193601360812913100
- MeyerRMGospodarowiczMKConnorsJMRandomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology GroupJ Clin Oncol200523214634464215837968
- BorchmannPHaverkampHDiehlVEight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEA-COPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage hodgkin’s lymphoma: final analysis of the HD12 trial of the German Hodgkin Study GroupJ Clin Oncol201129324234424221990399
- EngertAPlutschowAEichHTReduced treatment intensity in patients with early-stage Hodgkin’s lymphomaN Engl J Med2010363764065220818855
- RadfordJIllidgeTCounsellNResults of a trial of PET-directed therapy for early-stage Hodgkin’s lymphomaN Engl J Med2015372171598160725901426
- AdvaniRHHongFFisherRIRandomized phase III trial comparing ABVD plus radiotherapy with the stanford V regimen in patients with stages I or II locally extensive, bulky mediastinal Hodgkin lymphoma: a subset analysis of the North American Intergroup E2496 TrialJ Clin Oncol201533171936194225897153
- CardePKarraschMFortpiedCEight cycles of ABVD versus four cycles of BEACOPP escalated plus four cycles of BEACOPP baseline in stage III to IV, International Prognostic Score ≥3, High-Risk Hodgkin lymphoma: first results of the phase III EORTC 20012 Intergroup TrialJ Clin Oncol201634172028203627114593
- VivianiSZinzaniPLRambaldiAABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is plannedN Engl J Med2011365320321221774708
- MerliFLuminariSGobbiPGLong-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced Hodgkin lymphoma: a Study by Fondazione Italiana LinfomiJ Clin Oncol201634111175118126712220
- MounierNBricePBolognaSABVD (8 cycles) versus BEA-COPP (4 escalated cycles ≥4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trialAnn Oncol20142581622162824827123
- VassilakopoulosTPJohnsonPWTreatment of advanced-stage Hodgkin lymphomaSemin Hematol201653317117927496308
- HorningSJChaoNJNegrinRSHigh-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin’s disease: analysis of the Stanford University results and prognostic indicesBlood19978938018139028311
- LavoieJCConnorsJMPhillipsGLHigh-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in VancouverBlood200510641473147815870180
- GerrieASPowerMMShepherdJDSavageKJSehnLHConnorsJMChemoresistance can be overcome with high-dose chemotherapy and autologous stem-cell transplantation for relapsed and refractory Hodgkin lymphomaAnn Oncol201425112218222325149708
- LinchDCWinfieldDGoldstoneAHDose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomized trialLancet19933418852105110548096958
- ChopraRMcMillanAKLinchDCThe place of high-dose BEAM therapy and autologous bone marrow transplantation in poor-risk Hodgkin’s disease. A single-center eight-year study of 155 patientsBlood1993815113711458443375
- SchmitzNPfistnerBSextroMAggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomized trialLancet200235993232065207112086759
- JostingAMullerHBorchmannPDose intensity of chemotherapy in patients with relapsed Hodgkin’s lymphomaJ Clin Oncol201028345074508020975066
- YounesAGopalAKSmithSEResults of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphomaJ Clin Oncol201230182183218922454421
- GopalAKChenRSmithSEDurable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphomaBlood201512581236124325533035
- MoskowitzCHNademaneeAMassziTBrentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomized, double-blind, placebo-controlled, phase 3 trialLancet201538599801853186225796459
- KewalramaniTNimerSDZelenetzADProgressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphomaBone Marrow Transplant200332767367913130314
- SuredaARobinsonSCanalsCReduced-intensity conditioning compared with conventional allogeneic stem-cell transplantation in relapsed or refractory Hodgkin’s lymphoma: an analysis from the Lymphoma Working Party of the European Group for Blood and Marrow TransplantationJ Clin Oncol200826345546218086796
- HertzbergMRelapsed/refractory Hodgkin lymphoma: what is the best salvage therapy and do we need RIC-alloSCT?Hematol Oncol Clin North Am201428112314724287072
- ThomsonKJPeggsKSSmithPSuperiority of reduced-intensity allogeneic transplantation over conventional treatment for relapse of Hodgkin’s lymphoma following autologous stem cell transplantationBone Marrow Transplant200841976577018195684
- CorradiniPSarinaBFarinaLAllogeneic transplantation for Hodgkin’s lymphomaBr J Haematol2011152326127221155760
- BartlettNLNiedzwieckiDJohnsonJLGemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin’s lymphoma: CALGB 59804Ann Oncol20071861071107917426059
- AparicioJSeguraAGarceraSESHAP is an active regimen for relapsing Hodgkin’s diseaseAnn Oncol199910559359510416011
- ZelenetzADHamlinPKewalramaniTYahalomJNimerSMoskowitzCHIfosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin’s lymphomaAnn Oncol200314Suppl 1i5i1012736224
- SantoroABredenfeldHDevizziLGemcitabine in the treatment of refractory Hodgkin’s disease: results of a multicenter phase II studyJ Clin Oncol200018132615261910893294
- ZinzaniPLBendandiMStefoniVValue of gemcitabine treatment in heavily pretreated Hodgkin’s disease patientsHaematologica200085992692910980630
- VenkateshHDi BellaNFlynnTPVellekMJBoehmKAAsmarLResults of a phase II multicenter trial of single-agent gemcitabine in patients with relapsed or chemotherapy-refractory Hodgkin’s lymphomaClin Lymphoma20045211011515453926
- LittleRWittesRELongoDLWilsonWHVinblastine for recurrent Hodgkin’s disease following autologous bone marrow transplantJ Clin Oncol19981625845889469345
- DevizziLSantoroABonfanteVVinorelbine: an active drug for the management of patients with heavily pretreated Hodgkin’s diseaseAnn Oncol1994598178207531487
- MoskowitzAJHamlinPAJrPeralesMAPhase II study of bendamustine in relapsed and refractory Hodgkin lymphomaJ Clin Oncol201331445646023248254
- ClozelTDeauBBenetCPegylated liposomal doxorubicin: an efficient treatment in patients with Hodgkin lymphoma relapsing after high dose therapy and stem cell transplationBr J Haematol2013162684684823789905
- GodaJSMasseyCKuruvillaJRole of salvage radiation therapy for patients with relapsed or refractory hodgkin lymphoma who failed autologous stem cell transplantInt J Radiat Oncol Biol Phys2012843e329e33522672755
- FehnigerTALarsonSTrinkausKA phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphomaBlood2011118195119512521937701
- JohnstonPBInwardsDJColganJPA Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphomaAm J Hematol201085532032420229590
- YounesASuredaABen-YehudaDPanobinostat in patients with relapsed/refractory Hodgkin’s lymphoma after autologous stem-cell transplantation: results of a phase II studyJ Clin Oncol201230182197220322547596
- SteidlCConnorsJMGascoyneRDMolecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironmentJ Clin Oncol201129141812182621483001
- YamamotoRNishikoriMKitawakiTPD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphomaBlood200811163220322418203952
- SharpeAHWherryEJAhmedRFreemanGJThe function of programmed cell death 1 and its ligands in regulating autoimmunity and infectionNat Immunol20078323924517304234
- FreemanGJLongAJIwaiYEngagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activationJ Exp Med200019271027103411015443
- DongHZhuGTamadaKChenLB7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretionNat Med19995121365136910581077
- LatchmanYWoodCRChernovaTPD-L2 is a second ligand for PD-1 and inhibits T-cell activationNat Immunol20012326126811224527
- ParkJJOmiyaRMatsumuraYB7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell toleranceBlood201011681291129820472828
- DongHStromeSESalomaoDRTumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasionNat Med20028879380012091876
- RosenwaldAWrightGLeroyKMolecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphomaJ Exp Med2003198685186212975453
- RoemerMGAdvaniRHLigonAHPD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcomeJ Clin Oncol201634232690269727069084
- GreenMRMontiSRodigSJIntegrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphomaBlood2010116173268327720628145
- JoosSOtano-JoosMIZieglerSPrimary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL geneBlood1996874157115788608249
- BentzMBarthTFBruderleinSGain of chromosome arm 9p is characteristic of primary mediastinal B-cell lymphoma (MBL): comprehensive molecular cytogenetic analysis and presentation of a novel MBL cell lineGenes Chromosomes Cancer200130439340111241792
- JoosSKupperMOhlSGenomic imbalances including amplification of the tyrosine kinase gene JAK2 in CD30+ Hodgkin cellsCancer Res200060354955210676635
- SavageKJMontiSKutokJLThe molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphomaBlood2003102123871387912933571
- SteidlCShahSPWoolcockBWMHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancersNature2011471733837738121368758
- GreenMRRodigSJuszczynskiPConstitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and post-transplant lymphoproliferative disorders: implications for targeted therapyClin Cancer Res20121861611161822271878
- AnsellSMLesokhinAMBorrelloIPD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphomaN Engl J Med2015372431131925482239
- YounesASantoroAShippMNivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trialLancet Oncol20161791283129427451390
- KuppersRMolecular biology of Hodgkin lymphomaHematology Am Soc Hematol Educ Program2009149149620008234
- BrahmerJRDrakeCGWollnerIPhase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlatesJ Clin Oncol201028193167317520516446
- TopalianSLHodiFSBrahmerJRSafety, activity, and immune correlates of anti-PD-1 antibody in cancerN Engl J Med2012366262443245422658127
- BorghaeiHPaz-AresLHornLNivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancerN Engl J Med2015373171627163926412456
- BrahmerJReckampKLBaasPNivolumab versus Docetaxel in advanced squamous-cell non-small-cell lung cancerN Engl J Med2015373212313526028407
- MotzerRJEscudierBMcDermottDFNivolumab versus everolimus in advanced renal-cell carcinomaN Engl J Med2015373191803181326406148
- WeberJSD’AngeloSPMinorDNivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomized, controlled, open-label, phase 3 trialLancet Oncol201516437538425795410
- FoodUSAdministrationDrugModification of the Dose Regimen for NivolumabSilver Spring, MDUS Food and Drug Administration2016
- EigentlerTKHasselJCBerkingCDiagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapyCancer Treat Rev20164571826922661
- AgrawalSStatkevichPBajajGEvaluation of immunogenicity of nivolumab monotherapy and its clinical relevance in patients with metastatic solid tumorsJ Clin Pharmacol201757339440027557786
- AnsellSNivolumab in patients (Pts) with relapsed or refractory classical Hodgkin lymphoma (R/R cHL): clinical outcomes from extended follow-up of a Phase 1 Study (CA209-039)Blood2015126583
- TimmermanJEngertAYounesACheckmate 205 update with minimum 12 month follow up: A phase 2 study of Nivolumab in patients with relapsed/refractory classical Hodgkin LymphomaPresented at: American Society of Hematology MeetingDecember 3–6; 2016San Diego, CA abstract 1110
- MichotJMBigenwaldCChampiatSImmune-related adverse events with immune checkpoint blockade: a comprehensive reviewEur J Cancer20165413914826765102
- HofmannLForschnerALoquaiCCutaneous, gastrointestinal, hepatic, endocrine, and renal side effects of anti-PD-1 therapyEur J Cancer20166019020927085692
- PostowMACallahanMKWolchokJDImmune checkpoint blockade in cancer therapyJ Clin Oncol201533171974198225605845
- LarkinJChiarion-SileniVGonzalezRCombined Nivolumab and Ipilimumab or Monotherapy in Untreated MelanomaN Engl J Med20153731233426027431
- NaidooJPageDBLiBTToxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodiesAnn Oncol201526122375239126371282
- ArmandPNaglerAWellerEADisabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trialJ Clin Oncol201331334199420624127452
- MunakataWOhashiKYamauchiNTobinaiKFulminant type I diabetes mellitus associated with nivolumab in a patient with relapsed classical Hodgkin lymphomaInt J Hematol2017105338338627696192
- JohnsonDBBalkoJMComptonMLFulminant Myocarditis with combination immune checkpoint blockadeN Engl J Med2016375181749175527806233
- CarlosGAnforthRChouSClementsAFernandez-PenasPA case of bullous pemphigoid in a patient with metastatic melanoma treated with pembrolizumabMelanoma Res201525326526825831416
- WilgenhofSNeynsBAnti-CTLA-4 antibody-induced Guillain-Barre syndrome in a melanoma patientAnn Oncol201122499199321357649
- LoochtanAINickolichMSHobson-WebbLDMyasthenia gravis associated with ipilimumab and nivolumab in the treatment of small cell lung cancerMuscle Nerve201552230730825759003
- ReckMRodriguez-AbreuDRobinsonAGPembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancerN Engl J Med2016375191823183327718847
- MahoneyKMFreemanGJMcDermottDFThe next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in MelanomaClin Ther201537476478225823918
- ArmandPShippMARibragVProgrammed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failureJ Clin Oncol2016343137333739
- MoskowitzAZinzaniPLFanaleMPembrolizumab in Relapsed/Refractory Classical Hodgkin Lymphoma: Primary End Point Analysis of the Phase 2 Keynote-087 StudyBlood2016128 abstract 1107; American Society of Hematology Annual Meeting.
- AlinariLBlumKAHow I treat relapsed classical Hodgkin lymphoma after autologous stem cell transplantBlood2016127328729526576863
- SahaAAoyamaKTaylorPAHost programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethalityBlood2013122173062307324030385
- BlazarBRCarrenoBMPanoskaltsis-MortariABlockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanismJ Immunol200317131272127712874215
- ArmandPZinzaniPLCollinsGOutcomes of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) after Treatment with Nivolumab for Relapsed/Refractory Hodgkin LymphomaPresented at: American Society of Hematology Annual MeetingDecember 3–6, 2016San Diego, CA abstract 3502
- MerrymanRWKimHTZinzaniPLSafety and efficacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed/refractory lymphomaBlood2017129101380138828073785
- FujiokaTTamakiHIkegameKFrequency of CD4(+)FOXP3(+) regulatory T-cells at early stages after HLA-mismatched allogeneic hematopoietic SCT predicts the incidence of acute GVHDBone Marrow Transplant201348685986423165499
- YaredJAHardyNSinghZMajor clinical response to nivolumab in relapsed/refractory Hodgkin lymphoma after allogeneic stem cell transplantationBone Marrow Transplant201651685085226828905
- VillasboasJCAnsellSMWitzigTETargeting the PD-1 pathway in patients with relapsed classic Hodgkin lymphoma following allogeneic stem cell transplant is safe and effectiveOncotarget2016711132601326426848626
- AngenendtLSchliemannCLutzMNivolumab in a patient with refractory Hodgkin’s lymphoma after allogeneic stem cell transplantationBone Marrow Transplant201651344344526551782
- SinghAKPorrataLFAljitawiOFatal GvHD induced by PD-1 inhibitor pembrolizumab in a patient with Hodgkin’s lymphomaBone Marrow Transplant20165191268127027111048
- OnizukaMKojimaMMatsuiKSuccessful treatment with low-dose nivolumab in refractory Hodgkin lymphoma after allogeneic stem cell transplantationInt J Hematol Epub2017117
- AslanAArasTOzdemirESuccessful treatment of relapsed/refractory Hodgkins lymphoma with nivolumab in a heavily pretreated patient with progressive disease after both autologous and allogeneic stem cell transplantationLeuk Lymphoma201758375475527687237
- GodfreyJBishopMRSyedSHyjekEKlineJPD-1 blockade induces remissions in relapsed classical Hodgkin lymphoma following allogeneic hematopoietic stem cell transplantationJ Immunother Cancer201751128239465
- HaverkosBMSchowinksyJKaplanJCheckpoint Blockade for Treatment of Relapsed Lymphoma Following Allogeneic Hematopoietic Cell Transplant: Use May be Complicated By Onset of Severe Acute Graft Versus Host DiseaseBlood2016128 abstract 1163; American Society of Hematology Annual Meeting
- HerbauxCGauthierJBricePEfficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin’s lymphomaBlood Epub201737
- RaiolaADominiettoAVaraldoRUnmanipulated haploidentical BMT following non-myeloablative conditioning and post-transplantation CY for advanced Hodgkin’s lymphomaBone Marrow Transplant201449219019424185585
- AnsellSGutierrezMShippMA Phase I Study of Nivolumab in Combination with Ipilimumab for Relapsed or Refractory Hematologic Malignancies (CheckMate 039)Presented at: American Society of Hematology Annual MeetingDecember 3–6, 2016San Diego, CA abstract 183
- SharmaPAllisonJPImmune checkpoint targeting in cancer therapy: toward combination strategies with curative potentialCell2015161220521425860605
- HerreraAFBartlettNRamchandrenRPreliminary Results from a Phase 1/2 Study of Brentuximab Vedotin in Combination with Nivolumab in Patients with Relapsed or Refractory Hodgkin LymphomaPresented at: American Society of Hematology Annual MeetingDecember 3–6, 2016San Diego, CA abstract 1105
- DiefenbachCHongFDavidKAA Phase I Study with an Expansion Cohort of the Combination of Ipilimumab and Nivolumab and Brentuximab Vedotin in Patients with Relapsed/Refractory Hodgkin Lymphoma: A Trial of the ECOG-ACRIN Cancer Research Group (E4412 Arms D and E)Presented at: American Society of Hematology Annual MeetingDecember 3–6, 2016San Diego, CA abstract 1106
- ChiappinelliKBStrisselPLDesrichardAInhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retrovirusesCell2015162597498626317466
- FalchiLSawasADengCHigh rate of complete responses to immune checkpoint inhibitors in patients with relapsed or refractory Hodgkin lymphoma previously exposed to epigenetic therapyJ Hematol Oncol20169113227899158
- CoutreSEFurmanRRFlinnIWExtended treatment with single-agent ibrutinib at the 420 mg dose leads to durable responses in chronic lymphocytic leukemia/small lymphocytic lymphomaClin Cancer Res20172351149115528073846
- WangMLBlumKAMartinPLong-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy resultsBlood2015126673974526059948
- MaddocksKBlumKAIbrutinib in B-cell lymphomasCurr Treat Options Oncol201415222623724481980
- ByrdJCFurmanRRCoutreSETargeting BTK with ibrutinib in relapsed chronic lymphocytic leukemiaN Engl J Med20133691324223782158
- WangMLRuleSMartinPTargeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphomaN Engl J Med2013369650751623782157
- TreonSPXuLHunterZMYD88 mutations and response to ibrutinib in waldenstrom’s macroglobulinemiaN Engl J Med20153736584586
- TreonSPTripsasCKMeidKIbrutinib in previously treated Waldenstrom’s macroglobulinemiaN Engl J Med2015372151430144025853747
- Fernandez-VegaIQuirosLMSantos-JuanesJPane-FoixMMarafiotiTBruton’s tyrosine kinase (Btk) is a useful marker for Hodgkin and B cell non-Hodgkin lymphomaVirchows Arch2015466222923525433814
- DubovskyJABeckwithKANatarajanGIbrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytesBlood2013122152539254923886836
- HamadaniMBalasubramanianSHariPNIbrutinib in refractory classic Hodgkin’s lymphomaN Engl J Med2015373141381138226422743
