Abstract
Background
Thrombosis is one of the complications in the clinical course of multiple myeloma (MM). Vascular endothelial cells and/or the hemostatic-coagulatory system are thought to play an important role in thrombosis of MM. In addition to melphalan-prednisone (Mel-P) therapy, several new therapeutic drugs such as lenalidomide or bortezomib have been developed and show effectiveness against MM. However, these new drugs also have risk of therapy-related thrombosis.
Methods
We assessed 103 MM patients and 30 healthy controls, using enzyme-linked immunosorbent assays to evaluate five biomarkers: platelet-derived microparticles (PDMP), plasminogen activator inhibitor-1 (PAI-1), high mobility group box protein-1 (HMGB1), endothelial protein C receptor (EPCR), and soluble vascular cell adhesion molecule-1 (sVCAM-1). The effects of Mel-P, bortezomib, and lenalidomide on the plasma concentrations of these biomarkers were investigated.
Results
The plasma concentrations of PDMP, PAI-1, HMGB1, EPCR, and sVCAM-1 were higher in MM patients than in healthy controls. Mel-P, bortezomib, and lenalidomide therapies all reduced biomarker levels after treatment. However, when only patients with higher levels of EPCR were compared, differences were seen between the three therapies in the elevation of PDMP, HMGB1, and PAI-1.
Conclusion
These results suggest that both MM and therapies for MM can induce a hypercoagulable state. The elevated risk of thrombosis conferred by hypercoagulability increases patient morbidity and mortality. Attention should be paid to therapy-related thrombosis when new therapeutic regimens are selected for MM patients.
Introduction
Multiple myeloma (MM) is an incurable malignancy of the plasma cells.Citation1 The majority of MM patients relapse, regardless of their initial treatment.Citation1 Melphalan-prednisone (Mel-P) has long been the treatment of choice for MM patients over 65 years of age.Citation2 Recently, several new therapeutic drugs, such as lenalidomide and bortezomib, have been developed and show effectiveness against MM.Citation3,Citation4 However, the pathologic characteristics of individual MM patients differ, depending on the products secreted by MM cells. In addition, vascular endothelial cells and/or the hemostatic-coagulatory system are thought to play an important role in the clinical course of MM.Citation5,Citation6
Many individuals with cancer are in a hypercoagulable state, and the elevated risk of thrombosis conferred by hypercoagulability increases patients morbidity and mortality.Citation7–Citation9 Cancer patients frequently develop venous thromboembolism (VTE), and various potentially predictive biomarkers have been evaluated for association with VTE in cancer progression.Citation10–Citation12 For example, analysis of cell-derived microparticles (MP), high mobility group box protein 1 (HMGB1), plasminogen activator inhibitor-1(PAI-1), and soluble endothelial protein C receptor (sEPCR) can effectively predict the risk of VTE development.Citation13–Citation16 Previous studies have further demonstrated a significant relationship between these markers and thrombosis with MM.Citation17–Citation20 In addition, although new drugs such as bortezomib, lenalidomide, or monoclonal antibodies can improve the prognosis of MM, these drugs can also be accompanied by side effects such as VTE.Citation22–Citation26 Therefore, here we measured various thrombosis-related biomarkers in patients with MM to study the clinical significance of these biomarkers in MM and their relationship with MM therapy.
Patients and methods
Patients
The study group consisted of 103 patients newly diagnosed with symptomatic, measurable MM,Citation21 defined in accordance with the Guidelines for Diagnosis and Treatment of MM in Adults,Citation27 presenting at our hospital between May 2010 and August 2016. As a control group, 30 healthy volunteers were recruited from the hospital staff and other sources. All study participants provided signed informed consent, and this study was approved by the Ethics Committee at Kansai Medical University, Hirakata, Osaka, Japan (no 110788).
Study design
A total of 91 patients were assigned to receive Mel-P (n=23), bortezomib (n=31), or lenalidomide (n=37) (). The Mel-P regimen consisted of six 28-day cycles of melphalan (0.18 mg/kg body weight on days 1 through 4) and prednisone (2 mg/kg body weight on days 1 through 4). The bortezomib regimen consisted of subcutaneous bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11), oral cyclophosphamide (500 mg on days 1, 8, and 15), and oral dexamethasone (40 mg on days 1, 8, and 15). The cycle was repeated every 21 days.Citation28,Citation29 The lenalidomide regimen consisted of either oral lenalidomide at 25 mg daily on days 1–21 plus oral dexamethasone at 40 mg daily on days 1–4, 9–12, and 17–20 of each 28-day cycle, or the same schedule of lenalidomide plus oral 40 mg daily on days 1, 8, 15, and 22 of the cycle.Citation30,Citation31 Treatment was continued until disease progression stopped or unacceptable adverse effects developed. The primary study end point was an improvement in various biomarkers; secondary end points included response rate, response quality, and adverse events.
Plasma levels of platelet-derived microparticle (PDMP), cytokines, soluble factors, and PAI-1
Fasting blood samples were obtained from the peripheral veins of patients and controls, using 21-gauge needles to minimize platelet activation, and were transferred into vacutainers containing ethylenediaminetetraacetic acid-citrate dextrose (NIPRO Co. Ltd., Osaka, Japan). The samples were gently mixed by inverting the tubes once or twice and kept at room temperature for a maximum of 2–3 hours. Samples were centrifuged at 8,000 × g for 5 minutes, and 200 μL at the top of each 2 mL upper layer was withdrawn to avoid contamination by platelets. These plasma samples were stored at −40°C until analysis. PDMP was measured by enzyme-linked immunosorbent assay (ELISA; JIMRO Co. Ltd., Tokyo, Japan).Citation32,Citation33 Plasma concentrations of soluble vascular cell adhesion molecule-1 (sVCAM-1), tumor necrosis factor α (TNFα), and PAI-1 were measured using monoclonal antibody-based ELISA kits (Invitrogen International Inc., Camarillo, CA, USA). HMGB1 was measured using the HMGB1 ELISA kit (Shino-test Corp., Kanagawa, Japan). Plasma sEPCR levels were measured by ELISA (R&D Syetems Inc., Minneapolis, MN, USA). The recombinant products and standard solutions provided with the commercial kits were used as positive controls in each assay. All kits were used in accordance with the manufacturer’s instructions.
Statistical analysis
Data were expressed as mean ± SD and analyzed using multiple regression (stepwise method), as appropriate. Between-group comparisons were made using the Newman–Keuls test and Scheffe’s test. All statistical analyses were performed using Stat Flex (version 6; Artech Co., Ltd., Osaka, Japan) software, with p-values less than 0.05 considered statistically significant.
Results
The plasma concentrations of biomarkers in patients newly diagnosed with MM and in healthy controls are shown in . HMGB1, PDMP, sVCAM-1, PAI-1, and sEPCR concentrations were higher in patients than in controls.
Table 1 Plasma levels of cytokines, PDMP, and soluble factors
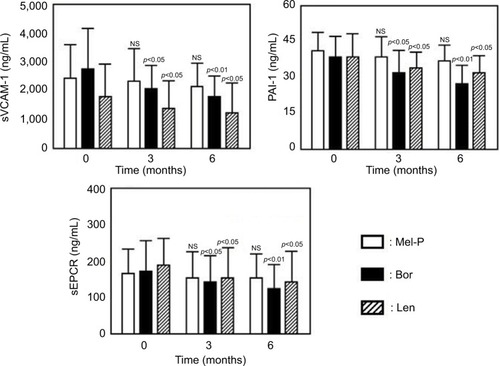
The demographic and baseline characteristics of the treated patients were similar among the three groups (data nor shown). Treatment with Mel-P for 6 months significantly reduced the plasma concentrations of PDMP, relative to baseline, but did not significantly alter the plasma concentrations of TNFα, HMGB1, sVCAM-1, PAI-1, or sEPCR ( and ). In contrast, treatment with bortezomib or lenalidomide alone improved the levels of all tested biomarkers other than TNFα ( and ).
Figure 2 Changes in the plasma levels of TNFα, HMGB1, and PDMP before and after treatments.
Abbreviations: Mel-P, melphalan and prednisone; Bor, bortezomib; Len, lenalidomide; TNFα, tumor necrosis factor α; HMGB1, high mobility group box protein 1; PDMP, platelet-derived microparticle; NS, not significant.
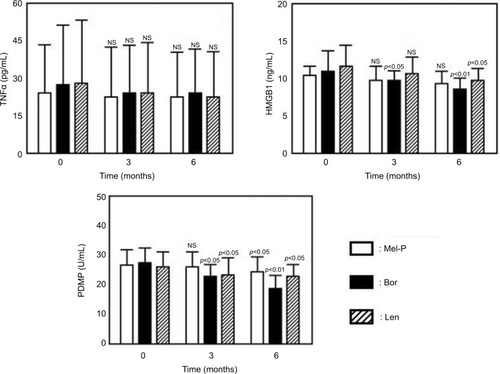
Figure 3 Changes in the plasma levels of sVCAM-1, PAI-1, and sEPCR before and after treatments.
Abbreviations: Mel-P, melphalan and prednisone; Bor, bortezomib; Len, lenalidomide; sVCAM-1, soluble vascular cell adhesion molecule-1; PAI-1, plasminogen activator inhibitor-1; sEPCR, soluble endothelial protein C receptor; NS, not significant.
Nineteen of 91 patients showed remarkable elevation of sEPCR (sEPCR >248 ng/mL) (including four patients treated with Mel-P, seven with bortezomib, and eight with lenalidomide). Four biomarkers (HMGB1, sVCAM-1, PAI-1, and PDMP) showed no significant concentration changes before and after treatment (). However, when only patients with higher levels of sEPCR were compared, differences were seen between the three therapies in the elevation of HMGB1, sVCAM-1, PAI-1, and PDMP (). Specifically, only lenalidomide treatment was associated with the significant elevation of these four biomarkers after 6 months (). However, these patients did not suffer from clinical thrombosis, because they received aspirin treatment.
Figure 4 Changes in HMGB1 (A), sVCAM-1 (B), PAI-1 (C), and PDMP (D) before and after treatment of patients with higher levels of sEPCR.
Abbreviations: HMGB1, high mobility group box protein 1; sVCAM-1, soluble vascular cell adhesion molecule-1; PAI-1, plasminogen activator inhibitor-1; PDMP, platelet-derived microparticles; Mel-P, melphalan and prednisone; Bor, bortezomib; Len, lenalidomide; NS, not significant; M, months.
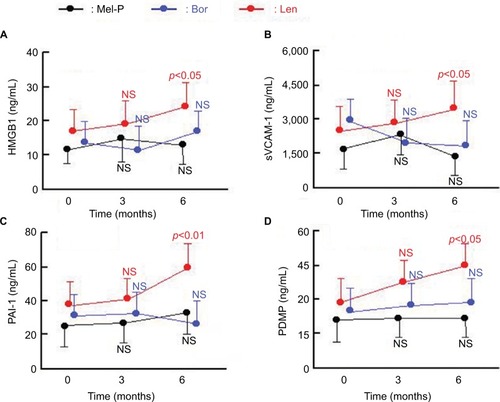
Table 2 Changes in the plasma levels of PDMP, soluble factors, and cytokines/chemokines before and after all treatments of patients with elevated sEPCR
Discussion
Bleeding and thrombosis are complications in patients with hematologic malignancies, with epidemiological, clinical, and pathophysiologic significance.Citation22,Citation23,Citation34 In patients with MM, several disease- and treatment-related factors have been found to affect the coagulation system, as well as increase the risks of bleeding and thrombotic complications.Citation34–Citation36 Similar to findings in other cancers, malignant clones in patients with MM induce a cytokine environment responsible for a hypercoagulable state.Citation22 Circulating monoclonal proteins increase blood viscosity and impair platelet and coagulation function, which are considered key mechanisms in the hemostatic abnormalities frequently detected in patients with MM.Citation6 This study assessed the plasma concentrations of several biomarkers of hemostasis, coagulation, and endothelial dysfunction in patients newly diagnosed with MM. We found that the concentrations of HMGB1, PDMP, sVCAM-1, PAI-1, and sEPCR were higher in MM patients than in healthy controls. These results suggest that patients with MM likely have coagulation- and/or endothelial cell activation-related risk factors for coagulation abnormalities.Citation6,Citation17–Citation19,Citation23–Citation25
Recently, the treatment strategy for MM has undergone a complete change.Citation37–Citation39 In particular, Mel-P plus bortezomib has been reported to improve progression-free survival and overall survival when compared with Mel-P alone.Citation3,Citation4,Citation30,Citation31,Citation40 Furthermore, lenalidomide also provides good therapeutic effects.Citation30,Citation31 Along with these treatments, new strategies using monoclonal antibodies can be anticipated.Citation41 However, an accurate understanding of therapeutic effects and/or side effects is necessary for the development of new MM treatments. This study therefore examined the response of various biomarkers to alternative treatments for MM. Treatment with Mel-P for 6 months significantly reduced the plasma concentrations of PDMP, relative to baseline, but did not significantly alter the plasma concentrations of TNFα, HMGB1, sVCAM-1, PAI-1, and sEPCR. In contrast, treatment with bortezomib or lenalidomide alone had a beneficial effect on all tested biomarkers other than TNFα. These results suggest the possibility that bortezomib and lenalidomide could improve the abnormality of thrombosis-related biomarkers that accompanies MM.
Activated protein C, combined with its cofactor protein S, acts as an anticoagulant, inactivating factor Va and factor VIIIa.Citation42 EPCR, which is a transmembrane glycoprotein found in the endothelium, enables activation of PC.Citation43 EPCR is also found in a soluble form, sEPCR, which binds activated protein C in competition with cell-surface EPCR.Citation44 Therefore, sEPCR can be considered to be a biomarker of cancer-related hypercoagulability in human malignancies.Citation16,Citation17,Citation45 In the present study, 19 of 91 patients showed remarkable elevation of sEPCR (sEPCR >248 ng/mL).
Four other biomarkers, HMGB1, sVCAM-1, PAI-1, and PDMP, showed no significant changes before and after treatment. However, when only patients with higher levels of sEPCR were compared, differences were seen in the elevation of these biomarkers between the three therapies described in this study. Only lenalidomide produced a significant elevation of the four biomarkers after 6 months of treatment. These results suggest the possibility that lenalidomide could cause the elevation of thrombosis-related biomarkers regardless of its effects on MM.Citation24,Citation25
The risk of venous thrombosis is increased not only in MM but also by chemotherapy, and is dependent on the combination of drugs administered.Citation46 In particular, VTE incidence greatly increases when thalidomide or lenalidomide is used in combination with dexamethasone or multiagent therapy.Citation47,Citation48 The hazard ratio for a specific VTE risk should be taken into account when choosing an appropriate anticoagulant prophylaxis, because the relative risk of VTE is quite heterogeneous.Citation47 Our patients received aspirin treatment. Although these patients did not exhibit clinical thrombosis, their thrombosis-related biomarkers were elevated. Therefore, when a new multiagent therapy in addition to lenalidomide is chosen, it may mandate the delivery of more aggressive prophylaxis, such as low-molecular-weight heparin, full-dose warfarin, or the new oral factor Xa anticoagulants.
The exact mechanism by which lenalidomide treatment leads to an increase in sEPCR levels remains unclear. However, at least one possibility can be inferred: the participation of TNFα-converting enzyme (TACE or ADMM17).Citation49 TACE is a metalloproteinase that plays a pivotal role in the shedding of many cellular receptors.Citation50 During MM treatment, lenalidomide has critical immunomodulatory activity, and increases inflammatory cytokines such as TNFα and IL-8.Citation51,Citation52 TNFα is closely linked to the production of TACE, and TACE ultimately causes the elevation of sEPCR.Citation49 A previous report demonstrated that the anti-inflammatory effect of lenalidomide may relate to its ability to block the production of TNFα in peripheral blood cells.Citation53 However, the effect of lenalidomide on TNFα is paradoxical, because an increasing level of TNFα after lenalidomide treatment has already been reported in chronic lymphoblastic leukemia patients.Citation51,Citation52 Our results support these findings. However, we could not confirm a direct correlation between the TNFα and sEPCR. Therefore, further examination is required to elucidate the mechanism of sEPCR elevation caused by lenalidomide.
Conclusion
These results suggest that a hypercoagulable state can be induced by both MM and the therapies used to treat it. Hypercoagulability elevates the risk of thrombosis and hence increases patient morbidity and mortality. The risk of therapy-related thrombosis should be considered when new therapeutic regimens are selected for patients with MM.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Dr Akiko Konishi, Dr Yukie Tsubokura, and Dr Yoshiko Azuma for technical assistance and for data collection. This study was supported in part by a grant from the Japan Foundation of Neuropsychiatry and Hematology Research, the Research Grant for Advanced Medical Care from the Ministry of Health and Welfare of Japan, and a grant (13670760 to SN) from the Ministry of Education, Science and Culture of Japan.
The abstract of this paper was presented as a poster presentation with interim findings at the 2017 Annual Meeting of the International Society for Laboratory Hematology, 4–6 May 2017, Honolulu, HI, USA, and was published in the International Journal of Laboratory Hematology, Volume 39, Issue S2 May 2017. The poster’s abstract was published in “Poster Abstracts” in the International Journal of Laboratory Hematology: http://onlinelibrary.wiley.com/doi/10.1111/ijlh.2017.39.issue-S2/issuetoc.
Disclosure
The authors report no conflicts of interest in this work.
References
- KyleRATherneauTMRajkumarSVA long-term study of prognosis in monoclonal gammopathy of undetermined significanceN Engl J Med2002346856456911856795
- PalumboAAndersonKMultiple myelomaN Engl J Med2011364111046106021410373
- San MiguelJFSchlagRKhuagevaNKBortezomib plus melphalan and prednisone for initial treatment of multiple myelomaN Engl J Med2008359990691718753647
- FayersPMPalumboAHulinCThalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trialsBlood201111851239124721670471
- Sanz-RodríguezFRuiz-VelascoNPascual-SalcedoDCharacterization of VLA-4-dependent myeloma cell adhesion to fibronectin and VCAM-1Br J Haematol199910782583410606891
- CoppolaATufanoADi CapuaMFranchiniMBleeding and thrombosis in multiple myeloma and related plasma cell disordersSemin Thromb Hemostas2011378929945
- SallahSWanJYNguyenNPVenous thrombosis in patients with solid tumors: determination of frequency and characteristicsThromb Haemost200287457557912008937
- BlomJWDoggenCJOsantoSMalignancies, prothrombotic mutations, and the risk of venous thrombosisJAMA2005293671572215701913
- KhoranaAAConnollyGCAssessing risk of venous thromboembolism in the patient with cancerJ Clin Oncol200927294839484719720906
- BlomJWVanderschootJPOostindiërMJIncidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage studyJ Thromb Haemost20064352953516460435
- SimanekRVormittagRAyCHigh platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS)J Thromb Haemost20108111412019889150
- MackmanNNew insights into the mechanisms of venous thrombosisJ Clin Invest201212272331233622751108
- FleitasTMartínez-SalesVVilaVCirculating endothelial cells and microparticles as prognostic markers in advanced non-small cell lung cancerPLoS One2012710e4736523077602
- NaumnikWNilklińskaWOssolińskaMChyczewskaESerum levels of HMGB1, survivin, and VEGF in patients with advanced non-small cell lung cancer during chemotherapyFolia Histochem Cytobiol200947470370920430742
- SuCYLiuYPYangCJPlasminogen activator inhibitor-2 plays a leading prognostic role among protease families in non-small cell lung cancerPLoS One2015107e013341126230665
- DucrosEMirshahiSSFaussatAMSoluble endothelial protein C receptor (sEPCR) is likely a biomarker of cancer-associated hypercoagulability in human hematologic malignanciesCancer Med20121226126723342274
- DriAPPolitouMGialerakiADecreased incidence of EPCR 4678G/C SNP in multiple myeloma patients with thrombosisThromb Res2013132340040123993723
- AuwerdaJJYuanaYOsantoSMicroparticle-associated tissue factor activity and venous thrombosis in multiple myelomaThromb Haemost20111051142021057704
- BenameurTChappardDFioleauEPlasma cells release membrane microparticles in a mouse model of multiple myelomaMicron201354557581
- KrishnanSRLukFBrownRDIsolation of Human CD138(+) microparticles from the plasma of patients with multiple myelomaNeoplasia2016181253226806349
- RoyMLiangLXiaoXLycorine downregulates HMGB1 to inhibit autophagy and enhances Bortezomib activity in multiple myelomaTheronostics201661222092224
- ChongBHLeeSHManagement of thromboembolism in hematologic malignanciesSemin Thromb Hemost200733443544817525901
- FranchiniMDario Di MinnoMHCoppolaADisseminated intravascular coagulation in hematologic malignanciesSemin Thromb Hemost201036438840320614391
- ValsamiSRufWLeikaufMSImmunomodulatory drugs increase endothelial tissue factor expression in vitroThromb Res2011127326427121159364
- RosovskyRHongFToccoDEndothelial stress products and coagulation markers in patients with multiple myeloma treated with lenalidomide plus dexamethasone: an observational studyBr J Haematol2013160335135823240658
- GaoYMaGLiuSThalidomide and multiple myeloma serum synergistically induce a hemostatic imbalance in endothelial cells in vitroThromb Res201513561154115925840743
- RajkumarSVKyleRATherneauTMSerum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significanceBlood2005106381281715855274
- DaviesFEWuPJennerMSrikanthMSasoRMorganGJThe combination of cyclophosphamide, velcade and dexamethasone induces high response rates with comparable toxicity to velcade alone and velcade plus dexamethasoneHaematologica20079281149115017650451
- BrownSHinsleySBallesterosMThe MUK five protocol: a phase II randomised, controlled, parallel group, multicentre trial of carfilzomib, cyclophosphamide and dexamethasone (CCD) vs. cyclophosphamide, bortezomib (Velcade) and dexamethasone (CVD) for first relapse and primary refractory multiple myelomaBMC Hematol2016161427190631
- RajkumarSVJacobusSCallanderNSLenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trialLancet Oncol2010111293719853510
- ReeceDEMasih-KhanEAtenafuEGPhase I–II trial of oral cyclophosphamide, prednisone and lenalidomide for the treatment of patients with relapsed and refractory multiple myelomaBr J Haematol20151681465425146584
- OsumiKOzekiYSaitoSDevelopment and assessment of enzyme immunoassay for platelet-derived microparticlesThromb Haemost200185232633011246556
- NomuraSUehataSSaitoSOsumiKOzekiYKimuraYEnzyme immunoassay detection of platelet-derived microparticles and RANTES in acute coronary syndromeThromb Haemost200389350651212624635
- EbyCPathogenesis and management of bleeding and thrombosis in plasma cell dyscrasiasBr J Haematol2009145215116319210509
- LeebeekFWKruipMJSonnevelsPRisk and management of thrombosis in multiple myelomaThromb Res2012129Suppl 1S88S9222682142
- WangXLiYYanXEfficacy and safety of novel agent-based therapies for multiple myeloma: a meta-analysisBioMed Res Int20162016684890226949704
- Martinez-LopezJBladeJMateosMVLong-term prognostic significance of response in multiple myeloma after stem cell transplantationBlood2011118352953421482708
- PalumboAHajekRDelforgeMContinuous lenalidomide treatment for newly diagnosed multiple myelomaN Engl J Med2012366191759176922571200
- MateosMVHernándezMTGiraldoPLenalidomide plus dexamethasone for high-risk smoldering multiple myelomaN Engl J Med2013369543844723902483
- PetrucciMTLeviABringhenSBortezomib, melphalan, and prednisone in elderly patients with relapsed/refractory multiple myeloma: a multicenter, open label phase 1/2 studyCancer2013119597197723096113
- TouzeauCMoreauPDumontetCMonoclonal antibody therapy in multiple myelomaLeukemia2017311927389053
- CastellinoFJPloplisVAThe protein C pathway and pathologic processesJ Thromb Haemost20097Suppl 114014519630787
- Van Hylckama VliegAMontesRRosendaalFRHermidaJAutoantibodies against endothelial protein C receptor and the risk of a first deep vein thrombosisJ Thromb Haemost2007571449145417439632
- FukudomeKKurosawaSStearns-KurosawaDJHeXRezaieAREsmonCTThe endothelial cell protein C receptor. Cell surface expression and direct ligand binding by the soluble receptorJ Biol Chem19962712917491174988663475
- AlthawadiHAlfarsiHBesbesSThe endothelial cell protein C receptor. Cell surface expression and direct ligand binding by the soluble receptorOncol Lep2015342603609
- Cesarman-MausGBraggioEFonsecaRThrombosis in multiple myeloma (MM)Hematology2012171S177S18022507814
- PalumboARajkumarSVDimopoulosMAPrevention of thalidomide- and lenalidomide-associated thrombosis in myelomaLeukemia200822241442318094721
- CarrierMLe GalGTayJWuCLeeAYRates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysisJ Thromb Haemost20119465366621255254
- QuDWangYEsmonNLEsmonCTRegulated endothelial protein C receptor shedding is mediated by tumor necrosis factor-alpha converting enzyme/ADAM17J Thromb Haemost20075239540217155946
- MossMLLambertMHShedding of membrane proteins by ADAM family proteasesEssays Biochem20023814115312463167
- Chanan-KhanAAChittaKErsingNBiological effects and clinical significance of lenalidomide-induced tumour flare reaction in patients with chronic lymphocytic leukaemia: in vivo evidence of immune activation and antitumour responseBr J Haematol2011155445746722010965
- MaigaSGomez-BougiePBonnaudSParadoxical effect of lenalidomide on cytokine/growth factor profiles in multiple myelomaBr J Cancer201310891801180623632478
- MullerGWChenRHuangSYAmino-substituted thalidomide analogs: potent inhibitors of TNF-alpha productionBioorg Med Chem Lett19999111625163010386948