 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
To evaluate the occurrence of sickle cell trait (SCT), assess patient awareness and evaluate the performance of a sickle cell hemoglobin-S (dithionate-qualitative solubility) point-of-care test among patients seeking care at Magale Health Center IV, Namisindwa District, Eastern Uganda.
Materials and methods
We conducted a cross sectional study, in which we consecutively enrolled participants aged ≥18 years at Magale Health Center IV. Four milliliters of EDTA blood were collected by venipuncture and screened for SCT using solubility testing, and confirmed with hemoglobin (Hb) electrophoresis at Central Public Health Laboratory (CPHL), Kampala, Uganda. A structured questionnaire was used to assess participants’ awareness of SCT. Data were presented as proportion, and measurements of diagnostic test performance were calculated.
Results
We enrolled 242 participants, of these 58.7% (N = 142) were females. Their mean age was 26.4 years (range 18–49). Of the 242 participants, 11, who represent 4.5% (95% CI: 3.3–5.9), tested positive. The sensitivity, specificity, positive predictive value and negative predictive value of the rapid sickle cell test were 63.64%, 100%, 100% and 98.30%, respectively. There was knowledge gap regarding sickle cell awareness.
Conclusion
The occurrence of SCT was high, and the point-of-care test showed a high diagnostic reliability. The risk of SCT is associated with genetic predisposition as indicated by Hb electrophoresis. Community sensitization is key to avert the associated risk of Hb defects.
Introduction
Hemoglobin (Hb) defects are by far the most diagnosed genetic disease, with over 10,000 variants that involve a single amino acid substitution.Citation1 A normal adult carries adult hemoglobin (HbA)Citation1,Citation2; however, mutations that cause substitution of glutamate for valine at position 6 of the β-globin chain result in Hb-S, a variant linked to life-long complications like sickle cell anemia.Citation3–Citation5 Globally, Hb disorders occur in about 7.87% of the population, representing 300 million individuals.Citation6–Citation8 The defects remain unacceptably high in sub-Saharan Africa where they affect 38% to 63% of the population.Citation7 In Uganda, studies done in Sironko and Tororo districts found a prevalence of 17.5% and 19.5%, respectively.Citation9–Citation11
Universal screening and early intervention are thought to offer a protective advantage of sickle cell defects,Citation12,Citation13 however, diagnostic barriers due to high cost, sophisticated equipment and reliance on electricity limit the scope of Hb-S screening in resource-limited settings.Citation8 This has necessitated tests that are inexpensive, reliable, rapid and fieldable.Citation12,Citation14–Citation15 Cognizant to these, point-of-care testing (PoCT) has been explored, and is hoped to avert preventable morbidities and mortalities. There are varied PoCT assays and techniques, notable among these are: SICKLEDEX® (Streck, Omaha, NE, USA) that relies on the solubility differences in the HbA and sickle hemoglobin (HB-S) molecules,Citation16 and the sickle cell hemoglobin-S (dithionate qualitative solubility) test (Bio Lab Diagnostics India Private Limited, Mumbai, Maharashtra, India) that is based on Hb solubility testing.Citation17 To ascertain their diagnostic use, validation was conducted,Citation12,Citation15,Citation18,Citation19 and HB-S-PoCT was found to be practical in a resource-limited setting. For example, a paper-based validation test in a cohort of 159 newborns in Angola reported HB-S diagnostic sensitivity of 81.8% and specificity of 83.3%; while in the USA, a sensitivity of 94.2%, specificity of 97.7% and an overall diagnostic accuracy of 96.9% was reported.Citation19
Although the invention of the sickle cell hemoglobin-S (dithionate-qualitative) test is pivotal to the diagnostic trend of sickle cell defect, its use remains at jeopardy due to scarcity of data on its validation in low resource settings. This coupled with limited awareness of Hb-S has led to immense sickle cell defects.Citation9,Citation10 This study established the occurrence of sickle cell trait (SCT), evaluated a point-of-care test and assessed sickle cell awareness among patients attending Magale Health Center IV, Namisindwa District in rural Eastern Uganda.
Materials and methods
Study design and setting
This was a cross sectional study that enrolled adults (>18 years) who were attending the out-patients department at Magale Health Center IV during the period of May to Novem-ber, 2017. Magale Health Center IV is a private-for-profit health facility located in Namisindwa District, with a bed capacity of 86. The facility receives 178,902 patients annually and supervises 12 lower health care units in the Eastern region. It carries out sickle cell screening and sends positive samples for confirmation to Central Public Health Laboratory (CPHL). This study did not include participants with known sickle cell disease or reported sickling crisis in the previous 48hours, those who reportedly had blood transfusion in the previous 4 months and those who declined to consent.
Sample size determination and sampling procedure
Using Kish and Leslie formulaCitation20 for sample size determination, given as:
where n represents the desired sample size, Z is the statistical level at 95% CI, p is the estimated prevalence of sickle cell trait reported at 13.3%Citation11 and d is the allowable error (at 5%), a total of 178 participants was calculated. We used a consecutive sampling technique in which participants were assessed for enrollment as they present to the health facility until the required sample size was attained.
Data collection
Data were obtained by laboratory analysis of blood for the HB-S as indicated in . In addition, an interviewer administered a questionnaire to assess the awareness of SCT.
Sample collection and dried blood spot (DBS) preparation
Four milliliters of venous blood were collected into EDTA a vac-utainer. Samples were kept at room temperature (22°C–27°C) and tested using rapid sickle cell test within 4 hours.
The preparation of a DBS was made by dispensing 100 µL of blood to a well labeled Whatman™ 903 Protein Saver Card (GE Healthcare Ltd, Cardiff, UK), and stored in a humid free zip-locked bag with desiccant packets at −20°C until shipped to CPHL for analysis.
Laboratory sample analyses
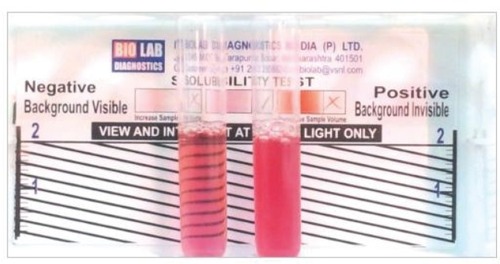
Rapid sickle cell hemoglobin-S (dithionate qualitative solubility) test (Bio Lab Diagnostics India Private Limited)Citation17 determines Hb containing variants in whole blood using visual inspection for turbidity due to difference in lysis of the insoluble HB-S and soluble form of HbA following a mixture of blood and a working solution in a test tube as shown in .
Figure 1 Showing the reactivity pattern of the rapid sickle cell hemoglobin-S (dithionate qualitative solubility) test
Constitution of the kit testing reagents
The Hb-S dithionate qualitative solubility kit comprises: reagent 1 (R1), composed of phosphate buffer; reagent 2 (R2), composed of 0.5 g sodium dithionate and reagent 3 (R3), composed of 0.5 g of white saponin. These were constituted to a working solution by transferring the contents of R2 and R3 to a bottle of R1, and were gently mixed for 15 minutes and well labeled.
Hb-S testing method
This involved addition of 2 mL of the working reagent to a labeled test tube, and 50 µL of blood. These were mixed well by gently shaking the test tube and incubated at room temperature (approximately 25°C) for 10 minutes. The test tube contents were visually examined and it was reported that the negative test was transparent, as seen in the background of the rapid sickle cell tube reader, while the background was not visible in the positive sample (HbAS or HbSS) given in .
Two test tube readings were done independently with unaided readers, and in case of a discrepancy, a third reader was considered, and a result was concluded.
Confirmation was done using Hb electrophoresis. To ensure quality of the test results, experimental procedures were conducted according to the manufacturers’ instructions. Known HbAS and HbSS blood samples were used as positive controls.
Awareness assessment
We used an interviewer administered questionnaire to determine awareness of SCT among study participants. The questionnaire was developed based on literature from the previous studies,Citation12,Citation15,Citation19–Citation24 and pre-tested at St Benedict Medical Center in Mayuge District to check for clarity and comprehension. Following this, changes were made to suit the intended use. The questionnaire comprised: demographics of the participant, knowledge of the family history and transmission mode.
Statistical analysis
Data were recorded in hard copy and transferred to Microsoft® Office Excel, version 2007. Proportion of participants with SCT was analyzed as the number of those with the SCT divided by the total number of participants. Performance evaluation was based on measurement of: sensitivity = true positives (TP)/[TP + false negatives (FN)]; specificity = TN/[false positives (FP) + TN]; positive predictive value (PPV) = TP/(TP + FP) and negative predictive value (NPV) = TN/(TN + FN).
Ethical considerations
We received ethical approval from the research and ethics committee of Clarke International University, Kampala, Uganda (formerly, International Health Sciences University). Further, we obtained a signed informed consent from each of our participants.
Results
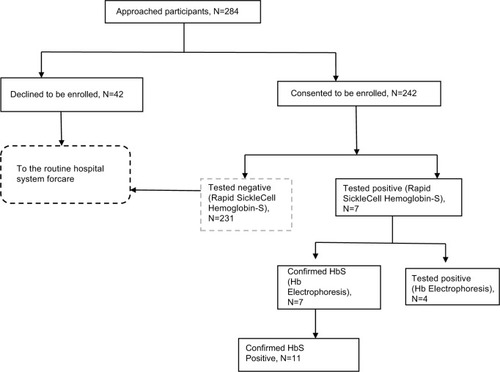
We approached 284 participants, of whom 242 were enrolled. The main reasons for exclusion were: being known HbSS, reported a sickling crisis in the previous 48 hours and history of blood transfusion in the previous 4 months. Participants’ mean age was 29.4 years (range 18–49), and they reported varied socio-demographic characteristics, as indicated in .
Table 1 Showing socio-demographic characteristics of respondents
Of the 242 participants, 11, who represent 4.5% (95% CI: 3.3–5.9), had SCT as reported by the Hb electrophoresis. The participant flow chart is presented in .
Figure 2 Study participant flow chart and reactivity pattern of tests. Abbreviations: Hb, hemoglobin; HB-S, sickle hemoglobin.
The diagnostic performance of the rapid sickle cell test against Hb electrophoresis as gold standard is presented in . From , the rapid test detected 7 cases of HbAS, while Hb electrophoresis reported 11 cases. The results have been summarized in to aid the computation of the sensitivity, specificity, NPV and PPV.
Sensitivity=TP/(TP + FN)=7/(7+4)=7/11=63.64%
Specificity=TN/(FP + TN)=231/(0+231)=231/231=100.00%
PPV= TP/(TP + FP)=7/(7+0)=7/7=100.00%
NPV=TN/(TN + FN)=231/(231+4)=231/235=98.30%
Table 2 Performance evaluation of the solubility rapid screening test method against Hb electrophoresis (gold standard)
Table 3 Participants’ responses regarding their awareness of the SCT
Participants’ response regarding the assessment of awareness of SCT indicated a knowledge gap, as presented in .
Discussion
From this study, the rapid sickle cell test accurately identified 7 true positives out of 11 confirmed by the gold standard diagnosis, giving a sensitivity and specificity of 63.64% and 100%, respectively. Further, the PPV and NPV were 100% and 98.30%, respectively. The sensitivity of the rapid sickle cell (dithionate qualitative solubility test) is low compared to the 84% of a density-based test using aqueous multiphase systems done in Zambia,Citation25 93% and 94% among children and post-partum women, respectively, in a study conducted in Angola,Citation19 98.4% sensitivity using an immunoassay,Citation14,Citation26 and 99% using the Sickle SCAN™ test (BioMedomics, Durham, NC, USA).Citation12 This assay has demonstrated a low sensitivity, which is attributed to interfering reactivity due to other Hb variants, particularly hemoglobin C (HbC)-Harlem and fetal hemoglobin (HbF).Citation17 On the other hand, our study detected a 100% specificity, thus a high reliability toward confirming the SCT, which coupled with its diagnostic returns like being simple to use and rapid, makes it very advantageous in a limited resource set up. In addition, its simplicity makes it fieldable even where electricity is not available. This is thought to bridge the gap in the widely thought community outreaches to increase awareness of Hb-S status.Citation8
Of the 242 participants, 231 (95.5%) tested negative, while 11 (4.5%) tested positive for SCT. This indicates a high burden of SCT, a finding comparable to a report from a review done in Africa.Citation4 Further, our findings are in agreement with the 4.6% prevalence that was earlier reported in Southwestern Uganda.Citation9 Four of the 11 participants who tested positive using the Hb electrophoresis were found to be negative when the Hb-solubility kit was used. This is attributable to the likely false negativity due to the high HbF variant interference.Citation17
As the Hb electrophoresis indicated the HbAS variant, it is irrefutable that genetics had a role in the Hb presentation. The same finding has been reported in Eastern and Western Uganda,Citation10 and in Ghana.Citation27 Although complex, the HbAS variant offers a protective advantage to infections common in our set up, such as infection with Plasmodium species,Citation6,Citation28–Citation30 and this risked 25% of the next generation to acquire HbSS if genetic counseling and testing is not emphasized.Citation21,Citation22 Further, findings from our study have indicated an information gap regarding the existence of the sickle cell defects, similar to what has been reported by other studies.Citation7,Citation9,Citation10 Lack of awareness may portend the effort to lessen the risk of genetic cross-over that could multiply the trait incidence as well as the number of individuals who may inherit the sickle cell disease. It ought to be remembered that premarital counseling and screening for genetic disorders is key to their reduction and possible elimination of defects as a result of two partners with a carier state.Citation4,Citation8 Thus, in a set-up where genetic screening is neither indicated nor done, the career likelihood is rather amplified and may promulgate the genotypes and the associated defects. Our study ought to be interpreted in light of these limitations. First, the rapid sickle cell test does not identify other hemoglobinopathies which may present with varied forms of anemia. Second, though our study population consisted of adults in the child bearing age, we did not carry out partner-to-partner screening. Thus, this study cannot independently conclude the attributable carry over risk to the next generation.
Conclusion
Performance evaluation of the rapid sickle cell test indicated a high diagnostic reliability, which makes it suitable for field set-ups. Further, the study reports a high prevalence of SCT, and it is associated with genetic predisposition. Community sensitization is key to avert the associated risk of Hb defects.
Author contributions
KM, SKT, BM, FW, CA and IMT participated in study conception and design, data acquisition, analysis and interpretation, manuscript drafting and revising. CA, BM and IMT critically revised the manuscript. All authors read and approved the final manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
The authors would like to acknowledge study participants and the management of Magale Health Center IV. We are grateful to the staff of Central Public Health Laboratories. We acknowledge the Uganda Sickle Cell Rescue Foundation who, with support from Hotel Africana, Roofings Group and AGT group, procured and donated the Sickle Cell screening kits to the study. This work did not receive any funding; however, we recognize the logistical support from Manafwa District Local Government.
Disclosure
The authors report no conflicts of interest in this work.
References
- HattonMFMolecular pathology of human haemoglobinNature2013219157902909
- PerutzMFRossmannMGCullisMGMuirheadHWillGNorthACStructure of haemoglobin: A three-dimensional Fourier synthesis at 5.5Å resolution, obtained by X-ray analysisNature20131854711416422
- LewisRGene mutations in human haemoglobin: the chemical difference between normal and sickle cell haemoglobinNature2006180326328
- MulumbaEWilsonSComprehensive care in sickle cell disease: its impact on morbidity and mortalitySemin Hematol2015283220226
- PielNNagelRLPlattOSDisorders of HemoglobinCambridgeCambridge University Press2010494526
- GamitDSickle cell trait and sudden death-bringing it homeJ Natl Med Assoc2014993300305
- JeneretteLMurdaughDNew human haemoglobin variant from southern ArabiaNature200821915911641166
- World Health OrganizationHemoglobin Disorders2013 Available from: http://apps.who.int/iris/bitstream/10665/44665/1/9789241548274_eng.pdfAccessedSeptember 5, 2018
- NdeeziGKiyagaCHernandezAGBurden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional studyLancet Glob Health201643e195e20026833239
- OkwiALByarugabaWNdugwaCMParkesAOcaidoMTumwineJKAn up-date on the prevalence of sickle cell trait in Eastern and Western UgandaBMC Blood Disord2010101520569434
- KiyagaCUganda Sickle Survey Results and Screening Program2014 Available from: http://www.hollyfoundationsicklecell.co.uk/wp-content/uploads/2015/06/The-Survey-in-Adobe-PDF-format.pdfAccessed July 17, 2017
- KanterJTelenMJHoppeCRobertsCLKimJSYangXValidation of a novel point of care testing device for sickle cell diseaseBMC Med201513122526377572
- KumarAAPattonMRHennekJWDensity-based separation in multiphase systems provides a simple method to identify sickle cell diseaseProc Natl Acad Sci201411141148641486925197072
- BondMHuntBFlynnBHuhtinenPWareRRichards-KortumRTowards a point-of-care strip test to diagnose sickle cell anemiaPLoS One2017125e017773228520780
- McgannPTSchaeferBAPaniaguaMHowardTAWareRECharacteristics of a rapid, point-of-care lateral flow immunoassay for the diagnosis of sickle cell diseaseAm J Hematol201691220521026537622
- Insert SickleDex®Kit StreckOmaha, NE, USA Available from: http://labs-inc.org/pdf/22_3.pdfAccessed July 10, 2018
- Rapid Sickle Cell Hemoglobin-S (HbAS/HbSS) Dithionate Qualitative Available from: http://biolabdiagnostics.com/image/catalog/pdf/Rapid%20Sickle%20Literature%20RSC%20R1+R2%209-13.pdfAccessed September 5, 2018
- BondMHuntBFlynnBHuhtinenPWareRRichards-KortumRTowards a point-of-care strip test to diagnose sickle cell anemia. Lam W, editorPLoS One2017125e017773228520780
- NathanielZPietyAGeorgeSA Paper-Based Test for Screening Newborns for Sickle Cell DiseaseSci Rep20174548828367971
- KishLSurvey SamplingNew YorkJohn Wiley and Sons, Inc Section 1.313.113.6 Available from: https://biblioteca.ibge.gov.br/visual-izacao/monografias/GEBIS%20-%20RJ/censocontinuo/Samples%20and%20Censuses.pdfAccessed July 10, 2018
- HarrisonSEWalcottCMWarnerTDKnowledge and Awareness of Sickle Cell Trait Among Young African American AdultsWest J Nurs Res20173991222123927550467
- ObedSAOppongSATortoMAboagyeSNuamahMAAsah-OpokuKAwareness of Sickle Cell Trait Status: A Cross-Sectional Survey of Antenatal Women in GhanaAm J Trop Med Hyg201796373574027994105
- CrearySAdanIStanekJSickle cell trait knowledge and health literacy in caregivers who receive in-person sickle cell trait educationMol Genet Genomic Med20175669269929178654
- PatilSSThikareAAWadhvaSKNarlawarUWShuklaSKnowledge, attitude and practice regarding sickle cell disease in adult sufferers and carriers in a rural areaInt J Community Med Public Health20174410751080
- KumarAAChunda-LiyokaCHennekJWEvaluation of a Density-Based Rapid Diagnostic Test for Sickle Cell Disease in a Clinical Setting in ZambiaPLoS One2014912e11454025490722
- PietyNZYangXKanterJVignesSMGeorgeAShevkoplyasSSValidation of a Low-Cost Paper-Based Screening Test for Sickle Cell AnemiaPLoS One2016111e014490126735691
- BrainJHereditary qualitative and quantitative erythrocyte defects in Ghana. An historical and geographical surveyGhana Med J20147118119
- DeoreMCokicVPSmithRDThe stereochemical mechanism of the cooperative effects in hemoglobin revisitedAnnu Rev Biophys Biomol Struct201327134
- VafaMTroye-BlombergMAnchangJGarciaAMigot-NabiasFMultiplicity of Plasmodium falciparum infection in asymptomatic children in Senegal: relation to transmission, age and erythrocyte variantsMalar J2008711718215251
- KwiatkowskiDPHow malaria has affected the human genome and what human genetics can teach us about malariaAm J Hum Genet200577217119216001361
