Abstract
Introduction
Copper (Cu) and zinc (Zn) are important trace elements that are also structural ions of superoxide dismutase (SOD), which reduce oxidative stress. Zinc deficiency and excess copper have been reported to be associated with inflammation. The human T-lymphotropic virus type 1 (HTLV-1) is a retrovirus, which is believed to cause systemic inflammation. The aim of this study is to measure levels of Zn, Cu, SOD, and prooxidant–antioxidant balance (PAB) in HTLV-1-positive patients and investigate the association between serum Zn and Cu concentrations and levels of oxidative stress in them.
Methods
The serum samples of 1,116 subjects who had participated in the “Mashhad Stroke and Heart Atherosclerotic Disorder” study, including 279 HTLV-1-positive and 837 HTLV-1-negative patients, were used. Levels of Zn, Cu, SOD, and PAB were measured.
Results
Zinc and SOD levels were lower in the HTLV-1-positive group; however, the difference was statistically significant only for the level of SOD (P=0.003). On the other hand, levels of copper and PAB were significantly higher in HTLV-1 positive subjects; P=0.004 and P=0.002, respectively.
Conclusion
In HTLV-infected patients, serum Zn concentration is lower and Cu concentration is higher than healthy controls. This altered situation might be either primary or secondary to HTLV-1 infection, which should be investigated in larger studies. We showed that SOD is significantly lower in HTLV-1-infected subjects. As in some other viruses that evolve different mechanisms to potentiate virus replication by changing the physiologic condition of host cells, HTLV-1 too probably decreases the activity of copper–zinc SOD1 by suppressing its gene.
Introduction
The human T-lymphotropic virus type 1 (HTLV-1) is a retrovirus that belongs to the Retroviridae family and is classified in the Deltaretrovirus genus.Citation1 This virus was discovered in 1979 in association with T-cell leukemia.Citation2,Citation3 Some regions in the world are highly endemic for this virus; including southwestern Japan, sub-Saharan Africa, South America, and the Caribbean area.Citation4 Northeastern Iran has also been introduced as an endemic region in which the prevalence of HTLV-1 infection was reported to be 2–3%.Citation5
Adult T-cell leukemia and an inflammatory condition called HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) are the two main diseases caused by this virus.Citation6 The modes of transmission of the virus are mainly through sexual contact with an infected person; mother-to-child, particularly through breastfeeding; and blood transfusion.Citation1
HTLV-1 causes systemic inflammation. The pathogenesis is either autoimmune antigen mimicry, in which the immune system attacks host cells because of antigen cross reaction with HTLV-1 antigens, or innocent bystander in which the cytokines secreted by HTLV-1-specific cytotoxic cells damage the surrounding tissues.Citation7
In patients diagnosed with HAM/TSP, signs of active and acute inflammation were shown to be associated with more severe form of HAM/TSP.Citation8
Reactive oxygen species (ROS) are metabolites of oxygen with strong oxidizing capabilities. They are produced by cells that are involved in the host-defense response and can cause endothelial dysfunction by oxidation of cellular signaling proteins.Citation9 While low concentrations of ROS serve as signaling molecules, chronic or prolonged ROS production is associated with the progression of inflammation.Citation9 In normal conditions, a balance is maintained between the production and elimination of the ROS. The role of oxidative stress (OS), which is the consequence of an imbalance between ROS and antioxidant factors,Citation10 has been approved in various inflammatory diseases and chronic infections.Citation11
Superoxide dismutase (SOD) is an antioxidant factor that removes the superoxide species.Citation12 Three forms of SOD are known so far, which include the cytosolic Cu/ZnSOD (SOD1), the mitochondrial MnSOD (SOD2), and the extracellular superoxide dismutase (SOD3).Citation13 The catalytic reaction of Cu/ZnSOD is performed by the cyclic reduction and oxidation of the copper ion (Cu2+).Citation12
The prooxidant–antioxidant balance (PAB), which can be measured in a single assay,Citation10 has been reported as a potential cardiovascular risk factor.Citation14
Copper (Cu) and zinc (Zn) are important trace elements that act as ion cofactors in proteins, hormones, and receptors and also as cofactors in numerous enzymatic reactions.Citation15 They are structural ions of SODCitation16 and reduce OS by induction of metallothionein synthesis.Citation17,Citation18 Because of their pivotal role in the redox mechanisms, their imbalanced status may lead to an increased susceptibility to oxidative damage.Citation19–Citation21
While acute Zn deficiency causes a decrease in innate and adaptive immunity, chronic deficiency increases inflammation.Citation22 On the other hand, excess Cu is probably associated with an inflammatory response, although it is not clear whether copper has prooxidant or antioxidant effects. This is because ceruloplasmin, as the main copper-containing protein, has been shown to act both as an antioxidant and prooxidant in different conditions.Citation23
Tax, which is an important regulatory protein encoded by the HTLV-1 genome, is essential for the replication of the virus. On the other hand, numerous studies indicate that continuous TAX production is associated with apoptosis, and OS is identified as the mediator of TAX-induced apoptosis. The oxidative damage induced by Tax is mediated by the transcriptional activation of nuclear factor kappa B (NF-κB) by the Tax. NF-κB is itself a prooxidant nuclear transcription factor.Citation24
One of the first proofs of the involvement of prooxidants in the functionality of Tax was the use of antioxidants. A radical scavenger called pyrrolidine dithiocarbamate was shown to strongly suppress the Tax-induced activation of the DNA-binding activity of NF-κB in Jurkat cells.Citation25
On the other hand, it has been shown that total antioxidant capacity (TAC) is depleted during HTLV-1 infection.Citation11
Considering the fact that both HTLV-1 infection and abnormal serum levels of Zn and Cu could lead to systemic inflammation, and taking into account that OS plays an important role in both of their mechanisms, in the current study, we measured levels of Zn and Cu in HTLV-1-positive patients in a control group. We also assessed the association between serum Zn and Cu concentrations and levels of OS in HTLV-1-infected subjects by measuring SOD-1 and PAB.
Materials and methods
Study population
In the current study, the serum samples of 1,116 subjects who had participated in the “Mashhad Stroke and Heart Atherosclerotic Disorder (MASHAD)” study were used, and the participants were selected through cluster-randomized allocation methodology.Citation26 The study’s protocol was approved by Mash-had University of Medical Sciences Ethics committee and all participants provided informed written consents. This study was conducted in accordance with the Declaration of Helsinki.
The inclusion and exclusion criteria of MASHAD study and the public features of the study’s population including marital status, occupation status, education level, drug use, and biochemical and anthropometry measurements were explained previously.Citation26 For the current study, 279 HTLV-1-positive patients as well as 837 HTLV-1-negative patients who matched the first group by age and sex were selected.
Sample collection
Twenty milliliters of blood was taken by venipuncture of an antecubital vein in vacuum tubes. Specimens were centrifuged at room temperature within 30–45 minutes of collection to separate the serum, which were then divided into aliquots and kept frozen at –20°C for future analysis.
HTLV-1 infection assessment
The serum samples of all participants of MASHAD study were screened for HTLV-1-specific antibodies by ELISA (Dia.Pro Diagnostic, Italy). Positive cases were assessed for the HTLV-1 genome using PCR for TAX- and LTR-specific primers to confirm the infection. Patients were confirmed to be infected by HTLV-1 if either of the genes were present. A total of 279 HTLV-1-infected patients were identified who were enrolled in the current study.
Measurement of serum zinc and copper concentrations
Serum samples were diluted with nitric acid at the ratio of 1:10. Using the flame atomic absorption (Varian AA240FS), the concentrations of Zn and Cu were evaluated. Also, using Zn and Cu standards (Merc and Co. Pharmaceutical Company), the Zn and Cu standard curves were created. The accuracy of the methods for Zn and Cu were 93%±4.8% and 95%±3.75%, respectively, which were assessed through measuring the confirmed reference material (MercK KGaA 64271 Darmstadt, Germany) comprising known values (1000±2 mg/ L) for Zn and Cu. The intra-assay and inter-assay coefficient of variation for Zn and Cu were also calculated and equal to 1.5%±0.2%, 2.6%±0.4%, 1.3%±0.12%, and 2.11%±0.32%, respectively.
PAB measurement
As mentioned before, the PAB test is the only assay capable of checking the balance of oxidants and antioxidants concurrently in a test. PAB values were evaluated in the serum samples through a modified PAB test that has been previously described.Citation10 Briefly, for making a standard solution, varying portions (0%–100%) of 250 µM hydrogen peroxide (H2O2) were mixed with 3 mM uric acid (in 10 mM NaOH). Sixty milligrams of TMB powder was dissolved in 10 mL DMSO. For making TMB cation, 400 µL of the TMB/DMSO was mixed with 20 mL acetate buffer (0.05 M buffer, pH 4.5). Subsequently, 70 µL of fresh chloramine T solution (100 mM) was added to this 20 mL of acetate buffer. Next, we mixed the solution and incubated it at the room temperature for 2 hours. Then, 25 U of peroxidase enzyme solution was mixed with TMB cation solution, distributed in 1 mL and stored at–20°C. Also, for making TMB solution, 200 µL of TMB/DMSO was mixed with 10 mL of acetate buffer (0.05 M buffer, pH 5.8) and the working solution was made by adding 1 mL TMB cation to 10 mL of TMB solution. Afterwards, the solution was incubated for 2 minutes at room temperature and immediately used. After that, 10 µL of each of the samples as well as the standard or blank (distilled water) were mixed with 200 µL of working solution in each well of a 96-well plate. The plate was incubated for 12 minutes at 37°C in a dark environment. After incubation, 100 µL of 2 N hydrochloric acid (HCL) was appended in each well and the OD was evaluated with an ELISA reader at 450 nm, with a reference wavelength of 620 or 570 nm. According to the values comparative to the standard samples, a standard curve was drawn. The values of PAB in the arbitrary HK unit are expressed as the percentage of hydrogen peroxide in the standard solution. Then, based on the amounts from the above standard curve, the values of unknown samples were calculated.Citation14
SOD measurement
Primarily, for making Tris–cacodylic acid buffer (0.05 M, pH 8.2) comprising 0.001 M diethylenetriamine pent acetic acid (DTPA), Tris (0.05 M, containing 0.001 M DTPA) was appended to cacodylic acid (0.05 M, containing 0.001 M DTPA) until pH =8.2 was obtained. Prior to use, the buffer was air-balanced for 1 hour. For pyrogallol preparation, a source of 0.02 M (100×) pyrogallol solution was constructed in water. To remove soluble oxygen, it was flushed with nitrogen for 1 hour, then aliquoted (100 µL per aliquot), and finally stored frozen until used. After that, 20 µL of each serum and control was added in duplicate wells. Also, using the equilibrated assay buffer, pyrogallol stock solution (0.02 M) was diluted 1:100, and 180 µL per well of the solution was added through a multichannel pipettor. The reactions were read on a plate reader at 405 nm at intervals of 5 minutes for 1 hour. The SOD level, which inhibited pyrogallol oxidation by 50% (relative to control), was defined as an SOD activity unit in the described conditions.Citation27
Statistical analyses
In the current study, SPSS Version 18 (SPSS Inc. Chicago, IL, USA) was used for all statistical analyses. The normality of the data was evaluated through the Kolmogorov–Smirnov test. Descriptive statistics containing mean, frequency, and SD were evaluated for all variables. Normally distributed variables were expressed by mean±SD, while parameters that were not normally distributed were expressed by median±IQR. Chi-square or Fisher’s exact assays were applied for the assessment of categorical parameters. To investigate the correlation between PAB, SOD, and HTLV-1, partial correlation and linear regression were utilized. A two-sided P-value of <0.05 was considered statistically significant. Figures were drawn using GraphPad Prism 6.
Results
Levels of zinc, copper, SOD, and PAB
shows the mean serum concentration of Zn and Cu and also the levels of SOD and PAB in both groups. Zn and SOD levels were lower in the HTLV-1-positive group; however, the difference was statistically significant only for the level of SOD (P=0.003). On the other hand, levels of Cu and PAB were significantly higher in HTLV-1-positive subjects (P=0.004 and P=0.002, respectively).
Table 1 Mean levels of zinc, copper, SOD, and PAB in HTLV-1-positive and -negative patients
The copper-to-zinc ratio was also significantly higher in the HTLV-1-positive group (P<0.001).
Correlation between zinc and SOD, SOD and PAB, and copper and SOD
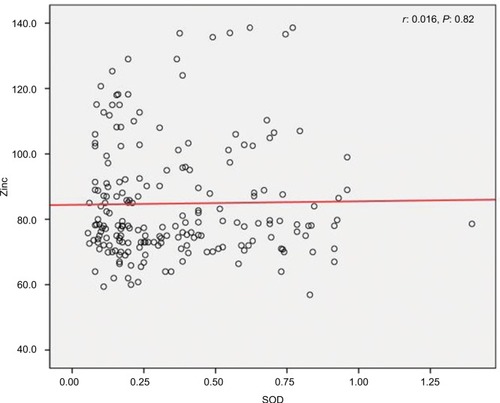
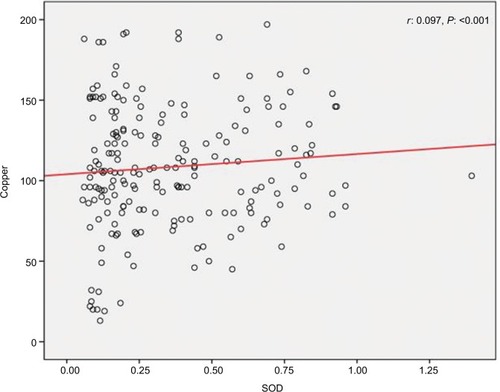
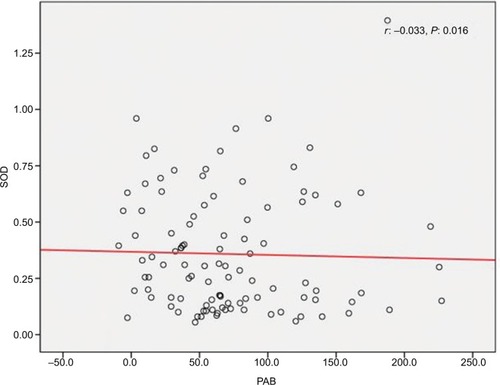
We found a positive relationship between levels of Zn and SOD in HTLV-1-positive patients; however, the relationship was not statistically significant (r=0.016, P=0.82) (). Also, it was shown that there is a significant positive correlation between Cu and SOD (r=0.097, P<0.001) (), as well as a significant reverse correlation between the level of SOD and PAB (r=−0.033, P=0.016) ().
Discussion
The results of this study show that in HTLV-infected patients, serum Zn concentration is lower and Cu concentration is higher than healthy controls. To the best of our knowledge, no other study has yet assessed the levels of these trace elements in HTLV-1-infected patients.
Although serum levels of both Zn and Cu are in normal ranges for the Iranian population in both cases and control groups,Citation28–Citation30 further studies are required to reveal whether the different serum Zn and Cu levels in HTLV-1 patients has led to the increased susceptibility to infection in them or the infection itself has led to different Zn and Cu levels.
Both Zn and Cu are claimed to protect organisms against infectious diseases and to regulate innate immune response.Citation31 However, the etiology of altered plasma Zn and Cu concentrations associated with proinflammatory conditions is yet to be determined.Citation32
The increased serum copper-to-zinc ratio is shown to be associated with an altered homeostatic status after a destabilizing event and is also associated with an inflammatory response.Citation32 Also, the combination of low serum Zn and high serum Cu is contributed to an increased risk of cardiovascular diseases.Citation33 Our results show a significant increase in the copper-to-zinc ratio in the HTLV-1-infected subjects in comparison to controls. This finding could be an explanation for the systemic inflammation seen in HTLV-1 infection, both in asymptomatic carriers and symptomatic patients.
We showed that SOD1 is significantly lower in HTLV-1-infected subjects. It is well known that viruses evolve different mechanisms to potentiate virus replication, by changing the physiologic condition of host cells. For example, it has been shown that influenza virus A increases superoxide anion level in human alveolar cells, mainly by suppressing the copper–zinc SOD1 gene.Citation34 In another example, the expression of the regulatory Tat protein from the human immunodeficiency virus type 1 (HIV-1) was shown to suppress the expression of cellular Mn-containing superoxide dismutase (Mn-SOD).Citation35 This might be the case for what is happening in HTLV-1 infection as well.
Also, the important regulatory protein Tax from HTLV-1 is shown to resemble other viral and cellular oncogenes like adenovirus E1A, simian virus 40 large tumor antigen, and the human papilloma virus E7 proteins, which aside from their transforming properties have the ability to induce apoptosis.Citation24 On the other hand, it has been demonstrated that the induction of OS was a prompt effect of TAX function and this OS is a mediator of TAX-induced apoptosis.Citation24 This effect is commonly observed in other viral proteins that cause apoptosis, such as HIV-1 Tat protein.Citation35 As HTLV-1 and HIV-1 share similar genomic organization and tropism for immune cells, in particular CD4+ and CD8+ T cells, their role in OS could be similar.
In a study that evaluated the serum level of trace elements Zn, Cu, Mg, and Se, and assessed the level of OS in children with type 1 diabetes, levels of all four elements and also the OS parameters were significantly lower in diabetic patients than in the controls.Citation36
We found a positive correlation between SOD and Cu and a negative correlation between SOD and PAB. We, therefore, assume that there might be a negative correlation between Cu and PAB. This assumption is in favor of what has been reported by Alamdari et al, which is a negative correlation between PAB and ceruloplasmin.Citation14 They have discussed that ceruloplasmin behaves as an antioxidant.
Regarding the association between HTLV-1 infection and antioxidant defense, in 2014, Shomali et al have investigated the TAC in the serum of HTLV-1-infected patients. They have reported that TAC is depleted during HTLV-1 infection. This reduction was seen in both asymptomatic carriers of HTLV-1 and those who had symptoms of HAM/TSP.Citation11 This finding is relevant with the lower SOD levels in HTLV-1-infected subjects, which is shown in the present study.
Revealing the exact mechanisms by which ROS are involved in the regulation of T-cell functions is important to achieve a good insight of the immune response, and to develop new treatments for the control of immune-mediated diseases.Citation37
Limitations
In this study, assessment of the relation between SOD and zinc and copper levels was not separately done in asymptomatic HTLV-1 carriers and those with symptoms. As symptomatic patients usually have higher viral loads, it is necessary to distinguish the two groups when studying the effects of the virus on different biologic mechanisms.
Conclusion
The decreased serum Zn and increased serum Cu in HTLV-1-infected subjects along with an increased Cu/Zn ratio confirms the fact that infection with this virus is associated with general inflammation. On the other hand, because the antioxidant capacity is reduced in these patients, supplying them with antioxidants could help postpone the incidence of symptoms.
Acknowledgments
This study was part of two Molecular Medicine PhD theses (codes: 940247 and 941175) sponsored by Mashhad University of Medical Sciences, Mashhad, Iran.
Disclosure
The authors report no conflicts of interest in this work.
References
- VerdonckKGonzálezEvan DoorenSVandammeAMVanhamGGotuzzoEHuman T-lymphotropic virus 1: recent knowledge about an ancient infectionLancet Infect Dis20077426628117376384
- PopovicMReitzMSSarngadharanMGThe virus of Japanese adult T-cell leukaemia is a member of the human T-cell leukaemia virus groupNature1982300588763666982418
- PoieszBJRuscettiFWMierJWWoodsAMGalloRCT-cell lines established from human T-lymphocytic neoplasias by direct response to T-cell growth factorProc Natl Acad Sci USA19807711681568196256763
- GessainACassarOEpidemiological aspects and world distribution of HTLV-1 infectionFront Microbiol2012338823162541
- RafatpanahHHedayati-MoghaddamMRFathimoghadamFHigh prevalence of HTLV-I infection in Mashhad, Northeast Iran: a population-based seroepidemiology surveyJ Clin Virol201152317217621840754
- SouzaATanajuraDToledo-CornellCSantosSCarvalhoEMImmunopathogenesis and neurological manifestations associated to HTLV-1 infectionRev Soc Bras Med Trop201245554555223152334
- BanghamCROsameMCellular immune response to HTLV-1Oncogene200524396035604616155610
- Puccioni-SohlerMGasparettoECabral-CastroMJHAM/ TSP: association between white matter lesions on magnetic resonance imaging, clinical and cerebrospinal fluid findingsArq Neuropsiquiatr201270424625122510735
- MittalMSiddiquiMRTranKReddySPMalikABReactive oxygen species in inflammation and tissue injuryAntioxid Redox Signal20142071126116723991888
- AlamdariDHPaletasKPegiouTSarigianniMBefaniCKoliakosGA novel assay for the evaluation of the prooxidant-antioxidant balance, before and after antioxidant vitamin administration in type II diabetes patientsClin Biochem2007403–424825417196578
- ShomaliSAvvalFZBoostaniRJarahiLYoussefiMSerum total antioxidant capacity status of HTLV-1 infected patientsActa Virol201559219920326104339
- BordoDDjinovićKBolognesiMConserved patterns in the Cu, Zn superoxide dismutase familyJ Mol Biol199423833663868176730
- FridovichISuperoxide anion radical (O2-.), superoxide dismutases, and related mattersJ Bio Chem19972723018515185179228011
- AlamdariDHGhayour-MobarhanMTavallaieSProoxidant-antioxidant balance as a new risk factor in patients with angiographically defined coronary artery diseaseClin Biochem200841637538018191639
- FleetJCZinc, copper and manganeseStipanukMBiochemical and Physiological Aspects of Human NutritionPhiladelphia, PASaunders2000
- FukuokaMTokudaENakagomeKWuZNaganoIFurukawaYAn essential role of N-terminal domain of copper chaperone in the enzymatic activation of Cu/Zn-superoxide dismutaseJ Inorg Biochem201717520821628780408
- RutherfordJCBirdAJMetal-responsive transcription factors that regulate iron, zinc, and copper homeostasis in eukaryotic cellsEukaryot Cell20043111314871932
- SaydamNAdamsTKSteinerFSchaffnerWFreedmanJHRegulation of metallothionein transcription by the metal-responsive transcription factor MTF-1: identification of signal transduction cascades that control metal-inducible transcriptionJ Biol Chem200227723204382044511923282
- SoinioMMarniemiJLaaksoMPyöräläKLehtoSRönnemaaTSerum zinc level and coronary heart disease events in patients with type 2 diabetesDiabetes Care200730352352817327315
- CerielloAOxidative stress and glycemic regulationMetabolism2000492272910693917
- KlotzLOKrönckeKDBuchczykDPSiesHRole of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stressJ Nutr200313351448S1451S12730440
- BonaventuraPBenedettiGAlbarèdeFMiossecPZinc and its role in immunity and inflammationAutoimmun Rev201514427728525462582
- BoSDurazzoMGambinoRAssociations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adultsJ Nutr2008138230531018203896
- ChlichliaKLosMSchulze-OsthoffKGazzoloLSchirrmacherVKhazaieKRedox events in HTLV-1 Tax-induced apoptotic T-cell deathAntioxid Redox Signal20024347147712215214
- SchreckRGrassmannRFleckensteinBBaeuerlePAAntioxidants selectively suppress activation of NF-kappa B by human T-cell leukemia virus type I Tax proteinJ Virol19926611628862931404592
- Ghayour-MobarhanMMoohebatiMEsmailyHMashhad stroke and heart atherosclerotic disorder (MASHAD) study: design, baseline characteristics and 10-year cardiovascular risk estimationInt J Public Health201560556157225943424
- AhmedHSchottEJGauthierJDVastaGRSuperoxide dismutases from the oyster parasite Perkinsus marinus: purification, biochemical characterization, and development of a plate microassay for activityAnal Biochem2003318113214112782041
- MashhadiMABakhshipourAZakeriZAnsari-MoghadamAReference range for zinc level in young healthy population in southeast of IranHealth Scope201761e18181
- DabbaghmaneshMHSalehiNMSiadatanJOmraniGRCopper concentration in a healthy urban adult population of southern IranBiol Trace Elem Res20111441–321722421573871
- ParizadehSMRKazemi-BajestaniSMRMoghaddamASSerum zinc and copper concentrations and socioeconomic status in a large Persian cohortAsian Biomedicine201153329
- StaffordSLBokilNJAchardMEMetal ions in macrophage antimicrobial pathways: emerging roles for zinc and copperBiosci Rep2013334541554
- MalavoltaMPiacenzaFBassoAGiacconiRCostarelliLMocchegianiESerum copper to zinc ratio: relationship with aging and health statusMech Ageing Dev20151519310025660061
- LeoneNCourbonDDucimetierePZureikMZinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortalityEpidemiology200617330831416570028
- PyoCWShinNJungKIChoiJHChoiSYAlteration of copperzinc superoxide dismutase 1 expression by influenza A virus is correlated with virus replicationBiochem Biophys Res Commun2014450171171624946209
- FloresSCMareckiJCHarperKPBoseSKNelsonSKMcCordJMTat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cellsProc Natl Acad Sci USA19939016763276368395050
- AlghobashyAAAlkholyUMTalatMATrace elements and oxidative stress in children with type 1 diabetes mellitusDiabetes Metab Syndr Obes201811859229618936
- BelikovAVSchravenBSimeoniLT cells and reactive oxygen speciesJ Biomed Sci2015228526471060