Abstract
Background and objectives
Myeloid leukemias (MLs) are clonal stem cell disorders affecting myeloid lineage cells. Advances in cytogenetic and molecular studies partially disclosed the mystery about risk factors and pathophysiology of MLs. Regarding incidence, risk factors, response to treatment, and overall survival of patients, research showed differences among different countries. However, the Western registry data are the basis for the documented description of MLs in medical textbooks. This research aimed to study MLs in Middle Eastern health centers. Egypt has the highest population in the Middle East; furthermore, 96.6% of the population is native Egyptians; accordingly the study focused on Egypt.
Patients and methods
Data of 468 patients with MLs were collected from hospital records at two big tertiary health centers. They were grouped into group 1 (chronic myeloid leukemia, CML) and group 2 (acute myeloid leukemia, AML); the latter was subgrouped into 2a (primary AML) and 2b (secondary AML).
Results and conclusions
The median age of patients was 43 years; males predominate in group 2a and females in groups 1 and 2b. 37.2% of group 1 patients were treated with Gleevec. Hematopoietic stem cell transplantation was planned for only 5% of group 2 and 18% relapsed. Of groups 1 and 2 patients, 25% and 12%, respectively, stopped follow up, and 15% and 35% died. ORR and overall survival were 53%, 27% and 7%, 0.4% for groups 1 and 2, respectively. Conclusively, this study showed a young age of ML patients, with female predominance in CML, and poor outcome. This reflected racial, ethnic and risk factor differences in incidence of MLs.
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Myeloid leukemias (MLs) are a heterogeneous group of disorders in which a leukemogenic event disrupts normal hematopoietic stem cell differentiation and enhances its proliferation. These events target myeloid lineage-derived cells and spare the lymphoid lineage cells. Leukemogenesis of MLs was found variable from one subtype to the other; therefore, the exact pathophysiology of MLs is a mystery.Citation1,Citation2 Chromosomal translocations producing a new chimeric protein with novel functions play an important role in the pathogenesis of MLs, as well as activating mutations such as nucleophosmin gene mutation which offers a proliferative advantage to cells.Citation3–Citation5
According to the WHO, MLs include acute myeloid leukemia (AML), chronic myeloid leukemia (CML), chronic eosinophilic leukemia and the hypereosinophilic syndrome, biphenotypic leukemia and myeloid sarcomas (extramedullary MLs). In addition, the WHO designed a specific term for ML in patients with Down syndrome (ML-DS), which is an erythromegakaryoblastic leukemia in a patient ˂ 5 years old.Citation6–Citation8
Research found that the variability of MLs extending from the underlying etiology to incidence and clinical presentation. The unique characteristic of MLs is uncontrolled proliferation of white blood cells (WBCs), with overproduction of immature forms. These cells could aggregate in blood vessels resulting in organ dysfunction in a clinical condition called leukostasis, which is mainly due to hyperviscosity and abnormally shaped WBCs.Citation9,Citation10 Moreover, the clinical presentation of extramedullary affection is markedly variable; erythematous eruptions could proceed development of ML by as long as one year in a condition known as aleukemic leukemia cutis.Citation11
Accurate diagnosis and classification of MLs requires the integration of clinical assessment, peripheral blood analyses, bone marrow aspirate and/or biopsy, immunophenotyping, cytogenetic and molecular analyses. In addition, immunohistochemistry and tissue biopsies with specific stains play an important role in diagnosing ML and differentiating them from lymphoid neoplasm.Citation12
Recent advances in understanding the pathophysiology of MLs led to the emergence of effective targeted therapies; furthermore, the treatment of certain types of MLs could be tailored for each patient. Bone marrow transplantation (BMT) is the hope for many cases with relapsed disease.Citation13,Citation14 This recent approach in the management of MLs is mainly based on cytogenetic and molecular analyses, which are unavailable in many centers worldwide.
The documented worldwide description of incidence, clinical picture, response to treatment, outcome, and survival probability of MLs was mainly dependent on Western registry data.Citation15,Citation16 This study aimed to study MLs in Middle Eastern health centers.
Patients and Methods
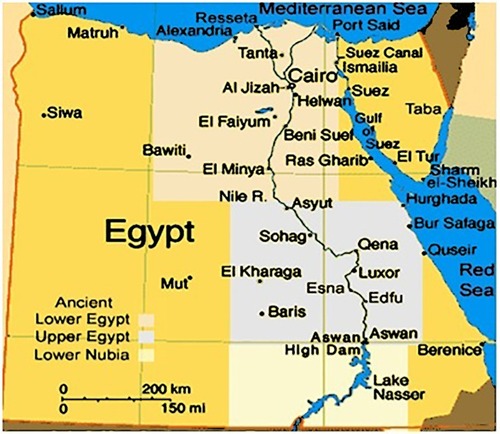
A total of 468 patients with MLs were included in the study. They were retrospectively recruited from patients who were referred and treated at the departments of Internal Medicine and Clinical oncology at Assiut University Hospital (AUH) and the Department of Medical Oncology at South Egypt Cancer Institute (SECI) in the period from January 2007 to November 2011. In order to obtain a proper sample size, another group of patients were collected from the Clinical Hematology Unit at AUH in the period from December 2011 to June 2016. SECI and AUH are tertiary healthcare centers located in Upper Egypt and provide highly specialized medical services to approximately eight Governorates of Egypt, including Assiut, El Minya, Sohag, Luxor, Al Wadi Al Jadeed, Qena, and Aswan and to some extent Red Sea Governorates, as shown in .
Figure 1 Geographic area of the study, from El Minya Governorate in the North to Aswan in the south together with Al Wadi Al Jadeed (El Karaga) in the west and Red Sea in the east.
Patients were grouped into group 1 (CML group), and group 2 (AML). Group 2 was further subdivided into group 2a (de-novo AML) and group 2b (secondary AML).
Sociodemographic, clinical, and data concerned with diagnosis, treatment, therapeutic responses and overall survival (OS) were gathered from patients’ hospital records. Hand-written records were reviewed for older cases from 2007 to 2011; from 2012 to 2016, data were retrieved from computer-based records.
Each patient was included in the study once, even if they was admitted more than one time. Different records for each patient were reviewed to obtain data concerned with response to treatment and OS. Accordingly, a patient diagnosed as CML and transformed to AML during follow-up was included in group 1, as CML in blastic transformation. Those who first presented to our center as AML secondary to CML were included in group 2b as secondary AML with CML considered a preleukemic disorder. Patients who were referred from AUH to SECI were also included once in the study according to first record.
Analysis of the collected data was done with SPSS software, version 17 (SPSS Inc., Chicago). Descriptive data were presented as median, mean and standard deviation (SD) if it is quantitative or as percentages for qualitative variables. The chi-square test was used to estimate association between qualitative variables. The one-way analysis of variance was used to differentiate between the three groups. Kaplan–Meier analysis of survival was used to asses OS. P value ˂0.05 was considered significant.
The study was consistent with the Declaration of Helsinki for medical research. Furthermore, the study protocol was approved by the board sections of the concerned departments and then by the research ethical committee at Faculty of Medicine, Assiut University. Prior permission of the head of the department was obtained before handling patients’ records. However, patients’ written informed consents to review their medical records was not required by the approving ethics committees, as that would be inappropriate due to the retrospective nature of the study, where a considerable proportion of patients stopped follow-up, died, or were living in distant governorates. Confidentiality was insured, when dealing with patients’ records, by substituting patients’ names with research codes and introducing data into a personal computer.
Results
and showed demographic characteristics of the study patients, revealing that 43 years was the median age of the study patients and nearly one-third of patients were in the age range 41–50 years; other peaks were in ranges 21–30 and 51–60 years, the latter for group 1 only. Interestingly, a considerable proportion (15.3%) of AML patients were students whose ages ranged from 17 to 20 years. Another astonishing finding was that females constituted 71.8% of secondary AML. Male predominance was obvious in de-novo AML, while female predominance was in CML and secondary AML; and the male to female ratios were 1.4, 0.8, and 0.4 in order.
Figure 2 Age groups of the study patients. Group 1 = CML patients (n= 226) and group 2 = AML patients (n = 242).
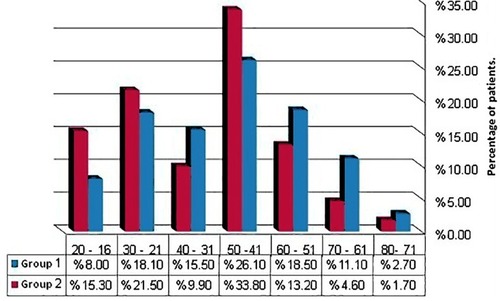
Table 1 Demographic Characteristics of Myeloid Leukemia Patients at SECI and AUH in the Period from January 2007 to December 2016 (Total Number = 468)
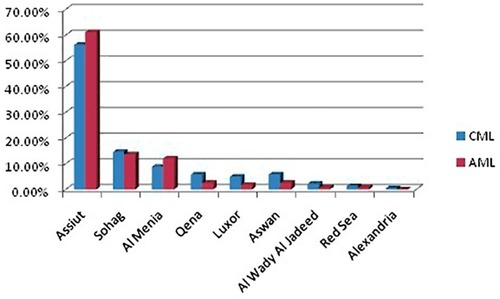
Most of the study patients were from Assiut governorate, 56.2% and 66.1% for groups 1 and 2, respectively. shows the distribution of the study patients over nine governorates of Egypt; one patient was from Alexandria, which is not in the geographic area of the study. As seen in , patients mostly lived in rural areas.
Figure 3 Distribution of the study patients over nine Governorates of Egypt. Group 1 = CML patients (n= 226) and group 2 = AML patients (n = 242).
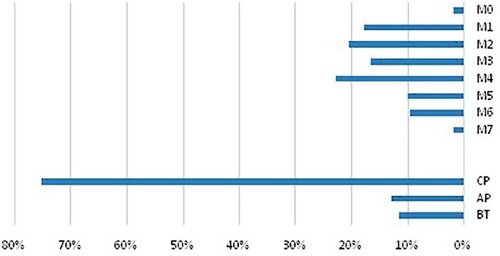
The most common preleukemic disorder in group 2b patients was CML followed by myelodysplastic syndrome (MDS) and lastly myelofibrosis (MF), as shown in . Nearly 3/4 of group 1 patients were in chronic phase. FAB M4, followed by M2 and M1, then M3 were the most prevalent FAB subtypes in group 2 patients as in .
Figure 4 Pleukemic disorder of group 2 patients.
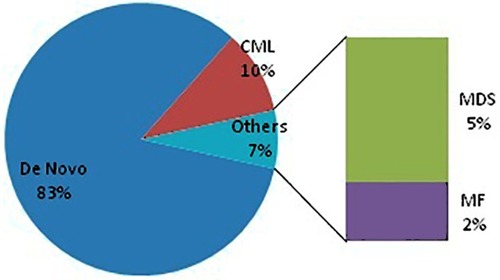
Figure 5 Distribution of leukemia phases and subtypes among the study patients. The upper panel showed subtypes of group 2 patients and the lower panel is phases of group 1 patients.
shows treatment modalities for myeloid leukemia cases in both SECI and AUH in the period from January 2007 to December 2016. It showed that only one-third or more of CML patients treated with tyrosine kinase inhibitors (TKIs) in the form of gleevec in 37.2% and dasatinib in one patient only, while 52% of patients were treated with hydroxyurea as first line. Of AML patients, 13.6% received consolidation therapy after induction and 12.8% were treated with low-dose ara-C.
Table 2 Treatment Modalities for Myeloid Leukemia Patients at SECI and AUH in the Period January 2007–December 2016 (Total Number = 468)
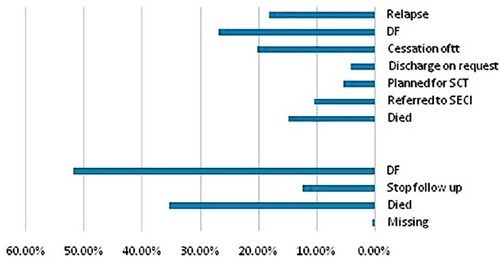
reveals that approximately 53% and 27% were the ORR for groups 1 and 2, respectively, where patients were disease-free until the end of the study. On the other hand, a considerable proportion of patients died during the study period (15% and 35% for groups 1 and 2, respectively). It is noticeable that the fate of more than one-third of group 2 (AML) patients was unknown due to cessation of treatment (20%), referral to SECI (10%), and discharge on request (5%), whereas of group 2 (CML) patients only 12% stopped follow-up, and only one patient was missed. Relapse was a problem in group 2 patients (18%), and only 5% of patients were planned for stem cell transplantation (SCT); however, data on whether the procedure was done or not, response rate or occurrence of any complications were unavailable.
Figure 6 Outcome of the study patients. The lower and upper panels showed outcome of groups 1 and 2, respectively.
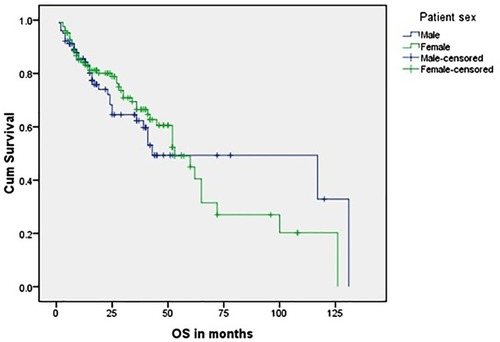
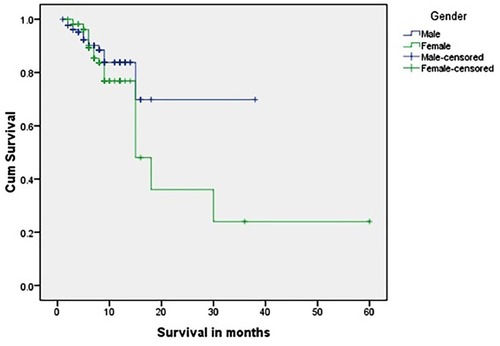
and show the OS of group 1 patients; nearly two-thirds of patients were censored, and the 5-year OS was 7%; 1.3% (three patients only) were still living and continued follow-up for ≥ 10 years. The longest OS was 12 years and 9 months, and there was no statistically significant difference in survival between male and female patients with a mean value 63.8 months.
Table 3 Overall Survival of Group 1 Patients
and show the OS of group 2 patients. Again, there was no significant difference in survival between male and female patients. The mean OS was 31.5 months and the longest OS was 5 years, which was observed in only one patient (0.4%). More than three-fourths of patients were censored.
Table 4 Overall Survival of Group 2 Patients
Discussion
MLs are the type of leukemias that affect WBCs except lymphocytes; red blood cells and platelets can also be affected. Chromosomal translocations and activated mutations offer proliferative advantages to leukemic cells and hinder apoptosis. Peripheral blood analysis, bone marrow aspirate/biopsy, flow cytometry and cytogenetic studies are crucial for the diagnosis of MLs.Citation3,Citation12,Citation17 This work aimed to study MLs in Middle Eastern health centers.
The results of the current study revealed a median age MLs (43 years) and female predominance was obvious for the CML group, most of whom were housewives. The vast majority of patients were from Assiut governorate, rural areas and most of them were farmers and unemployed or low socioeconomic class.
These results were contradictory to some Western studies. In Europe, the first case of ML was detected in a 63-year-old male patient. According to Cancer Research UK, the peak age range for MLs is 85–89 years and females comprised 45% and 44% of CML and AML cases, respectively; similar findings were proved by other European studies.Citation18–Citation21 In USA during 2000–2002, the incidence of AML in those <65 years old was 1.8 per 100,000 persons, while in persons aged ≥65 years it was 17 per 100,000 persons.Citation22 Similar results were obtained in Canada; AML is therefore primarily a disease of later adulthood.Citation23,Citation24 The distribution of the proportion of prevalent cases of all leukemias in the UK shows that 42.8% of patients are >65 years age. Furthermore, patients newly diagnosed with AML were found to have a median age of 65 years. AML is rarely diagnosed before the age of 40 years; thereafter, the incidence increases progressively with age.Citation25 According to SEER cancer statistics in the period from 1975 to 2009, the median age of AML patients at diagnosis was 66−71 years old.Citation26 When considering CML there was a discrepancy between clinical trials and cancer registry data regarding the median age of CML patients at presentation, where it was 50 years in the former and 10 years older in the latter.Citation27
On the contrary, our results were consistent with the results of studies in other Middle Eastern countries; however, male predominance was aberrant in those studies. An exception is a study that was done in United Arab Emirates and reported female predominance in AML.Citation28–Citation32
These differences between Western registry data and the results of the current study could not be explained by differences in diagnosis and data recording; rather, they reflect a true difference in risk factors. Another logical explanation is the shorter life expectancy of Middle Eastern people compared with the Western people. Furthermore, elderly Easterners used to receive care from other family members, unlike Westerners who have to take care of themselves, rely on paid caregivers, or reside in homes for the elderly. These social circumstances make elderly Western people complain more compared with Eastern people.Citation33 Another possible but serious explanation is the deficient knowledge of our patients regarding hematological cancers. In our society, diagnosis with leukemia is associated with fear of untreatable, fatal diseases, and that chemotherapy worsens a patient’s condition. Accordingly, elderly patients refuse referral to tertiary health centers and prefer to die in peace.Citation34,Citation35 The latter assumption was reaffirmed by the obvious distribution of the vast majority of patients at Assiut Governorate where AUH and SECI present. The last explanation would be the possibility of ethnic differences in incidence of MLs.Citation36
As it was indicated in this work, social circumstances and knowledge about MLs of our population judge their behavior in seeking medical advice. In addition, the limited resources in healthcare centers delay diagnosis and treatment of these patients. Accordingly, we assumed that even prolongation of the life expectancy of people living in developed countries will not change the demographic characteristics of ML patients.
The obvious female predominance, in this study, observed in group 1 and group 2b was consistent with other studies and contradictory to others.Citation20,Citation25,Citation26 These females were mostly housewives who lived in rural areas. In Egypt, farmers’ wives used to raise birds such as ducks, chickens, geese and pigeons, they feed them and clean their living places with pesticides and insecticides, and accordingly they keep them near to their houses. Furthermore, farmers used to store insecticides and chemical fertilizers in a room within their homes. These living circumstances showed longer exposure of a housewife to these hazardous chemicals. These findings denoted that environmental pollution is the most important risk factor for MLs in our patients. This was contradictory to Western studies, where exposure to radiation and prior chemotherapy are the most important risk factors for MLs.Citation37–Citation39 On the other hand, our findings reaffirmed the findings of Kachuri et al., who concluded increased incidence of hematopoietic malignancies among agricultural workers and attributed this to overexposure to hazardous chemicals.Citation40
Unlike other studies CML was the most prevalent preleukemic disorder in our patients.Citation20 This could be related to differences in registration of data.
In this study only 37.6% of group 1 patients were treated with TKIs. This was not the case in many Western centres, where TKIs are the mainstay of treatment for CML patients. This difference could be explained by the results of previous study where cost, side effects, and incompliance were the main drawbacks of TKIs in our patients.Citation41 Many researchers found that nearly 89% is the remission rate of patients with CML treated with TKIs.Citation42 In this study only 53% of CML patients achieved remission, these differences were because the current study did not focus on TKIs. When considering group 2 patients only, 13.7% received consolidation therapy, more than half were treated with 3/7 induction alone. Moreover, the outcome of a considerable proportion of the study patients was unknown, mainly due to patient incompliance when presented with cessation of treatment, cessation of follow-up and earlier discharge from the hospital. Assessment of the reasons for incompliance was difficult due to the retrospective nature of the study. Nevertheless, it was apparent from patient characteristics that the main etiology of incompliance is deficient knowledge and awareness of their disease, treatment plan and expected prognosis.
Although SECI and AUH offer health services to people of Upper Egypt without expense or with minimal fees, many of our patients who achieved complete remission did not receive HSCT. This could be attributed to many reasons, the most important being unavailability of matched donors, as stem cell banking from volunteered donors is not available in our locality. Furthermore, HSCT is based on governmental coverage of expenses and requires a lengthy administrative procedure; most of our patients developed complications during this period and died either from bleeding or intractable infections. Another important reason is the limited number of transplantation centers in our locality.Citation43
Visser and colleagues assessed incidence and survival of myeloid neoplasms in Europe, and found the 5-year OS for patients with CML was 62%, and 19% for AML.Citation44 This was markedly higher than in our study, 7% and 0.4%, respectively. These differences may be mainly due to irregular follow-up of our patients where more than three-fourths were censored. Why did our patients not follow-up regularly? Again, the answer would be related to their socioeconomic circumstances that were mentioned before, besides the bad reputation of chemotherapy or even BMT in our patients.Citation45
Conclusions
The results of this study showed a younger median age at presentation of patients with MLs in our centers compared with other studies. Furthermore, the study as proven that chemical and environmental pollution could be major risk factors for MLs in Middle Eastern centers, compared to secondary leukemia after chemotherapy or exposure to radiation in Western ones. Furthermore, this study proved ethnic and racial differences in incidence of MLs. In respect to management, MLs still carry poor prognosis in our locality, which was mainly apparent for AML patients.
In light of this study, we recommended further wide-scale prospective studies to uncover ML risk factors in our region. Additionally, mass health education programs about MLs have to be delivered to the population about risk factors of MLs, encouraging early diagnosis and avoiding underrecording. These programs aimed to improve the image of chemotherapy and give hope to patients in light of recent advances on treatment. Another aim was to encourage normal subjects to donate stem cells and help AML patients who achieved complete remission.
Also we recommended revision of cultivation techniques in our society, particularly preservation and utilization of insecticides and fertilizers.
Acknowledgments
The authors wish to express great thanks and deep gratitude to the HODs of the departments at AUH and SECI who facilitated and permitted accessing patient’s records after understanding the value of this research. Great thanks to Jennifer H. Mesde at Shaqra University for revision of English language in this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Speck NA, Gilliland DG. Core-binding factors in haematopoiesis and leukaemia. Nat Rev Cancer. 2002;2:502–513. doi:10.1038/nrc84012094236
- Mastuno N, Osato M, Yasmashita N, et al. Dual mutations in the AML1 and FLT3genes are assoicated with leukaemogenesis in acute myeloblastic leukaemia of the M0 subtype. Leukaemia. 2003;17:2492–2499. doi:10.1038/sj.leu.2403160
- Ramsey H, Zhang D-E, Richkind K, et al. Fusion of AML1/Runx1to CopineVIII, a novel member of the copine family, in an aggressive acute myelogenous leukaemia with t(12;21)translocation. Leukaemia. 2003;17:1665–1685. doi:10.1038/sj.leu.2403048
- Khaled SA, Burthem J, Abo ElNoor E, et al. Differences between nucleophosmin isoforms in de-novo acute myeloid leukemia: possible implications in developing targeted therapy for acute myeloid leukemia with normal karyotype. Egyptian J Hematol. 2015;40(41):190–194. doi:10.4103/1110-1067.170220
- Khaled SA, Burthem J, Abo ElNoor E, et al. Role of nucleophosmin gene mutation in leukemogenesis of acute myeloid leukemia. J Hematol. 2018;7(1):7–13. doi:10.14740/jh365w
- Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951.19357394
- Chang KL, Weiss LM. Lymph Nodes in Modern Surgical Pathology by Noel Weidner, Richard J. Cote, Saul Suster, and Lawrence M. Weiss (Second Ed.). Elsevier Inc; 2009.
- Najfeld V. Conventional and Molecular Cytogenomic Basis of Hematologic Malignancies in Hematology (Ebook Seventh Edition), 2018. In: Hoffman R, Benz EJ, Jr, Abutalib SA, editors. New York: Elsiever Inc.; 2018.
- Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the haematological malignancy research network. Br J Cancer. 2011;105(11):1684–1692. doi:10.1038/bjc.2011.45022045184
- Cosmai L, Mourizio O. Gallieni, Acute Kidney Injury in Oncology and Tumor Lysis Syndrome in Critical Care Nephrology (Ebook Third Edition). Elsiever Inc; 2019:234–250.
- Jacqueline M, Junkins –hopkins JL, Klaus J, et al. Hematopoietic Neoplasms in Dermatopathology. ebook1sted. Saunders/Elsiever Inc;2010.
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2012 update on diagnosis, monitoring, and management. Am J Hematol. 2012;87:1037–1045. doi:10.1002/ajh.v87.1123090888
- Schlenk RF, Do¨hner K, Kneba M, et al. Gene mutations and response to treatment with all-trans retinoic acid in elderly patients with acute myeloid leukemia. Results from the AMLSG trial AML HD98B. Haematol`ogica. 2009;94(1):54–60.
- Lahaye T, Riehm B, Berger U, et al. Response and resistance in 300 patients with BCR-ABL-positive leukemia treated with imatinib in a single center: a 4.5-year follow up. Cancer. 2005;103:1659–1669. doi:10.1002/cncr.2092215747376
- Deschler B, Lübbert M. Acute myeloid leukemia: epidemiology and etiology cancer. Cancer. 2006;107(9):2099–2107. doi:10.1002/cncr.2223317019734
- Juliusson G, Lazarevic V, Horstedt AS, et al. Acute myeloid leukemia in the real world: why population-based registries are needed. Blood. 2012;119(17):3890–9.29. doi:10.1182/blood-2011-12-37900822383796
- Khaled SA, Burthem J, Abo ElNoor E, et al. The mutated cytoplasmic form of nucleophosmin expressed by the cell line OCI-AML3 is phosphorylated on Thr199: implications for the pathogenesis of primary acute myeloid leukaemia (AML). Available from: https://www.eposters.net/poster/the-mutated-cytoplasmic-form-of-nucleophosmin. Accessed 604, 2019.
- Hehlmann R, Hauchhaus A, Baccarani M. Chronic myeloid leukemia. Lancet. 2007;370:342–350. doi:10.1016/S0140-6736(07)61165-917662883
- Cancer research Uk. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/leukaemia-cml/incidence. Accessed 3, 2019.
- Hulegardh E, Nilsson C, Lazarevic V, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish acute leukemia registry. Am J Hematol. 2015;90(3):208–14.26. doi:10.1002/ajh.2390825421221
- Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. 2012;3:268.27. doi:10.3389/fgene.2012.00268
- Ries LAG EM, Eisner MP, Kosary CL, et al. Edwards BK (Eds). SEER Cancer Statistics Review, 1975–2002. Bethesda, MD:National Cancer Institute; 2005 Available from: https://seer.Cancer.gov/csr/1975_2002/. Based on November 2004 SEER data submission, posted to the SEER website.
- Xie Y, SM D, Xiang Y, et al. Trends in leukemia incidence and survival in the United States (1973–1998). Cancer. 2003;97:2229–2235. doi:10.1002/cncr.1131612712476
- The incidence of acute myeloid leukemia … (PDF Download Available). Available from: https://www.researchgate.net/publication/318990878_The_incidence_of_acute_myeloid_leukemia_in_Calgary_Alberta_Canada_A_retrospective_cohort_study. Accessed 404, 2018.
- Phekoo KJ, Richards MA, Moller H, et al. The incidence and outcome of myeloid malignancies in 2112 adult patients in southeast England. Haematologica. 2006;91(10):1400–4. 26.17018393
- Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish acute leukemia registry. Blood. 2009;113(18):4179–4187. doi:10.1182/blood-2008-07-17200719008455
- Druker BJ, Guilhot F, O’Brien SG. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi:10.1056/NEJMoa06286717151364
- Khan MQ, Shivarudrappa AS, el-Bialy S, et al. Leukemia cases in central Hospital Riyadh (Saudi Arabia). J Indian Med Assoc. 1991;89(2):38–42.2056174
- Azzazi MO, Fahmy O, Mattar M, et al. Second report of arab leukemia net (ALN) registry for chronic myeloid leukemia (CML) in the Middle East &North Africa Region (AFME). Epidemiology of CML and Additional Chromosomal Abnormalities (ACAs) in Egypt, Multicenter Results. Blood. 2014;124(21):5539. doi:10.1182/blood.V124.21.5539.5539
- Hassan IB, Islam SI, Alizadeh H, et al. Acute leukemia among the adult population of United Arab Emirates: an epidemiological study. Leuk Lymphoma. 2009;50(7):1138–1147. doi:10.1080/1042819090291918419557635
- Allahyari A, Tajeri T, Sadeghi M. Prognostic factors and survival in acute myeloid leukemia cases a report from the Northeast of Iran. Asian Pac J Cancer Prev. 2016;17(3):1547–1551. doi:10.7314/APJCP.2016.17.3.154727039804
- Al-Bahar S, Pandita R, Al-Muhannaha A, et al. The epidemiology of leukemia in Kuwait. Leuk Res. 1994;18(4):251–255. doi:10.1016/0145-2126(94)90027-28170169
- Pavlova M. Europe is going grey: can EU countries work together to care for their elderly? Available from: https://theconversation.com/europe-is-going-grey-can-eu-countries-work-together-to-care-for-their-elderly-80416. Accessed 64, 2019.
- Kav S, Tokdemir G, Tasdemir R, et al. Patients with cancer and their relatives beliefs, information needs and information –seeking behaviour about cancer and treatment. Asian Pac J Cancer Prev. 2012;13:6027–6032. doi:10.7314/APJCP.2012.13.12.602723464398
- Taha NM, Zatton HK, Zatton HI. Nurses knowledge and practice regarding educational needs for patients with leukemia. J Nurse Care. 2017;6:406–413.
- Halpern MT, Ward EM, Pavluck AL, et al. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–231. doi:10.1016/S1470-2045(08)70032-918282806
- Smith RE, Bryant J, DeCillis A, et al. National surgical adjuvant breast and bowel project experience. acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the national surgical adjuvant breast and bowel project experience. J Clin Oncol. 2003;21(7):1195–1204. doi:10.1200/JCO.2003.03.11412663705
- Carli PM, Sgro C, Parchin-geneste N, et al. Increase therapy-related leukemia secondary to breast cancer. Leukemia. 2000;14:1014–1017. doi:10.1038/sj.leu.240178710865966
- Van Leeuwen FE. Risk of acute myelogenous leukaemia and myelodysplasia following cancer treatment. Baillieres Clin Haematol. 1996;9:57–85. doi:10.1016/S0950-3536(96)80037-08730551
- Kachuri L, Harris MA, MacLeod JS, et al. Cancer risks in a population based study of 70, 750 agricultural workers : results from the Canadian census of health and environment cohort (CanCHEC). BMC Cancer. 2017;17(1):343. doi:10.1186/s12885-017-3346-x28525996
- Khaled SA. Treatment with tyrosine kinase inhibitors in chronic myeloproliferative neoplasms: pros and cons. Am J Intern Med. 2016;4(4):66–74. doi:10.11648/j.ajim.20160404.12
- Kantarijian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia:2-year follow-up from a randomized phase 3 trial(DASISION). Blood. 2012;119(5):1123–1129. doi:10.1182/blood-2011-08-37608722160483
- Mahmoud H, El-Haddad A, Fahmy O, et al. Hematopoietic stem cell transplantation in Egypt. Bone Marrow Transplant. 2008;42(Suppl 1):S76–S80. doi:10.1038/bmt.2008.13618724311
- Visser O, Trama A, Maynadié M, et al. Incidence, survival, and prevelance of myeloid malignancies in Europe. Eur J Cancer. 2012;48(17):3257–3266. doi:10.1016/j.ejca.2012.05.02422770878
- Zayed HA, Saied SM, El-Sallamy RM, et al. Knowledge, attitudes and practices of safe handling of cytotoxic drugs among oncology nurses in tanta university hospitals. Egypt J Occup Med. 2019;43(1):75–92. doi:10.21608/ejom.2019.25119