Abstract
Purpose
Red blood cell transfusion is a key element of treatment among patients with transfusion-dependent thalassemia (TDT). Volume overload and HCC syndrome (hypertension, convulsion, and intracranial hemorrhage) are fatal complications related to transfusion. Furosemide has been widely used to prevent hypertension secondary to volume overload with unclear supportive evidence. This study aimed to evaluate the efficacy of furosemide to prevent volume overload among children and young adults diagnosed with TDT.
Methods
Patients diagnosed with TDT were enrolled and randomized to receive either furosemide pretransfusion or no furosemide pretransfusion. After 3 weeks to 4 months of wash-out periods, those patients underwent the alternate regimens as per crossover design of the study. Clinical and laboratory parameters including blood pressure and NT-proBNP levels were measured before and after each transfusion. The difference of those parameters between two randomized groups and their potential associated factors were analyzed.
Results
In all, 30 patients undergoing 60 red blood cell transfusions were enrolled in the study. All were randomized and crossover was designed as receiving and not receiving furosemide pretransfusion. No transfusion reactions, symptoms of volume overload and HCC syndrome were observed. No statistically significant correlation was found between pretransfusion furosemide and the difference between pre- and posttransfusion systolic blood pressure (2 mmHg systolic blood pressure difference in pretransfusion furosemide and 1.5 mmHg in no pretransfusion furosemide; p-value = 0.721), as well as between pretransfusion furosemide and the difference between pre- and posttransfusion NT-proBNP levels (−3.8 pg/mL NT-proBNP level difference in pretransfusion furosemide and −2.4 pg/mL in no pretransfusion furosemide; p-value = 0.490). No significant correlation was also observed even in selected patients with high NT-proBNP levels (p-value = 0.262). Associated factors affecting the difference between pre- and posttransfusion NT-proBNP levels were analyzed, and none of those were affected concerning the difference in the levels.
Conclusion
Furosemide has been included in standard transfusion guidelines in many institutions. Our study provided important evidence of the unnecessary use of the drug in preventing volume overload particularly in pediatric and young adult patients with TDT.
Thai Clinical Trials Registry (Tctr) Number
TCTR20180209001. Registered 6 February 2018, https://www.clinicaltrials.in.th/.
Introduction
Thalassemia is a common congenital hemolytic anemia found worldwide. The disease is characterized by genetic mutations of globin genes resulting in impaired hemoglobin synthesis and eventually reduced hemoglobin level. Thalassemia can be genotypically categorized as alpha- and beta-thalassemia depending on types of defective globin genes (alpha versus beta globin genes).Citation1,Citation2 The prevalence of thalassemia trait varies geographically with approximately 40% prevalence in Thailand, including 20 to 30% for alpha-thalassemia and 3 to 9% for beta-thalassemia. The hematologic manifestations of thalassemia are heterogeneous, ranging from an asymptomatic silent carrier with normal clinical findings to patients with severe microcytic anemia requiring regular red blood cell transfusion or those with life-threatening fatal conditions at birth.Citation3 The current classification of thalassemia is generally based on red blood cell transfusion requirement in which the disease can be divided as transfusion-dependent thalassemia (TDT) and nontransfusion dependent thalassemia (NTDT).
Although hematopoietic stem cell transplantation (HSCT) and gene therapy are considered curative treatment for patients with transfusion-dependent type thalassemia, red blood cell transfusion is still considered the key element of care among those patients awaiting HSCT and gene therapy as well as the remaining patients ineligible for curative treatment.Citation4,Citation5 Transfusion strategies in treating thalassemia are generally divided into two types. First, occasional transfusions involve red blood cell transfusion given irregularly at a dose of 10 to 15 mL/kg when patients develop anemic symptoms and have hemoglobin level less than 7 g/dL. Second, frequent transfusion involves red blood cell transfusion given regularly to maintain high pretransfusion hemoglobin levels of 9 to 10 g/dL with the aim to suppress erythropoiesis, decrease iron absorption from the gastro-intestinal tract and eradicate anemic symptoms as well as their complications.Citation6 Unlike patients receiving an occasional transfusion, most patients undergoing frequent transfusion will have a normal level of activity, appropriate growth for their age as well as normal facial architecture and lower likelihood of requiring splenectomy.Citation6 These findings have resulted in the dramatic improvement of quality of life among these patients and eventually, their longevity.
However, transfusion-related complications anticipated among most patients with thalassemia receiving regular and frequent transfusions include iron overload, volume overload and HCC syndrome (hypertension, convulsion, and intracranial hemorrhage).Citation7–Citation9 Transfusion-associated circulatory overload (TACO) is a potential fatal transfusion-related complication leading to significant morbidity and mortality.Citation10 Classical clinical presentations of TACO include dyspnea and features of fluid overload. Although the pathophysiology of this condition has been unclear, inappropriate management of patient’s volume status, preexisting cardiac and/or renal dysfunctions, numbers of red blood cell product transfused and an extreme of age are associated with increased risk of TACO.Citation11–Citation13 HCC syndrome is a transfusion-related complication and can be found among patients with thalassemia requiring either occasional, regular or frequent transfusion regimens.Citation14–Citation21 The syndrome has been found to be associated with prolonged chronic anemia; however, posttransfusion hypertension is more common and can be found in 16.7% of patients with beta thalassemiaCitation22 in which some patients reported headaches or even developed seizures.Citation23 Iron chelation has been well studied and effectively used to manage iron overload. Unlike iron overload, the standard treatment and prevention of volume overload and HCC syndrome have unfortunately been unclear.
Furosemide is a loop diuretic and has been used to treat volume overload and high blood pressure.Citation24 The mechanism of action of furosemide is similar to other loop diuretics, ie, inhibiting the luminal Na+, K+, Cl2- cotransporter at the thick ascending segment of the loop of Henle causing inhibition of Na+, K+, Cl2- re-absorption and resulting in those electrolytes lost in urine. The onset of action of intravenous furosemide is 5 minutes with the peak effect of 30 minutes and duration of action of 2 hours.Citation25 Indications for the practical use of furosemide include acute pulmonary edema, chronic congestive heart failure, hypertension and hyperkalemia.Citation26,Citation27 The medication has been used in many institutions as a premedication to supplement red blood cell transfusion to promote diuresis and prevent high blood pressure secondary to volume overload as well as HCC syndrome among transfusion-dependent thalassemia (TDT) patients.Citation28,Citation29 The role of furosemide in preventing or treating hypertension secondary to red blood cell transfusion among patients with TDT was explored in several studies; however, the results remain controversialCitation7–Citation9,Citation30 and its use varies among institutions.Citation31–Citation33 In addition, several side effects of furosemide have been reported in the literature including hypotension, hyperuricemia, hypokalemia, nephrotoxicity and ototoxicity which make the determination of the real clinical benefits of this drug truly necessary.Citation24,Citation34–Citation36
N-terminal prohormone of brain natriuretic peptide (NT-proBNP or BNPT) is a biomarker in the blood used to screen, diagnose, and monitor heart failure.Citation37,Citation38 This biomarker can be measured by rapid assay technique called fluorescence immunoassay. The key stimulus for increased synthesis and secretion of NT-proBNP is myocardial wall tension which could lead to abnormal left ventricular systolic ejection fraction and ventricular diastolic function.Citation39 Elevated plasma concentration of NT-proBNP is typically found among patients with asymptomatic or symptomatic heart failure. Given its half-life of 120 minutes, this biomarker represents an immediate effect of myocardial wall stress and has been widely used to diagnose and determine severity of heart failure.Citation39 The appropriate cut-off values for NT-proBNP to differentiate dyspnea secondary to heart failure from dyspnea secondary to other causes differ depending on patients’ age ranges.Citation37,Citation40,Citation41 The sensitivity and specificity of this biomarker in predicting heart failure are 90 and 84%, respectively, with a negative predictive value of 98% using the level less than 300 pg/mL to exclude heart failure.
Herein, we investigated the clinical usefulness of furosemide to prevent volume overload in children and young adults with a diagnosis of TDT using clinical and laboratory parameters. This is the first study to use NT-proBNP to assess cardiovascular volume status pre- and posttransfusion among children and young adults with TDT.
Methods
Patient Selection
Thirty TDT patients who attended the Hematology Clinic, Department of Pediatrics, Phramongkutklao Hospital from February 14, 2018 to February 13, 2019 were enrolled in this study. Written informed consent and assent forms to take part in the study were obtained from all participants including the children and young adults themselves as well as their parents or legal guardians before engaging in the study. This randomized, open-label, pre/post, crossover trial was approved by the Institutional Review Board, Royal Thai Army Medical Department according to the ethical principles of the Declaration of Helsinki (1975) and its revision (reference number: R012h/57). The study was also registered and approved by the Thai Clinical Trials Registry (TCTR20180209001). The study’s inclusion criteria included patients with TDT aged between 1 to 25 years undergoing treatment at the Phramongkutklao Hospital. Patients contraindicated for furosemide such as those with signs or symptoms of dehydration, a history of sulfonamide allergy, baseline systolic blood pressure more than 90th percentile for their age, underlying epilepsy or a history of intracranial pathology, heart disease or chronic kidney disease and patients who had taken oral furosemide, other diuretics, anti-hypertensive drugs, anti-platelets or anti-coagulants were excluded from the study. The aim of this study was to evaluate the efficacy of furosemide to prevent volume overload in children and young adults with TDT. Although the study’s actual endpoint was the occurrence of volume overload and HCC syndrome, systolic blood pressure and NT-proBNP levels were used as surrogate markers to assess cardiovascular volume status instead according to ethical consideration, given the high mortality of the complications.
Randomization Strategy and Crossover Design
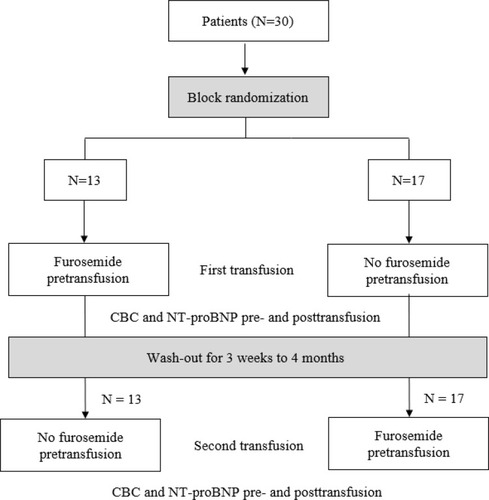
The study adhered to CONSORT guidelines. Nonblinded block randomization was employed in this study as shown in the flow diagram in . After informed consent and assent were obtained, participating patients were randomized to receive two different red blood cell transfusion regimens based on the time-points of furosemide administration. Patients randomized to receive the first transfusion regimen (furosemide pretransfusion) were given furosemide as premedication at 1 hour before red blood cell transfusion. Since furosemide is practically provided for children and young adults with TDT in Thailand including our institution in order to prevent hypertension, volume overload and HCC syndrome, it was unethical to have an experimental group of patients not receiving furosemide. Therefore, those patients who were randomized to receive the second transfusion regimen (no furosemide pretransfusion) were then given furosemide at the end of study (4 hours after the initiation of transfusion) after clinical assessments and laboratory testing were obtained.
Crossover design was implemented for the second red blood cell transfusion as shown in in which the interval between first and second transfusion ranged from 3 weeks to 4 months (wash-out periods). Patients who were initially randomized to receive furosemide pretransfusion on the first transfusion then ”crossed-over” to receive no furosemide pretransfusion but were given furosemide at the end of study due to the ethical consideration described previously. On the other hand, those patients initially randomized to receive no furosemide pretransfusion, were then given furosemide as premedication on the second red blood cell transfusion. Our pediatric nurses served as the third party who administered furosemide, performed red blood cell transfusion, drew blood and monitored patients during their participation for the study’s duration.
Red Blood Cell Transfusion Regimen and Clinical Monitoring Procedures
Red blood cell transfusions were administered to all participating patients in our daycare unit in the form of either leukocyte depleted packed red blood cell (LDPRC) or leukocyte poor packed red blood cell (LPRC) according to each individual patient’s transfusion protocol. The institutional guideline for red blood cell transfusion dosing was calculated based on the degree of anemia in each individual patient. Red blood cell transfusion dosing of 10 mL/kg, 12 mL/kg and 15 mL/kg were administered to children and young adults with a diagnosis of TDT who had pretransfusion hematocrit more than 30, 25 to 30 and less than 25%, respectively. The total duration of transfusion was 4 hours. Vital signs (body temperature, heart rate, respiratory rate and blood pressure) and pulse oximetry were measured before initiating red blood cell transfusion and repeated every 15 minutes during the first hour of transfusion, every 30 minutes during the second hour of transfusion and continued hourly thereafter. In addition, level of consciousness and other transfusion-related complications including headache, seizure, signs and symptoms of volume overload and other transfusion reactions were closely monitored and recorded for the entire duration of transfusion. In the case of high blood pressure observed during red blood cell transfusion, furosemide at the dose of 1 mg/kg/dose with the maximum dosing of 40 mg would be administered intravenously. If the blood pressure was still elevated, patients would then be hospitalized and treated according to standard hypertensive guidelinesCitation42 until hypertension subsided.
Measurement of NT-proBNP and Other Laboratory Parameters
Laboratory tests included complete blood count (CBC) and NT-proBNP performed on all enrolled patients at 1 hour before initiating red blood cell transfusion and repeated at the end of transfusion (4 hours after the start of transfusion). NT-proBNP levels were measured using the quantitative Elecsys proBNP II assay on Elecsys and Cobas e immunoassay analyzers (Roche Diagnostics, 2019) in which the proBNP II CalSet was used to calibrate the assay according toRoche Diagnostics’ protocol. According to the study from Delaporta et al,Citation43 the average NT-proBNP levels among patients with thalassemia major with and without cardiac hemochromatosis were 185.1±78.0 and 128.9±20.2 pg/mL, respectively. Therefore, the cut-off NT-proBNP value of 129 pg/mL was used in our study.
Furosemide Administration Regimen
Furosemide dose was calculated based on patient’s weight and administered by our pediatric nurses at 1 mg/kg/dose intravenously with the maximal dosing of 40 mg either before red blood cell transfusion (furosemide pretransfusion regimen) or at the end of transfusion (no furosemide pretransfusion regimen) depending on the study’s randomization.
Statistical Analysis
The randomized, open-label, crossover study was designed to accrue 26 patients with 10% estimated drop out, providing 90% power to detect different risks of NT-proBNP of 185.1 to 128.9 pg/mL at the two-sided level of significance. Baseline values of selected variables were analyzed and presented as mean with standard deviation (SD) or median (range) for continuous variables and calculated using frequency and percentage for categorical variables. Comparison between two independent datasets was analyzed using Mann–Whitney nonparametric test. Univariate and multivariate analyses were performed using logistic regression to analyze the associated factors affecting the difference between pre- and posttransfusion NT-proBNP levels. Statistical Package for the Social Science (SPSS), Version 23 Software (IBM, NY, USA) was used and a p-value <0.05 was considered statistically significant.
Results
Patient Characteristics
Patient characteristics including age, sex, thalassemia type, red blood cell transfusion requirement, type of red blood cell product, iron status and iron chelation requirement are summarized in . Most participating patients were primary school aged, nonsplenectomized and started on red blood cell transfusion at earlier ages. Males were more predominant than females at a ratio of 2:1. All patients with thalassemia were transfusion-dependent in which hemoglobin E/β-thalassemia was the most common diagnosis followed by AE Bart’s disease, homozygous β-thalassemia and hemoglobin H disease, by rank. Their average ferritin levels were above 1000 ng/mL and most patients required iron chelation. None of the patients experienced transfusion-related complications including transfusion reactions and HCC syndrome.
Table 1 Patients’ Demographic Data
Determination of Sufficient Red Blood Cell Transfusion
To evaluate the adequacy of red blood cell transfusion among all participating patients, hemoglobin levels before and at the end of red blood cell transfusion sessions were collected and analyzed. The median increase of hemoglobin levels was at 2.45 g/dL as shown in , which differed significantly compared with pretransfusion levels with p-value <0.001.
Table 2 Difference of Pre- and Posttransfusion Hemoglobin Levels
Correlation Between Pretransfusion Furosemide and Systolic Blood Pressure
Vital signs were measured and recorded before red blood cell transfusion and during the transfusion according to our institutional guideline described previously. Systolic blood pressure measured at pre- and posttransfusion among patients receiving furosemide as premedication before red blood cell transfusion and those not receiving furosemide before transfusion was analyzed as shown in . No statistically significant difference was found of systolic blood pressure at pretransfusion (median systolic blood pressure of 107.5 mmHg in pretransfusion furosemide and 100 mmHg in no pretransfusion furosemide; p-value = 0.122), posttransfusion (median systolic blood pressure of 106.5 mmHg in pretransfusion furosemide and 104.5 mmHg in no pretransfusion furosemide; p-value = 0. 223) as well as any difference between pre- and posttransfusion blood pressures (median systolic blood pressure difference of 2 mmHg in pretransfusion furosemide and 1.5 mmHg in no pretransfusion furosemide; p-value = 0.721) between those two groups of patients.
Table 3 Effect of Pretransfusion Furosemide on Systolic Blood Pressure and NT-proBNP
Correlation Between Pretransfusion Furosemide and NT-proBNP Levels
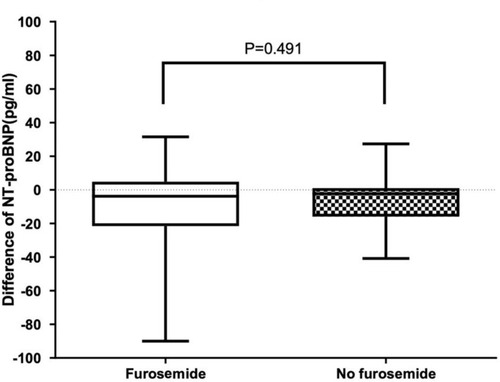
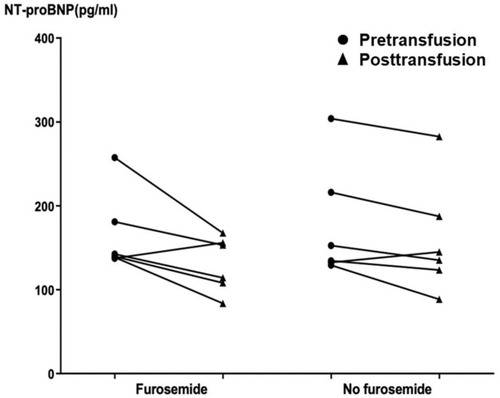
NT-proBNP levels obtained at pre- and posttransfusion among patients receiving furosemide as premedication before red blood cell transfusion and those not receiving furosemide before transfusion were analyzed as shown in and . No significant difference was found of NT-proBNP levels at pretransfusion (median NT-proBNP level of 70.2 pg/mL in pretransfusion furosemide and 64.5 pg/mL in no pretransfusion furosemide; p-value = 0.550), posttransfusion (median NT-proBNP level of 61.2 pg/mL in pretransfusion furosemide and 64.7 pg/mL in no pretransfusion furosemide; p-value = 0.590) as well as the difference between pre- and posttransfusion levels (median NT-proBNP level difference of −3.8 pg/mL in pretransfusion furosemide and −2.4 pg/mL in no pretransfusion furosemide; p-value = 0.490) between those two groups of patients. In addition, six patients who had high NT-proBNP levels, above 129 pg/mL according to the report from Delaporta et alCitation43 described previously, were selected and analyzed. Interestingly, still no significant difference was found of NT-proBNP levels at pretransfusion (median NT-proBNP level of 141.1 pg/mL in pretransfusion furosemide and 143.5 pg/mL in no pretransfusion furosemide; p-value = 0.631), posttransfusion (median NT-proBNP level of 133.7 pg/mL in pretransfusion furosemide and 140.0 pg/mL in no pretransfusion furosemide; p-value = 0.522) as well as the difference between pre- and posttransfusion levels (median NT-proBNP level difference of −30.0 pg/mL in pretransfusion furosemide and −2.4 pg/mL in no pretransfusion furosemide; p-value = 0.262) among those selected patients with high NT-proBNP levels when they underwent red blood cell transfusion with and without furosemide administered pretransfusion ( and ).
Associated Factors Affecting the Difference Between Pre- and Posttransfusion NT-proBNP Levels
Potential relevant factors correlated with the difference of pre- and posttransfusion NT-proBNP levels from all participating patients with thalassemia were examined using univariate analysis as shown in . Interestingly, patient’s age and total duration of red blood cell transfusion were found to be influential factors affecting the difference between pre- and posttransfusion NT-proBNP levels. However, multivariate analysis was used, revealing no associated factors affecting any differences of those levels.
Table 4 Factors Affecting the Difference Between Pre- and Posttransfusion NT-proBNP Levels
Discussion
Overt cardiac disease including heart failure can be found in 6.9% of patients with thalassemia majorCitation44 and heart failure can present at any time after the age of 10 years. Circulatory overload and HCC syndrome are fatal complications anticipated among most patients with TDT and highly associated with significant morbidity and mortality. According to the Thalassemia International Federation (TIF) guidelines for the management of transfusion dependent thalassemia,Citation45 furosemide infusion is indicated for alleviating congestive symptoms of acute heart failure in patients with TDT; however, prophylactic strategies for volume overload and HCC syndrome using loop diuretics premedication prior to or during transfusions are routinely performed in many institutions worldwide with insufficient supportive evidence. It was poorly understood how single pre-transfusion furosemide has an impact on HCC syndrome, which could occur as late as two weeks after transfusion. However, pathophysiology of HCC syndrome in TDT patients is believed to be from accumulated effect of patient’s elevated baseline intravascular pressure during each repetitive cycle of red blood cell transfusion. The syndrome could then be aggravated by blood pressure surge during red blood cell transfusion while patients are in hospital or with high-risk physical activities/exercises while patients are outside of hospital. The surge of blood pressure could result in elevated intracranial pressure and eventually lead to headache or convulsion.Citation46 Therefore, a single pre-transfusion dose of furosemide administered prior to each repetitive cycle of red blood cell transfusion is believed to lower the patient’s baseline intravascular pressure and minimize the likelihood of development of HCC syndrome.
However, loop diuretics are presently not recommended as first-line treatment in current hypertension guidelines largely due to the lack of outcome data.Citation47 In addition, several side effects of loop diuretics have been reported which make the determination of the real therapeutic advantage of these drugs truly necessary.Citation24,Citation34–Citation36 Herein, we evaluated the clinical benefits of furosemide to prevent volume overload among children and young adults with TDT. Because most of the transfusion practices and guidelines in Thailand including our institution require furosemide to be administered before red blood cell transfusion in all thalassemia cases, participating patients not receiving furosemide as premedication before transfusion would have the medication given at the end of the study (4 hours after the initiation of transfusion) instead. A wash-out period was required among all patients depending on their actual transfusion requirements before switching to another furosemide regimen according to the crossover design of the study. Thirty TDT patients with different globin gene mutations were enrolled in this study and randomized to receive either pre- or posttransfusion furosemide. A total of 60 red blood cell transfusions were analyzed in this study. Adequate red blood cell transfusion was affirmed by having significant elevation of posttransfusion hemoglobin. None of the patients experienced transfusion-related complications including transfusion reactions and HCC syndrome. Clinical assessments and laboratory parameters including NT-proBNP level were performed and recorded. Given the half-life of 120 minutes which represents an immediate effect of myocardial wall stress,Citation39 NT-proBNP level was obtained at 4 hours after starting the transfusion. Interestingly, no significant differences were observed in clinical and laboratory parameters associated with volume overload consisting of systolic blood pressure and NT-proBNP levels between patients receiving furosemide before red blood cell transfusion and those who did not. In addition, patients at risk, with high NT-proBNP levels, were chosen and re-analyzed. Again, no significant differences were found in NT-proBNP levels among selected patients (N = 7) receiving furosemide before transfusion and those who did not. 48of 7 patients. According to the study from Menis et al,Citation49 the risk of TACO was correlated with the number of red blood cell units transfused with odds ratio (OR) of 2.00 for 2–4 red blood cell units, OR of 3.10 for 5–19 red blood cell units and OR of 3.55 for more than 9 red blood cell units. However, potential associated factors affecting the difference between pre- and posttransfusion NT-proBNP levels including total duration of red blood cell transfusion were analyzed and none of those factors affected any difference in the levels.
This is the first study to use non-invasive surrogate markers including systolic blood pressure and NT-proBNP to assess cardiovascular volume status pre- and posttransfusion among children and young adults with TDT. According to the results from this study, no real evidence was found of clinical benefits of furosemide administered before red blood cell transfusion to prevent volume overload among children and young adults with TDT, given no significant differences of pre- and posttransfusion systolic blood pressure as well as NT-proBNP levels. These results are consistent with the systematic review of Sarai and Tejani,Citation29 which reported no sufficient evidence to support therapeutic advantages of prophylactic furosemide to prevent transfusion-related morbidity. Moreover, the medication also failed to prevent hypertension during perioperative splenectomy in thalassemic children.Citation30 Besides patients diagnosed with TDT, minimal clinical benefits of pretransfusion administration of furosemide were also observed in preterm infants.Citation48 Therefore, revising the transfusion guideline should be considered to eliminate the risks of developing serious side effects from this medication by omitting furosemide used as premedication in patients with low risk thalassemia with normal cardiac function and preserving the use of this drug only for patients at high risk such as those with cardiac iron overload or a history of transfusion-associated circulatory overload (TACO) or HCC syndrome.
The limitation of this study included small sample size of participating patients which might have resulted in no significant differences found among clinical and laboratory parameters between patients receiving furosemide as premedication before red blood cell transfusion and those who did not. Therefore, the small patient numbers might preclude a final conclusion on the effect of pre-transfusion furosemide on NT-proBNP levels and blood pressure. In addition, more comprehensive and precise measurements of volume overload such as pulmonary capillary wedge pressure, extravascular lung water index or pulse contour cardiac output (PiCCO) system were not performed and used in this study since those measurements were invasive and associated with serious complications. Moreover, this study could not have an experimental group of patients not receiving furosemide since this diuretic has been routinely used for children and young adults with TDT in Thailand including our institution in order to prevent hypertension, volume overload and HCC syndrome. Therefore, it was unethical to have a group of patients truly not receiving furosemide. Thus, those patients who were randomized to receive no furosemide pretransfusion were then given furosemide at the end of study after clinical assessments and laboratory testing were obtained.
Conclusion
Thalassemia disease has been considered one of the major global health concerns especially in developing countries where their abilities to control and treat the disease are restricted due to a lack of general knowledge of the disease’s true prevalence, and limited financial support.Citation50,Citation51 More understanding of pathophysiology of the disease and its complications is important because treatment-related side effects could be avoided thereby optimizing economic burdens nationally by securing budget cuts from unnecessary treatment with no real evidence of clinical benefits and allocating those funds to essential treatment. Although the use of furosemide is included in the standard transfusion practices and guidelines in many institutions, our study provided important evidence of the unnecessary use of this drug in preventing volume overload particularly in pediatric patients with TDT. Due to the study’s limitations previously described, further well-designed and constructed study with a larger sample size in patients with broader age ranges is required to affirm the role of furosemide in this specific indication.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available since these datasets are being used in a different ongoing study; however, the datasets are available from the corresponding author on reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
Funding from the Phramongkutklao College of Medicine and Hospital, Royal Thai Army was used to conduct the study, analyze and interpret the study results and submit the study for publication.
Disclosure
All authors report no conflicts of interest in this work.
References
- Martin A, Thompson AA. Thalassemias. Pediatr Clin North Am. 2013;60(6):1383–1391. doi:10.1016/j.pcl.2013.08.00824237977
- Marengo-Rowe AJ. The thalassemias and related disorders. Proc (Bayl Univ Med Cent). 2007;20(1):27–31. doi:10.1080/08998280.2007.1192823017256039
- Baldini M. Thalassemia major: the present and the future. N Am J Med Sci. 2012;4(3):145–146.22454829
- Olivieri NF, Brittenham GM. Management of the thalassemias. Cold Spring Harb Perspect Med. 2013;3(6):a011767–a011767. doi:10.1101/cshperspect.a01176723732853
- Chonat S, Quinn CT. Current standards of care and long term outcomes for thalassemia and sickle cell disease. Adv Exp Med Biol. 2017;1013:59–87.29127677
- Cappellini M, Cohen A, Eleftheriou A, Piga A, Porter J, Taher A. Guidelines for the Clinical Management of Thalassaemia [Internet]. Thalassaemia International Federation TIF; 2008.
- Suddock JT, Crookston KP. Transfusion Reactions. Treasure Island (FL): StatPearls; 2020.
- Sahu S, Hemlata VA, Verma A. Adverse events related to blood transfusion. Indian J Anaesth. 2014;58(5):543–551. doi:10.4103/0019-5049.14465025535415
- Muschinske D. The nonwhite as child: G. Stanley Hall on the education of nonwhite peoples. J Hist Behav Sci. 1977;13(4):328–336. doi:10.1002/1520-6696(197710)13:4<328::AID-JHBS2300130405>3.0.CO;2-H336680
- Lieberman L, Maskens C, Cserti-Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(4):206–212. doi:10.1016/j.tmrv.2013.07.00224075097
- Alam A, Lin Y, Lima A, Hansen M, Callum JL. The prevention of transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(2):105–112. doi:10.1016/j.tmrv.2013.02.00123465703
- Klanderman RB, Bosboom JJ, Maas AAW, et al. Volume incompliance and transfusion are essential for transfusion-associated circulatory overload: a novel animal model. Transfusion. 2019;59(12):3617–3627. doi:10.1111/trf.1556531697425
- Murphy EL, Kwaan N, Looney MR, et al. Risk factors and outcomes in transfusion-associated circulatory overload. Am J Med. 2013;126(4):357 e29–357 e38. doi:10.1016/j.amjmed.2012.08.019
- Constantopoulos A, Matsaniotis N. Hypertension, convulsion, and cerebral haemorrhage in thalassaemic patients after multiple blood transfusions. Helv Paediatr Acta. 1980;35(3):269–271.7410113
- Incorpora G, Di Gregorio F, Romeo MA, Pavone P, Trifiletti RR, Parano E. Focal neurological deficits in children with beta-thalassemia major. Neuropediatrics. 1999;30(1):45–48. doi:10.1055/s-2007-97345710222462
- Masood SA, Zaidi A. Post-transfusion hypertension, convulsion and intracranial haemorrhage in beta-thalassemia major. J Coll Physicians Surg Pak. 2012;22(7):473–475.22747874
- Sonakul D, Fucharoen S. Brain pathology in 6 fatal cases of post-transfusion hypertension, convulsion and cerebral hemorrhage syndrome. Southeast Asian J Trop Med Public Health. 1992;23(Suppl 2):116–119.
- Wasi P, Na-Nakorn S, Pootrakul P, Sonakul D, Piankijagum A, Pacharee P. A syndrome of hypertension, convulsion, and cerebral haemorrhage in thalassaemic patients after multiple blood-transfusions. Lancet. 1978;2(8090):602–604. doi:10.1016/S0140-6736(78)92824-680527
- Wiwanitkit V. Brain pathology in a syndrome of hypertension, convulsion, and cerebral haemorrhage in thalassaemic patients after multiple blood-transfusions: a summary in reported Thai autopsy cases. J Hypertens. 2006;24(3):601. doi:10.1097/01.hjh.0000203844.49561.fd16467665
- Chuansumrit A, Isarangkura P, Hathirat P, Thirawarapan S. A syndrome of post-transfusion hypertension, convulsion and cerebral hemorrhage in beta-thalassemia Hb E disease: a case report with high plasma renin activity. J Med Assoc Thai. 1986;69(Suppl 2):1–5.
- Gurgey A, Kalayci O, Gumruk F, Cetin M, Altay C. Convulsion after blood transfusion in four beta-thalassemia intermedia patients. Pediatr Hematol Oncol. 1994;11(5):549–552. doi:10.3109/088800194091416947826850
- Tabatabaie M, Hooman N, Arjmandi-Rafsanjani K, Isa-Tafreshi R. Ambulatory blood pressure monitoring for children with beta-thalassemia major: a preliminary report. Iran J Kidney Dis. 2013;7(4):299–303.23880807
- Thirawarapan SS, Snongchart N, Fucharoen S, Tanphaichitr VS, Dhorranintra B. Study of mechanisms of post-transfusion hypertension in thalassaemic patients. Southeast Asian J Trop Med Public Health. 1989;20(3):471–478.2699089
- Oh SW, Han SY. Loop diuretics in clinical practice. Electrolyte Blood Press. 2015;13(1):17–21.26240596
- Ponto LL, Schoenwald RD. Furosemide (frusemide). A pharmacokinetic/pharmacodynamic review (Part I). Clin Pharmacokinet. 1990;18(5):381–408.2185908
- Reubi FC. Clinical use of furosemide. Ann N Y Acad Sci. 1966;139(2):433–442. doi:10.1111/j.1749-6632.1966.tb41217.x5230286
- Ellison DH, Felker GM, Ingelfinger JR. Diuretic Treatment in Heart Failure. N Engl J Med. 2017;377(20):1964–1975. doi:10.1056/NEJMra170310029141174
- Nand N, Gupta MS, Sharma M. Furosemide supplemented blood transfusion in cases of chronic severe anemia. Jpn Heart J. 1986;27(2):177–182. doi:10.1536/ihj.27.1773723795
- Sarai M, Tejani AM. Loop diuretics for patients receiving blood transfusions. Cochrane Database Syst Rev. 2015;(2):CD010138.25685898
- Suwanchinda V, Pirayavaraporn S, Yokubol B, Tengapiruk Y, Laohapensang M, Udomphunthurak S. Does furosemide prevent hypertension during perioperative splenectomy in thalassemic children? J Med Assoc Thai. 1995;78(10):542–546.8576661
- Siddon AJ, Kenney BC, Hendrickson JE, Tormey CA. Delayed haemolytic and serologic transfusion reactions: pathophysiology, treatment and prevention. Curr Opin Hematol. 2018;25(6):459–467. doi:10.1097/MOH.000000000000046230124474
- Py JY, Cabezon B, Sapey T, Jutant T. Unacknowledged adverse transfusion reactions: are they a mine to dig? Transfus Clin Biol. 2018;25(1):63–72. doi:10.1016/j.tracli.2017.06.01628690037
- Carman M, Uhlenbrock JS, McClintock SM. CE: a review of current practice in transfusion therapy. Am J Nurs. 2018;118(5):36–44. doi:10.1097/01.NAJ.0000532808.81713.fc
- Joannidis M, Klein SJ, Ostermann M. 10 myths about frusemide. Intensive Care Med. 2019;45(4):545–548.30643933
- Carone L, Oxberry SG, Twycross R, Charlesworth S, Mihalyo M, Wilcock A. Furosemide. J Pain Sympt Manag. 2016;52(1):144–150. doi:10.1016/j.jpainsymman.2016.05.004
- Greenberg A. Diuretic complications. Am J Med Sci. 2000;319(1):10–24. doi:10.1016/S0002-9629(15)40676-710653441
- Mir TS, Marohn S, Laer S, Eiselt M, Grollmus O, Weil J. Plasma concentrations of N-terminal pro-brain natriuretic peptide in control children from the neonatal to adolescent period and in children with congestive heart failure. Pediatrics. 2002;110(6):e76. doi:10.1542/peds.110.6.e7612456943
- Wu Y-R, Chen S-B, Huang M-R, Zhang Y-Q, Sun K, Chen S. N-terminal pro-brain natriuretic peptide in the diagnosis of congestive heart failure in pediatric patients with ventricular septal defect. World J Pediatr. 2006;1:40–44.
- Weber M, Mitrovic V, Hamm C. B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide - diagnostic role in stable coronary artery disease. Exp Clin Cardiol. 2006;11(2):99–101.18651043
- Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89(8):875–878. doi:10.1136/heart.89.8.87512860862
- Raymond I, Groenning BA, Hildebrandt PR, et al. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003;89(7):745–751. doi:10.1136/heart.89.7.74512807847
- Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140(3):e20171904. doi:10.1542/peds.2017-190428827377
- Delaporta P, Kattamis A, Apostolakou F, et al. Correlation of NT-proBNP levels and cardiac iron concentration in patients with transfusion-dependent thalassemia major. Blood Cells Mol Dis. 2013;50(1):20–24. doi:10.1016/j.bcmd.2012.09.00223017692
- Aessopos A, Farmakis D, Hatziliami A, et al. Cardiac status in well-treated patients with thalassemia major. Eur J Haematol. 2004;73(5):359–366. doi:10.1111/j.1600-0609.2004.00304.x15458515
- Cappellini MD, Cohen A, Porter J, Taher A, Viprakasit V. Guidelines for the Management f Transfusion Dependent Thalassemia (TDT). 3rd ed. Thalassaemia International Federation; 2014.
- Gasparini S, Ferlazzo E, Sueri C, et al. Hypertension, seizures, and epilepsy: a review on pathophysiology and management. Neurol Sci. 2019;40(9):1775–1783. doi:10.1007/s10072-019-03913-431055731
- Malha L, Mann SJ. Loop diuretics in the treatment of hypertension. Curr Hypertens Rep. 2016;18(4):27. doi:10.1007/s11906-016-0636-726951244
- Balegar VK, Kluckow M. Furosemide for packed red cell transfusion in preterm infants: a randomized controlled trial. J Pediatr. 2011;159(6):913–8 e1. doi:10.1016/j.jpeds.2011.05.02221784442
- Menis M, Anderson SA, Forshee RA, et al. Transfusion-associated circulatory overload (TACO) and potential risk factors among the inpatient US elderly as recorded in Medicare administrative databases during 2011. Vox Sang. 2014;106(2):144–152. doi:10.1111/vox.1207023848234
- Williams TN, Weatherall DJ. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med. 2012;2(9):a011692. doi:10.1101/cshperspect.a01169222951448
- De Sanctis V, Kattamis C, Canatan D, et al. Beta-thalassemia distribution in the old world: an ancient disease seen from a historical standpoint. Mediterr J Hematol Infect Dis. 2017;9(1):e2017018. doi:10.4084/mjhid.2017.01828293406