Abstract
The rollout of the SARS-CoV-2 vaccine is underway, and millions have already been vaccinated. At least 25 reports of “immune thrombocytopenia” (ITP) or “thrombocytopenia” following the Moderna or Pfizer vaccine have been added to the Vaccine Adverse Event Reporting System (VAERS) in the US. ITP is a rare but known complication of several vaccinations. SARS-CoV-2 vaccine is new, with a novel mechanism of action, and understanding the epidemiology, clinical manifestations, treatment success and natural history of post-vaccination thrombocytopenia is evolving. We report a 74-year-old man who developed refractory thrombocytopenia within one day of receiving the Moderna SARS-CoV-2 vaccine. Several hours after vaccination, he developed significant epistaxis and cutaneous purpura. Severe thrombocytopenia was documented the following day, and he developed extremity weakness and encephalopathy with facial muscle weakness. Over a 14-day period, thrombocytopenia was treated first with high dose dexamethasone, intravenous immunoglobulin, platelet transfusions, rituximab, plasma exchange (for presumed acute inflammatory demyelinating polyneuropathy (AIDP)), and four daily doses of the thrombopoietin receptor agonist (TPO-RA) eltrombopag (Promacta™), without a platelet response. Three days later, he received the TPO-RA romiplostim (Nplate™). Five days later, his platelet count began to rise and by post-vaccination day 25, his platelet count was in the normal range. Thrombocytopenia was refractory to frontline and second-line treatment. The eventual rise in his platelet count suggests that one or both TPO-RAs may have impacted platelet recovery. Possibly, but less likely given the temporality, the drug-induced thrombocytopenia was subsiding. The aggressive use of immunosuppressive treatment may jeopardize the intended purpose of the SARS-CoV-2 vaccine, and earlier use of non-immunosuppressive second-line treatment for vaccine-related severe thrombocytopenia, such as with TPO-RAs, should be considered. While it is imperative to continue the global vaccination program, vigilance to the occurrence of post-vaccination severe thrombocytopenia is warranted.
Introduction
Primary Immune Thrombocytopenic Purpura (ITP) is an autoimmune disorder characterized by increased platelet destruction and decreased platelet production.Citation1 The incidence of ITP is 6 per 100,000 adults/year.Citation1 ITP has been documented as a result of viral illnesses or vaccinations that are given for prevention of infectious illnesses.Citation2–Citation11 The recent global pandemic of SARS-CoV-2, a novel coronavirus, has caused many deaths worldwide and a global vaccination program is imperative to achieving herd immunity.Citation12,Citation13 Platelet aggregation and activation appear to be deranged in hospitalized patients with COVID-19, however the effect on the platelet count is not significantly correlated, although severe (presumed immune-mediated) thrombocytopenia during COVID-19 has been reported in a small number of patients.Citation14–Citation17 To date, the SARS-CoV-2 vaccine appears to rarely cause an immune-mediated platelet destruction, not unlike that seen with the rubella, pneumococcus, and influenza vaccines.Citation2,Citation4,Citation5 The mechanism of post-vaccination thrombocytopenia, in both live and inactivated vaccines, is presumed to be immune mediated and may be similarly related to hyperfunction of B-cells observed in ITP.Citation18,Citation19 Tens of millions of people have been vaccinated with the new SARS-CoV-2 vaccine. A small but growing number of cases of “immune thrombocytopenia” or “thrombocytopenia” following the administration of the SARS-CoV-2 vaccine have been reported to the FDA’s VAERS.Citation20 Review of available information regarding post SARS-CoV-2 vaccination-related thrombocytopenia suggests a heterogenous onset, severity, and duration. At least one reported patient had a good platelet response to frontline treatment with corticosteroids and IVIg.Citation21 Here we report a case of severe, multi-drug, refractory immune thrombocytopenia shortly after the initial dose of the Moderna SARS-CoV-2 vaccine. The thrombocytopenia eventually relented after treatment with the thrombopoietin receptor agonist (TPO-RA) romiplostim.
Case Report
A 74-year-old male with hypertension, gout, hyperlipidemia and nonischemic cardiomyopathy, presented with acute epistaxis and diffuse cutaneous purpura a few hours after receiving the first dose of the Moderna SARS-CoV2 vaccine. He received the influenza and pneumococcal vaccines three months and six weeks, respectively, prior to his presentation.
Two months prior to vaccination, the patient had a platelet count of 224 x 109/L. The SARS-CoV-2 vaccine was administered on 19 Jan 2021 and that evening he experienced severe epistaxis and diffuse purpura. The next day, he presented to a local emergency department with uncontrolled epistaxis, and was noted to have a platelet count of 10 x 109/L. He was hospitalized for five days and received high-dose dexamethasone (40 mg/day), five daily doses of intravenous immunoglobulin (400 mg/kg/day), three daily platelet transfusions, and two weekly doses of rituximab (375 mg/m2/dose). Immune suppression with dexamethasone (20 mg/day) was continued after hospital discharge. (See ) Due to persistent severe thrombocytopenia, platelet count 21 × 109/L, the TPO-RA eltrombopag (50 mg/day) was initiated on post-vaccination day ten.
Figure 1 Post-vaccination platelet count.
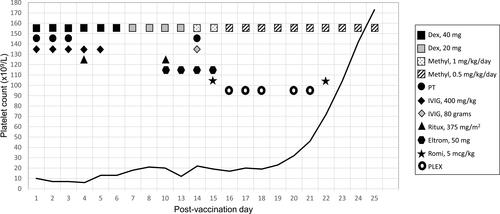
On post-vaccination day 13, he again presented to the emergency department with severe thrombocytopenia, platelet count 12 × 109/L, but now with progressive, generalized weakness that first involved the left arm and the lower extremities bilaterally, back pain causing inability to ambulate, urinary retention, constipation and encephalopathy with dysarthria. These findings, although concurrent with the persistent, refractory severe thrombocytopenia, were thought to be unrelated. Due to suspicion of acute inflammatory demyelinating polyneuropathy (AIDP), plasma exchange was initiated on post-vaccination day 15. Magnetic resonance imaging on post-vaccination day 16 revealed severe L1-5 stenosis with multi-level disc herniation and fluid collections within the lumbar, posterior and paraspinal musculature.
On post-vaccination day 14, he received one pheresis unit of platelets for a platelet count of 22 × 109/L without improvement in his platelet count 1-hour post transfusion, and an additional dose of IVIg of 80 grams was given. On post-vaccination day 15, he received high dose methylprednisolone (1 mg/kg/day) and romiplostim (5 mcg/kg) with no increase in the platelet count for the next five days (See ).
Examination of the peripheral blood smear on post-vaccination day 14 revealed normal-to-large sized platelets. Blood and urine cultures grew methicillin-susceptible Staphylococcus aureus (MSSA) on post-vaccination day 13 that was treated with cefazolin. SARS-CoV-2 by a polymerase chain reaction assay was not detected on post-vaccination days 13 and 14. Additionally, tests for human immunodeficiency virus, Hepatitis B virus (HBV), Hepatitis C virus, Epstein-Barr virus, cytomegalovirus, and parvovirus B19 were negative.
On post-vaccination day 19, after the third episode of plasma exchange, his facial weakness improved. On post-vaccination day 22, his platelet count was 72 × 109/L, a second dose of romiplostim 5 mcg/kg was administered, and a corticosteroid taper was begun. He was transferred to a skilled nursing facility on post-vaccination day 25 with a platelet count of 173 × 109/L.
Discussion
Previous studies have shown that ITP is a rare complication following routine vaccinations, primarily rubella but also pneumococcus, Haemophilus influenza type B, HBV, human papilloma virus, varicella-zoster, diphtheria, tetanus, pertussis, and polio.Citation2–Citation11
Our patient presented with severe thrombocytopenia within one day of receiving the Moderna SARS-CoV-2 vaccine with virtually no response to standard ITP treatment, suggesting refractoriness or a different pathophysiology for the severe thrombocytopenia. The patient’s refractory thrombocytopenia ultimately responded after treatment with romiplostim (Nplate), much like many cases of ITP with an onset of action as soon as four days after the subcutaneous first dose.Citation22,Citation23 This patient’s poor response to frontline and second-line treatments suggests that TPO-RA agents may be useful in refractory ITP post SARS-CoV-2 vaccination.
Because of the concurrent, suspected AIDP and concern for jeopardizing the effect of the SARS-CoV-2 vaccine, thereafter, nonimmune suppressive treatment for severe thrombocytopenia was chosen. The deleterious effects of aggressive immunosuppression for thrombocytopenia should not be overlooked. In prior studies, immunosuppressive therapies post vaccination resulted in attenuation of the immune response to the administered vaccine.Citation24 TPO-RAs are not immunosuppressive and very effective for persistent or chronic ITP.Citation25 Knowing that TPO-RAs may increase the risk of venous or arterial thrombosis in a small proportion of patients, and that patients with SARS-CoV-2 infection are at higher risk for thrombotic events, patients selected for TPO-RA therapy for SARS-CoV-2 related severe thrombocytopenia should be chosen judiciously.Citation26,Citation27
Conclusion
Given the SARS-CoV-2 vaccine is new, with a novel mechanism of action, there exists the uncertainty of whether or not refractory ITP will be a rare adverse event. Notwithstanding that post-vaccination induced ITP is a rare adverse event, it should not limit the use of vaccines, including the SARS-CoV-2 vaccine. However, further investigation is imperative to explicate the pathological mechanism, epidemiology, clinical manifestations, and treatment outcomes.
Consent
Written informed consent for publication of their details was obtained from the patient. The governing institutional review board (IRB) for St. Francis Medical Center, the University of Illinois College of Medicine- Peoria IRB, gave approval for this case report.
Acknowledgments
All of the authors wish to acknowledge the diligent work of the clinical and laboratory staffs of OSF Sacred Heart of Mary and OSF Saint Francis Medical Center.
Disclosure
Dr Michael D Tarantino reports personal fees for consulting and/or speaking from and past clinical trials investigator for Amgen and Dova, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- Weycker D, Hanau A, Hatfield M, et al. Immune thrombocytopenia in US clinical practice: incidence and healthcare burden in the first 12 months following diagnosis. J Med Econ. 2020;23(2):184–192. doi:10.1080/13696998.2019.1669329
- Black C, Kaye JA, Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. Br J Clin Pharmacol. 2003;55(1):107–111. doi:10.1046/j.1365-2125.2003.01790.x
- Garbe E, Andersohn F, Bronder E, et al. Drug induced immune thrombocytopaenia: results from the Berlin Case-Control Surveillance Study. Eur J Clin Pharmacol. 2012;68:821–832. doi:10.1007/s00228-011-1184-3
- Tseng HF, Sy LS, Quan L. Pneumococcal conjugate vaccine safety in elderly adults. Open Forum Infect Dis. 2018;5(6):1–8.
- Nagasaki J, Manabe M, Ido K, et al. Postinfluenza vaccination idiopathic thrombocytopenic purpura in three elderly patients. Case Reports in Hematol. Epub 2016 February 21.
- Jin C, Dong H, Sun Z, et al. Acute immune thrombocytopenic purpura as adverse reaction to oral polio vaccine (OPV). Hum Vaccin Immunother. 2013;9(8):1739–1740. doi:10.4161/hv.24847
- Genovese C, La Fauci V, Squeri A, Trimarchi G, Squeri R. HPV vaccine and autoimmune diseases: systematic review and metaanalysis of the literature. J Prev Med Hyg. 2018;59:E194–E199. doi:10.15167/2421-4248/jpmh2018.59.3.998
- Miller E, Waight P, Farrington P, et al. Idiopathic thrombocytopenic purpura and MMR vaccine. Arch Dis Child. 2001;84:227–229. doi:10.1136/adc.84.3.227
- Cecinati V, Principi N, Brescia L. Vaccine administration and the development of immune thrombocytopenic purpura in children. Human Vacc Immunotherap. 2013;9(5):1158–1162. doi:10.4161/hv.23601
- France EK, Glanz J, Xu S, et al.; Vaccine Safety Datalink Team. Risk of immune thrombocytopenic purpura after measles-mumps-rubella immunization in children. Pediatrics. 2008;121(3):e687–e92. doi:10.1542/peds.2007-1578
- Isai A, Durand J, Le Meur S, Hidalgo-Simon A, Kurz X. Autoimmune disorders after immunisation with Influenza A/H1N1 vaccines with and without adjuvant: eudraVigilance data and literature review. Vaccine. 2012;30(49):7123–7129. doi:10.1016/j.vaccine.2012.09.032
- Centers for disease control and prevention.. Available from: https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed February. 15, 2021
- Omer SB, Yildirim I, Forman HP. Herd Immunity and Implications for SARS-CoV-2 Control. JAMA. 2020;324(20):2095–2096. doi:10.1001/jama.2020.20892
- Leopold V, Pereverzeva L, Schuurman AR, et al. Platelets are hyperactivated but show reduced glycoprotein VI reactivity in COVID-19 patients. Thromb Haemost. 2021. doi:10.1055/a-1347-5555
- Comer SP, Cullivan S, Sklanna PB, et al. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19(2):e3001109. doi:10.1371/journal.pbio.3001109
- Slomka A, Kowalewski M, Zekanowska E. Coronavirus disease 2019 (COVID-19): a short review on hematological manifestations. Pathogens. 2020;9(6):493. doi:10.3390/pathogens9060493
- Bhattacharjee S, Banerjee M. Immune thrombocytopenia secondary to COVID-19: a systematic review. SN Comp Clin Med. 2020;2:2048–2058. doi:10.1007/s42399-020-00521-8
- Giordano P, Cascioli S, Lassandro G, et al. B-cell hyperfunction in children with immune thrombocytopenic purpura persists after splenectomy. Ped Res. 2016;79(2):262–270. doi:10.1038/pr.2015.211
- Yokomichi H, Tanaka-Taya K, Koshida R, et al. Immune thrombocytopenic purpura risk by live, inactivated and simultaneous vaccinations among Japanese adults, children and infants: a matched case-control study. Int J Hematol. 2020;112(1):105–114. doi:10.1007/s12185-020-02866-1
- Vaccine adverse event reporting system. Available from: https://vaers.hhs.gov. Accessed February 15, 2021.
- Tarawneh OH, Tarawneh HS. Immune thrombocytopenia in a 22-year-old post Covid-19 vaccine. Am J Hematol. 2021. doi:10.1002/ajh.26106
- Kuter DJ, Bussel JB, Lyons RM, et al. Randomized, controlled, 6-month evaluation of AMG-531 in patients with chronic immune thrombocytopenic Purpura. Lancet. 2008;371(9610):395–403. doi:10.1016/S0140-6736(08)60203-2
- Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. 2004;76(6):628–638. doi:10.1016/j.clpt.2004.08.010
- Papp KA, Haraoui B, Kumar D, et al. Vaccination guidelines for patients with immune-mediated disorders on immunosuppressive therapies. J of Cut Med Surg. 2019;21(1):50–74. doi:10.1177/1203475418811335
- Tarantino MD, Chalmers S. Therapeutic Thrombopoietin Mimetics. In: Gresele P, Kleiman NS, Lopez JA, Page CP, editors. Platelets in Thrombotic and Nonthrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics, 2nd. Cambridge University Press; 2016: 1417–1429.
- Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenia: safety and efficacy. Brit J of Haematol. 2013;161:411–423. doi:10.1111/bjh.12260
- Pavord S, Thachil J, Hunt BJ, et al. Practical guidance for the management of adults with immune thrombocytopenia during the COVID-19 pandemic. Brit J of Haematol. 2020;189(6):1038–1043. doi:10.1111/bjh.16775
