Abstract
Rare, chronic diseases such as hemophilia and other congenital coagulation disorders require coordinated delivery of services for optimal outcomes. Hemophilia Treatment Centers (HTCs) are specialized, multidisciplinary health-care centers providing team-based care to meet the physical, psychosocial, and emotional needs of people with hemophilia (PWH) and may serve as a model for other rare coagulation disorders. Health-care purchasers, as well as the general medical community, may not appreciate the breadth and quality of services provided by HTCs. They exemplify the acculturalization and actualization of integrated care by providing comprehensive diagnostic and treatment services that reduce morbidity, mortality, avoidable emergency room visits, hospitalizations, and overall costs, while promoting a longer lifespan and improved patient functioning and outcomes. This is accomplished by a team-based approach relying upon a shared decision-making model to effectively prevent complications and manage symptoms in PWH, who are dependent on high-cost treatments. This article provides a concise yet comprehensive description of the core components of an HTC and the regional and national networks in the United States, which together achieve their incomparable value for all stakeholders.
Graphical abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Plain Language Summary
Hemophilia Treatment Centers (HTCs) and the regional and national network to which they each belong deliver integrated, patient-centered care that reduces morbidity, mortality, and overall costs, while promoting a longer lifespan and improved outcomes. However, a comprehensive presentation of the HTC Network of care in the United States has not been published since 2005.
During the intervening years, there have been dramatic changes in the diagnosis and treatment of hemophilia and other rare coagulation disorders and rapid evolution in the importance of patient-centered, integrated, value-oriented care. For example, there is more widespread acceptance of prophylaxis as the standard of care for severe hemophilia and there are many more factor and non-factor therapies available for patients to consider. In addition, there has been a dramatic shift in the patient populations served by HTCs, including many more middle-aged and older adults, women, and people with other rare coagulation disorders. Moreover, there are new organizations, such as the American Thrombosis and Hemostasis Network (ATHN), that did not exist in 2005 and which now play a central role, and advances in information technology and telemedicine that have revolutionized the ways in which care is provided.
This article was written to help inform the medical and public health communities about the inner workings of a complex but highly coordinated approach to delivery of health-care services to this unique population, and to advance widespread recognition of the benefits of this model of care to ensure that sufficient funding and support continues into the future.
Introduction
Hemophilia is a rare inherited, bleeding disorder caused by a deficiency of coagulation factor VIII (FVIII) or factor IX (FIX), known as hemophilia A or B, respectively. The prevalence (per 100,000 males) is 17.1 cases for all severities of hemophilia A, and 3.8 for hemophilia B.Citation1 Bleeding occurs most commonly in joints, soft tissues, and muscles; it can be serious, causing debilitating pain and musculoskeletal complications, resulting in morbidity or even death.Citation1,Citation2 Acute and chronic complications result in a major impact on health-related quality of life (HRQoL) for people with hemophilia (PWH).Citation2 The mainstay of treatment in PWH is intravenous factor replacement therapy, for either prevention of bleeding (prophylaxis) or its treatment on demand.Citation1 Prophylaxis has been proven to maintain joint status and function but requires maintenance therapy over a lifetime.Citation3 Moreover, PWH who develop hemophilic arthropathy require a multimodal approach, including medical treatment, surgery, rehabilitation, and exercise.Citation4 Women with hemophilia and bleeding require the same care as males during hemostatic challenges such as surgery, pregnancy, childbirth, and menstruation.Citation5
Over the past 4 decades, Hemophilia Treatment Centers (HTCs) have emerged as specialized, multidisciplinary health-care centers with unique expertise to meet the physical, psychosocial, and emotional needs of PWH and other congenital coagulation disorders. Optimal outcomes are achieved with a team-based, multidisciplinary, shared decision-making model. Specifically, HTCs offer integrated and comprehensive diagnostic and treatment services, including prevention education, counseling, case management, care coordination, outreach, research, surveillance, and outpatient pharmacy services. These services ensure that PWH and other congenital coagulation disorders have access to highly specialized care to reduce morbidity and enhance wellness, promote a longer lifespan, and improve patient/family functioning; while at the same time reducing avoidable emergency room visits, hospitalizations, and overall costs.Citation6,Citation7
In 2020, the last full year for which we have complete data, HTCs in the United States (US) provided care for 37,541 people with bleeding disorders, compared with 25,450 in 2012.Citation8 In the US, HTCs are at a crossroads: hemophilia treatment is rapidly evolving toward greater complexity, utilization of HTC services by those with other congenital coagulation disorders is on the rise,Citation9 and the generation of sufficient revenue to fund integrated care services has moved to the forefront.Citation10–Citation13 The evolution and increased complexity of hemophilia treatment, coupled with the introduction of precision medicine principles, molecular medicines, and requisite surveillance to monitor these developments, along with the ability to provide sufficient education to engage in shared decision-making, requires ever more expertise on the part of clinicians to ensure that PWH receive state-of-the-art care as HTC teams incorporate these advances.Citation14
Furthermore, several populations are growing overall HTC utilization. First is the increasing number of PWH who reach older ages over the last 20 years.Citation15 Previously, viral contamination of hemophilia medications with both hepatitis C and human immunodeficiency virus (HIV) decimated the community.Citation16 Viral comorbidities, plus progressive painful musculoskeletal deterioration, combine with aging to add complexity to the population’s disease management.Citation17 The lifespan of PWH has extended from 13 years in the early 1900s to approximately 70 years today.Citation18 Finally, HTCs are increasingly serving diverse populations: between 1990 and 2010, the number of women treated at HTCs has increased by 346%, and the proportion of other congenital coagulation disorders, such as von Willebrand disease (VWD), has also substantially increased.Citation9
Purchasers of health care, as well as the general medical community, may not have a complete appreciation of the breadth and quality of services provided by US HTCs. This article is intended to provide a concise yet comprehensive description of the core components of all US HTCs and illustrate how they function as a highly effective national network within a regional structure. Furthermore, it articulates for all stakeholders the value chain of services resulting in the lowest total cost of care while achieving optimal health outcomes for affected individuals and families.
The HTC Model
Integrated Care
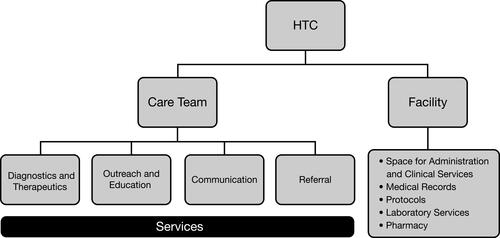
HTCs provide value to PWH and other congenital coagulation disorders, their caregivers, health-care providers (HCPs), insurers, and policymakers in the form of integrated disease management. This integration is possible through a core care team () including a hematologist/medical director, nurse coordinator, physical therapist, and social worker, and access to a specialized coagulation laboratory.Citation7,Citation19,Citation20 This is complemented by an extended team, comprising professionals in related disciplines such as orthopedic surgery, as well as nutritionists, genetic counselors, psychologists, and others available upon referral (, ).Citation7,Citation21 Other extended team members include those who facilitate research activities at the HTC, such as data managers and clinical research nurses.Citation22
Table 1 Requirements for HTC Funding Through the Hemophilia Care Act of 1975
Figure 1 MASAC Standards and Criteria for the Care of Persons with Congenital Bleeding Disorders. Data from Reding and Kenney.Citation21
Expertise
The sustainability of provider expertise is ensured at HTCs, where clinicians focus on congenital coagulation disorders, and where training is regularly provided. Private practice hematologists and health maintenance organizations do not have the clinical experience, teamwork-based model,Citation23 or capacity to make the investment in quality and expertise that HTCs have, nor do they have ready access to clinical trials that most HTCs have.Citation24
Adherence to Clear Guidelines
Guidelines inform and set a foundation for standardization and assurance of baseline levels of care. To this end, the National Hemophilia Foundation (NHF) partnered with McMaster University to develop an evidence-based clinical practice guideline on “Care Models for Hemophilia Management,” which have been implemented at HTCs across the country.Citation19 The current HTC standard of care is in line with these clinical practice guidelines, ensuring consistent care for PWH within the HTC network.Citation22,Citation25 Moreover, HTCs also adhere to treatment guidelines, standards, and recommendations established by the Medical and Scientific Advisory Council (MASAC) of the NHFCitation26 as well as the World Federation of Hemophilia (WFH) Guidelines for the Management of Hemophilia, which include recommendations for 24-hour patient care and access to safe and effective hemostasis products.Citation20
Specialty Coagulation Laboratory Services
Laboratory services and interpretation of test results are a large part of diagnosis, management, and monitoring for PWH and represent a core service in HTCs.Citation27 Large HTCs have their own specialty laboratories, while smaller HTCs have access to the laboratory services at larger HTCs, as well as those of the Centers for Disease Control and Prevention (CDC). Samples can be shipped from centers within the region, or when necessary, to HTCs outside the region. The quality of care of PWH and others with congenital coagulation disorders depends on the highest quality and timeliness of laboratory services. Speed of results and relationship with hospital-based coagulation laboratories as opposed to commercial laboratories is vital to ensuring optimal outcomes for PWH.Citation28
Coordinated Pharmacy Services
Coordination of care reaches beyond communication between HCPs and PWH. Embedding pharmacy services into HTCs enhances care coordination and real-time response, facilitates shared decision-making, and reduces fragmentation that occurs when pharmacy services are handled by a separate entity. Notably, the pharmacies within the HTCs are consistent with the MASAC’sCitation26 minimum standards for specialty pharmacies serving PWH, including knowledgeable staff, comprehensive access to ancillary services and medications that treat hemophilia, timeliness, accuracy, accessibility, safety, and thoroughness.Citation29 Most HTCs participate in the federal 340B program, a discounted prescription drug purchasing program, and are registered with Health Resources Services Administration (HRSA) as comprehensive HTC 340B programs (https://www.hrsa.gov/opa/index.html). In accordance with the principal aim of the program to enable covered entities to reach more patients and provide more comprehensive services, any income generated through 340B drug discounts is to be used for the direct benefit of PWH, thereby providing a critical revenue stream to support HTC services including patient care and education, staff salaries, and care coordination.Citation30
Although PWH may use any pharmacy allowed by their insurer, using the HTC pharmacy facilitates case management and the opportunity for collaboration between the patient and the medical and pharmacy teams, enabling more agile care coordination in real time, ensuring accurate and complete reporting while achieving the lowest total cost of care and optimal health outcomes.Citation31,Citation32
HTCs Function Within a National Network
In addition to the expertise within each HTC, their value is enhanced by the existence of the US Hemophilia Treatment Center Network (USHTCN), which supports fully integrated and consistent disease management and education across all HTCs. The network not only provides operational organization and administration of HTCs, but also facilitates education and training of HCPs and PWH.Citation25
Operational Organization
Eight regional HRSA grantees administer and support 149 HTCs, comprising the USHTCN.Citation33 Core centers serve many functions including disbursement of funds and monitoring to ensure that HTCs conform to the HRSA and CDC requirements.Citation29 Regional HTC networks are administered via a Regional Director and Regional Administrator, roles with standardized responsibilities in accordance with the HRSA and the CDC. Regional core centers organize Executive Committees, promulgate policies and procedures, convene ad hoc working groups, engage with partners, and liaise with community-based organizations. These functions provide a consistent foundation for organized communication and education among and between HTCs.Citation34
Provider Education and Training
Within the United States, there are a limited number of expert HCPs who are concentrated in the 149 US HTCs, necessitating a highly organized and efficient network model to maximize these scarce resources and support cost-effective educational opportunities. The WFH Guidelines also acknowledge this need and recommend networking strategies to provide educational opportunities benefiting HCPs including regional conferences, discipline-specific work groups, information distribution lists, shadow-experiences in HTC clinics, and mentorship of new colleagues.Citation20 Beyond educating HCPs who are focused on treating people with congenital coagulation disorders, the network model also supports the education of community physicians and physical therapists, homecare nurses, emergency room staff, school personnel, and others.Citation11,Citation34,Citation35
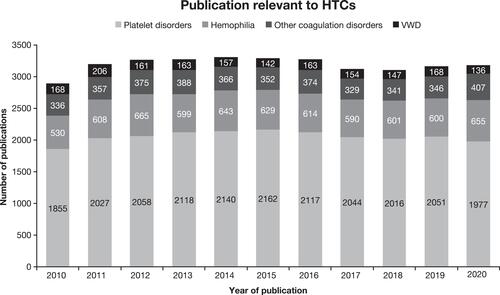
In summary, the USHTCN provides operational organization and excellence to enhance communication and education, thereby creating a living and learning network that constantly evaluates and revises treatment standards and recommendations. For example, only through this network approach could over 35,000 publications in the past decade on various types of coagulation disorders (), many of which were generated by HTCs, be assimilated, vetted, and utilized to advance patient care and improve patient outcomes. Importantly, much of this quality improvement is neither reimbursed nor paid for by federal grants and exists as unfunded mandates to ensure the highest quality of care for PWH.
Figure 2 Proliferation of Knowledge Generation in Hemophilia and Other Coagulation Disorders.
Unique Benefits of Integrated Care Provided by HTCs
Comprehensive Care and Educational Support Across the Lifespan
The operational organization of US HTCs enables responsiveness to patient needs, identifying gaps in care and planning educational opportunities around these gaps. The benefits extend to safety and surveillance efforts to enhance outcomes and HRQoL for PWH by reducing the likelihood of serious adverse events due to the bleeding disorder or its treatment while enhancing shared decision-making and self-sufficiency focusing on the PWH as a partner rather than only as a consumer of health care.Citation34
Treatment plan development beginning in early childhood and extending through adulthood includes disease and treatment education; case management; rehabilitation; genetic counseling and testing; dental and orthopedic care; pain management; and nutritional, psychosocial, and vocational counseling. Education is provided to parents of newborns and continues as children develop the capacity to participate in decisions regarding their own treatment (eg, self-infusion education). HTCs facilitate the transition from adolescent to adult health services and preparedness for health-care independence.Citation34
As adults, PWH are directly engaged in their medical care using the shared decision-making model and are fully supported in their aspiration to live as normal a life as possible with respect to education, employment, physical activity, and all other aspects of living.Citation36,Citation37 Comprehensive case management by the HTC’s core care team enables the provision of genetic testing and counseling, surgical coordination, rehabilitation and physiotherapy expertise, and advanced training.Citation7,Citation38 Across the lifespan, women with bleeding disorders require specialized care that includes attention to heavy menstrual bleeding pre-menopause,Citation39 as well as pre- and post-surgery planning to control for heavy blood loss associated with surgery, pregnancy, and postpartum and during times of spontaneous and traumatic joint bleeding.Citation40–Citation42
The lifespan of PWH is increasing, necessitating development of care expertise for elderly PWH. Three issues associated with aging for PWH are general age-associated disease, motor coordination/falls, and specific symptomatology associated with hemophilia such as renal insufficiency and joint disease increasing the incidence of falls. Elderly PWH express comorbidities associated with general aging, such as obesity and hypertension. However, the presentation of these diseases can look different in PWH than the general population.Citation43,Citation44
Improving Patient Outcomes by Evidence Generation
Comprehensive and strategic collection, curation, analysis, and dissemination of data concerning the care and outcomes of PWH and other congenital coagulation disorders is achieved through a collaboration between the PWH, HTCs, CDC, American Thrombosis and Hemostasis Network (ATHN; https://athn.org/), and the USHTCN. Initially, the focus of this effort was on infectious disease (such as viral hepatitis and HIV) and health complications associated with joint disease, but has expanded to examine specific bleeding complications for women and babies, as well as HRQoL.Citation45–Citation47 For example, the Community Counts Project, administered by ATHN in partnership with the USHTCN, collects data for public health tracking of the complications of congenital coagulation disorders ().Citation45,Citation48 Near real-time reporting of data in an electronic data visualization tool allows for faster detection of trends and potentially faster response times.Citation45
Table 2 Comparison of the Universal Data Collection and the Community Counts Surveillance Systems
Beyond patient care data, the HTC infrastructure also provides PWH with an opportunity to participate in clinical research trials, including novel therapeutics; this is the standard of care at many centers. For example, from 2012 to 2018, the “My Life, Our Future” (MLOF) Research Repository processed information from over 11,000 male and female participants who contributed their genetic data and biologic samples to create the world’s largest hemophilia scientific resource. This rich dataset was realized through extensive partnership between PWH, ATHN, Bloodworks Northwest, the NHF, and Bioverativ.Citation49 ATHN is currently housing the MLOF Research Repository on behalf of the community. Examples of ongoing projects include understanding the genetic, epigenetic, and antibody signatures of inhibitor responses in PWH, and studying factors associated with bleeding in women with hemophilia.Citation49 Another example of using data to demonstrate better outcomes comes from the 3-year retrospective data abstraction study conducted by the Hemophilia Surveillance System, including data on PWH from 6 states. This study indicated a 40% reduction in mortality in PWH who were treated at HTCs, compared with those treated in other health-care settings.Citation50 Additionally, a 20% reduction in bleed-related hospitalizations among PWH cared for at an HTC who were on a medically supervised self-infusion program was also reported.Citation51 More recently, a small study of PWH in Indiana showed a reduction in the use of emergency department services from 33.3% of those treated outside of an HTC to 17.6% of those who were being treated through an HTC.Citation52
Quality improvement (QI) is also a key function of the network, with a focus on 3 areas: patient engagement, medical home, and adolescent transition. The data and quality metrics are essential; the network continues to strive to improve the principles and practices used to diagnose and treat PWH so that the manifestations of their disease and its complications are minimized to achieve better outcomes and reduce disease burden on the individual and his/her family, while achieving optimal health outcomes at the lowest total cost of care—all steps toward health equity.Citation53
Assessing Patient Satisfaction
Improvements in HRQoL such as reduced unemployment and a reduction in mortality are important outcomes, but patient satisfaction with treatment can have a profound impact on the patient’s experience and subsequent treatment adherence. HTCs conducted national patient satisfaction surveys in 2015 and 2018, with another underway in 2021.Citation54 The 2015 and 2018 surveys engaged 5006 and 4767 PWH, respectively. Nationally, over 90% of respondents, regardless of age, sex, race, ethnicity, diagnosis, disease severity, or where they lived, reported being usually or always satisfied with overall HTC care; components of HTC care (each >90% satisfaction nationally) were core team members, HTC care processes and services, and adolescent transition to adult care guidance.Citation55,Citation56 Addressing patient satisfaction also involves finding areas for improvement. In 2013, the National HTC Patient Needs Assessment Survey was mailed to PWH being actively treated at 129 HTCs. Question topics included management and information, access and barriers to care, coping, resources, and transition. Over 90% of respondents agreed or strongly agreed that care at HTCs was patient centered and reported HTC care as important or very important; <3% reported not receiving the services they need. The survey also identified gaps in care including dietary advice, genetic testing, information on aging with hemophilia, sexual health, and basic needs resources; additionally, minority respondents reported more barriers.Citation57
Focusing on Value of Care and Collaboration with Payers
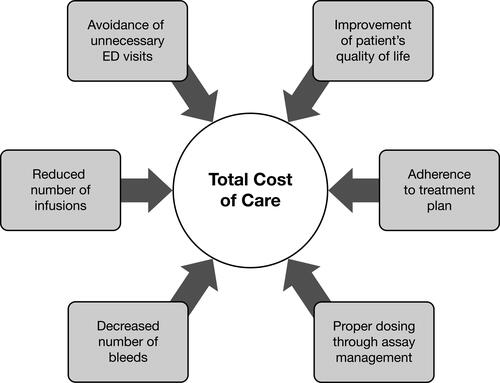
Integrated care brings value from a public health perspective, in the form of potential cost savings to the system and to PWH, which can dramatically impact HRQoL. illustrates how many of the features of HTC care and its clinical benefits contribute to lowering the total cost of care for PWH treated at an HTC. Payers have acknowledged that they did not fully recognize the comprehensive medical and support services that HTCs provide and the positive impact those services have on patient HRQoL and cost of care.Citation58
Figure 3 Benefits of Care at an HTC are Associated with Lower Total Cost of Care. Data from National Hemophilia Foundation.Citation6
Strengthening relationships and fostering mutual understanding between health plans and HTCs could further improve outcomes for PWH while providing a more favorable cost-to-benefit ratio. The Comprehensive Care Sustainability Collaborative (CCSC), initiated by NHF in 2014, has served as a springboard for bringing payers and HTCs together to explore and develop opportunities to work collaboratively and enhance communication and understanding between payers and providers to assure optimal patient outcomes at the lowest total cost of care.Citation11 As of 2020, the CCSC advisory board comprises a wide-ranging group of stakeholders, including medical and operational directors representing 8 HTCs, national and regional health plans, and the voice of the self-funded employer. One key learning from the CCSC inaugural consensus meeting was that HTCs are providing a level and range of multidisciplinary care that is far more comprehensive than what has generally been recognized by purchasers of health-care services. After multiple iterations in the CCSC, participants agreed on 2 sets of reporting metrics (payer and HTC). These metrics are being assessed in pilot programs ().Citation59
Table 3 Final HTC- and Payer-Reported Metrics for Use in Pilot Programs
Advocating for PWH Locally, Regionally, and Nationally
HTCs are actively involved in collaborating with the NHF and other patient advocacy organizations to advance the interests of PWH and their families.Citation60 Families and PWH rely on the NHF for its infrastructure, which includes 50 chapters that provide community support. Through the NHF and its chapters, families can connect with support networks, essential education, and advocacy efforts. In addition to national advocacy efforts, HTCs build partnerships with local foundations and support advocacy at state and national levels;Citation61 HTC staff serve in many advisory and other leadership capacities locally and at NHF.
Funding Mechanisms for HTCs
HRSA has identified specific criteria to qualify for a limited grant; these criteria ensure consistency within the network and support a specific standard of care at each center. HTCs that receive HRSA grant funding are required to provide evidence of optimal care using a multidisciplinary team-based approach.Citation62
With the passage of the Veterans Health Care Act of 1992, regional HRSA grantees and subcontracting HTCs were included as entities eligible to participate in the 340B drug discount program. Though HTCs receive some federal support, they depend on revenue from their pharmacy programs to help finance clinical, educational, counseling, surveillance, and many laboratory services. Most of the federally designated comprehensive HTCs with established 340B programs who participated in a survey indicated that they rely on their 340B-associated income to help fund the staff hours of their on-site social workers, physical therapists, and nurses.Citation30 At issue is the fact that many essential facets of integrated care that are typically delivered via the HTC staff are not billable or are minimally billable. For example, 29 centers in the survey reported that in 2013 alone, 62,640 medical coordination encounters and 57,072 urgent/emergent telephone triage encounters occurred, most of which were non-billable services. In the same survey, 28,880 encounters for psychosocial and vocational services were provided by 30 centers in 2013, again mostly non-billable.Citation30
Charting a Course for the Future of HTC-Based Care
The HTC model can be adapted to the changing health-care needs of PWH as it is primed to take advantage of the evolving technology of the twenty-first century. For example, telehealth and telemedicine at HTCs help to overcome access barriers, allowing HTC care to be offered to anyone regardless of their proximity to a center. These interventions are supported by preliminary evidence of their feasibility and effectiveness, especially for those who face transportation, mobility, or economic barriers, either in the United States or in developing countries;Citation63 however, the benefits remain to be conclusively demonstrated. Telemedicine increases opportunities for collaboration, promoting communication between HTC staff and local HCPs to ensure seamless care.Citation64
One example of successful telehealth implementation includes telerehabilitation for people who need musculoskeletal rehabilitation. Telerehabilitation can greatly improve a patient’s experience and attitude toward essential physical movement needed for recovery. Videoconferencing can also be useful for remotely evaluating bleeding or providing integrated care with multiple providers on the line at the same time.Citation65 The third national HTC Patient Satisfaction Survey, underway in Q1 2021, asks respondents about satisfaction with telehealth/telemedicine, and the extent to which COVID-19 was a barrier to obtaining needed HTC services. Adding these questions illustrates the capacity of the USHTCN to rapidly assess its ability to respond to emerging events (eg, the pandemic) from the patient perspective in a uniform way across centers nationwide.Citation54
Monitoring and self-auditing procedures for PWH in HTCs are also evolving with technology. Cellphone tools and apps have been developed to record information, including details on bleeding episodes, pain, and the use of coagulation factors, potentially allowing for virtual treatment supervision.Citation65 Enhanced real-world evidence collection would stand to improve patient outcomes through increased health surveillance, and potentially offer payers more evidence in support of the advantages of integrated care offered by HTCs.Citation6 HTCs serve PWH and their families who express high satisfaction, a high level of needs met, and improved outcomes spanning HRQoL to cost savings.Citation21,Citation56
The value of the HTC model and the USHTCN are clear; but HTCs are in a state of financial jeopardy, currently heavily reliant on 340B pharmacy-generated income. To continue to provide essential services for PWH and other rare coagulation disorders, it is imperative not only that HTCs receive optimal and fair reimbursement but also that PWH have access to HTCs. Revisions to federal regulations and funding schemes are essential to maintain the quality and level of services currently being offered to PWH. Increased reimbursement for existing billing codes, as well as new codes that more accurately reflect the services delivered, would be an important step toward securing the future of HTCs. Another critical step would be reducing or eliminating exclusionary practices that limit access within health-care networks.
Looking to the future, HTCs are poised to partner on the critical health priorities of the country. With the current structure, HTCs could serve a larger number of people with VWD and other rare coagulation disorders, who currently make use of the integrated care model, along with PWH. Refinement over 40 years has created the foundation for sustainability and the possibility of growth, with the potential for expanded services to PWH and individuals with other rare coagulation disorders that require a similar level of integrated, multidisciplinary care.
Conclusion
People with congenital coagulation disorders have been well served by the HTCs and USHTCN operating in the United States for over 40 years. Herein, we have described what HTCs do and how they do it through a team-based, multidisciplinary approach that incorporates the affected person in shared decision making. We have shown that PWH/caregivers are highly satisfied and have presented evidence for why this model of care has been and will remain the gold standard in the United States. This article can serve as a reference document for all stakeholders in the care of PWH and other rare coagulation disorders. The unique benefits of the HTC network system of care have been clearly demonstrated. Taking all the benefits into account, we believe that purchasers of health care will conclude, as we do, that HTCs provide the highest-quality care for their beneficiaries, delivering optimal health outcomes at the lowest total cost of care.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Ellen Riker and Johanna Gray for their thoughtful input on this review. Medical writing assistance was provided by Bill Kadish, MD of Parexel International and funded by the National Hemophilia Foundation.
Disclosure
LAV, JRB, NF, KK, JNP, and MRi declare no conflicts of interest. All grant support declared in the following statements was paid to the author’s institution. RB received grant support from Health Resources & Services Administration (HRSA) and American Thrombosis and Hemostasis Network (ATHN), and honoraria from Genentech and Pfizer for advisory panel participation and from Medscape for a webinar series. ME received institutional support for clinical research from ATHN, Novo Nordisk, Sanofi, Takeda, and uniQure; payments for educational lectures from Bayer, BioMarin, Genentech, Novo Nordisk, and Pfizer; and has participated on Advisory Boards of Bayer, CSL Behring, Genentech, Kedrion, NHF, Novo Nordisk, Pfizer, and Sanofi; all outside the submitted work. SK has been reimbursed for travel to and attendance at meetings of the Hemophilia Alliance, for which she was the unpaid board chair (2018–2020) and is a retiree from the University of California, San Francisco. SL has received a grant from HRSA and from ATHN and honoraria from the National Hemophilia Foundation (NHF) for speakers’ bureau/educational events and from uniQure for participation in an advisory board. DN is co-chair of the Steering Committee and member of the Executive Committee of CONNECTS (National Heart, Lund, and Blood Institute), president and founder of the Center for Inherited Blood Disorders, and a member of the Board of Directors of the National Association of Sickle Cell Centers and Pediatric Subspecialty Faculty at CHOC Children’s Hospital. MRe reports grants from Spark Therapeutics during the preparation of this manuscript. Outside the submitted work, he has received grants from Bayer, Grifols, LFB, and Octapharma; personal fees from Catalyst Biosciences and Kedrion; and grants and personal fees from BioMarin, CSL Behring, Genentech, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, and uniQure. In addition, he is on the board of directors for Foundation for Women and Girls with Blood Disorders and Partners in Bleeding Disorders. MTR has received grant support from Bayer and BioMarin; honoraria for being on the Speakers’ Bureau of Bayer, CSL Behring, Sanofi, and Takeda; and honoraria for participation on Advisory Boards of Bayer, CSL Behring, Novo Nordisk, Sanofi, and Takeda, all outside the submitted work; and is a member of NHF’s Medical and Scientific Advisory Council. CBT has received honoraria for virtual educational presentations from InFuCare RX and vWD Connect Foundation and sits on the NHF Pain Committee and Social Work Working Group. MS has received consulting fees to his institution from NHF. He is on the Board of Directors of the World Federation of Hemophilia USA and on the governing board of the Institute for Clinical and Economic Review. The authors report no other conflicts of interest in this work.
Additional information
Funding
References
- Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males: a meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):540–546. doi:10.7326/M19-1208
- O’Hara J, Walsh S, Camp C, et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes. 2018;16(1):84. doi:10.1186/s12955-018-0908-9
- Pipe SW. New therapies for hemophilia. Hematology Am Soc Hematol Educ Program. 2016;2016(1):650–656. doi:10.1182/asheducation-2016.1.650
- Nacca CR, Harris AP, Tuttle JR. Hemophilic arthropathy. Orthopedics. 2017;40(6):e940–e946. doi:10.3928/01477447-20170619-05
- McLintock C. Women with bleeding disorders: clinical and psychological issues. Haemophilia. 2018;24(Suppl 6):22–28. doi:10.1111/hae.13501
- National Hemophilia Foundation. Comprehensive hemophilia management: a payer’s guide to the hemophilia comprehensive care model. Available from: https://www.managedcarehemo.com/wp-content/uploads/2017/09/Payeru2019s-Guide-to-the-Hemophilia-Comprehensive-Care-Model.pdf. Accessed February 9, 2021.
- Aledort LM. The evolution of comprehensive haemophilia care in the United States: perspectives from the frontline. Haemophilia. 2016;22(5):676–683. doi:10.1111/hae.12970
- Centers for Disease Control and Prevention. Community counts: HTC population profile. Available from: https://www.cdc.gov/ncbddd/hemophilia/communitycounts/data-reports/2021-03/table-1-patient-characteristics-by-calendar.html. Accessed July 28, 2021.
- Baker JR, Riske B, Drake JH, et al. US Hemophilia Treatment Center population trends 1990–2010: patient diagnoses, demographics, health services utilization. Haemophilia. 2013;19(1):21–26. doi:10.1111/j.1365-2516.2012.02915.x
- Dalton DR. Hemophilia in the managed care setting. Am J Manag Care. 2015;21(6 Suppl):S123–S130.
- Comprehensive Care Sustainability Collaborative. HTC success in a value-based health care environment: findings from the Comprehensive Care Sustainability Collaborative (CCSC). Available from: https://www.ccschemo.com/webcast20/. Accessed September 17, 2021.
- Croteau SE, Cheng D, Cohen AJ, et al. Regional variation and cost implications of prescribed extended half-life factor concentrates among U.S. haemophilia treatment centres for patients with moderate and severe haemophilia. Haemophilia. 2019;25(4):668–675.
- Goodman C, Rangarao S, Rubin RJ; The Lewin Group. National hemophilia foundation: strategic summit report. Available from: https://www.hemophilia.org/sites/default/files/document/files/HemophiliaSummitFinalReportOct2012.pdf. Accessed February 4, 2021.
- Di Minno G, Tremoli E. Tailoring of medical treatment: hemostasis and thrombosis towards precision medicine. Haematologica. 2017;102(3):411–418. doi:10.3324/haematol.2016.156000
- Mazepa MA, Monahan PE, Baker JR, et al. Men with severe hemophilia in the United States: birth cohort analysis of a large national database. Blood. 2016;127(24):3073–3081. doi:10.1182/blood-2015-10-675140
- White GC. Hemophilia: an amazing 35-year journey from the depths of HIV to the threshold of cure. Trans Am Clin Climatol Assoc. 2010;121:61–73.
- Mejia-Carvajal C, Czapek EE, Valentino LA. Life expectancy in hemophilia outcome. J Thromb Haemost. 2006;4(3):507–509. doi:10.1111/j.1538-7836.2006.01776.x
- National Hemophilia Foundation. History from 2AD to the present. Available from: https://www.hemophilia.org/bleeding-disorders-a-z/overview/history. Accessed July 29, 2021.
- Pai M, Key NS, Skinner M, et al. NHF-McMaster guideline on care models for haemophilia management. Haemophilia. 2016;22(Suppl):36.
- Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(Suppl 6):1–158. doi:10.1111/hae.14046
- Reding MT, Kenney J. Hemophilia care coordination and current treatment options: the latest insights for managed care and specialty pharmacy. Available from: https://www.managedcarehemo.com/cmece/. Accessed February 4, 2021.
- National Hemophilia Foundation. Comprehensive medical care: HTCs. Available from: https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/comprehensive-medical-care. Accessed October 24, 2020.
- Mitchell P, Wynia M, Golden R, et al. Core principles & values of effective team-based health care. Presented at: National Academy of Medicine (Discussion Paper); October 2, 2012; Washington, DC.
- Weill Cornell Medicine. Comprehensive hemophilia treatment center: research. Available from: https://hemophilia.weill.cornell.edu/research. Accessed April 17, 2021.
- Center for Disease Control. Hemphilia treatment centers. Available from: https://www.cdc.gov/ncbddd/hemophilia/documents/a-simmons_htc-brochure-508c.pdf. Accessed July 30, 2021.
- Medical and Scientific Advisory Council. MASAC documents. Available from: https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/masac-documents. Accessed July 30, 2021.
- University of Vermont Larner Colllege of Medicine. The vermont regional hemophilia treatment center: coagulation lab. Available from: https://www.med.uvm.edu/medicine/hemonc/patient_care_-vermont_regional_hemophilia_treatment_center. Accessed April 17, 2021.
- National Hemophilia Foundation. MASAC Document 262 - MASAC resolution on off-site hemostasis testing. Available from: https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-262-masac-resolution-on-off-site-hemostasis-testing. Accessed May 5, 2021.
- Skinner MW, Soucie JM, McLaughlin K. The National Haemophilia Program standards, evaluation and oversight systems in the United States of America. Blood Transfus. 2014;12(Suppl 3):e542–e548.
- Malouin RA, McKernan L, Forsberg A, et al. Impact of the 340B pharmacy program on services and supports for persons served by hemophilia treatment centers in the United States. Matern Child Health J. 2018;22(9):1240–1246. doi:10.1007/s10995-018-2545-7
- New York Blood Center. About New York Blood Center pharmacy: pharmacy services. Available from: https://www.nybloodcenter.org/products-and-services/medical-services/hemophilia-services/pharmacy-services/. Accessed April 17, 2021.
- National Hemophilia Foundation. MASAC Document 188 - recommendations regarding standards of service for pharmacy providers of clotting factor concentrates for home use to patients with bleeding disorders. Available from: https://www.hemophilia.org/healthcare-professionals/guidelines-on-care/masac-documents/masac-document-188-recommendations-regarding-standards-of-service-for-pharmacy-providers-of-clotting-factor-concentrates-for-home-use-to-patients-with-bleeding-disorders. Accessed November 21, 2020.
- Centers for Disease Control and Prevention. Hemophilia treatment center directory. Available from: https://dbdgateway.cdc.gov/HTCDirSearch.aspx. Accessed July 30, 2021.
- Baker JR, Crudder SO, Riske B, Bias V, Forsberg A. A model for a regional system of care to promote the health and well-being of people with rare chronic genetic disorders. Am J Public Health. 2005;95(11):1910–1916. doi:10.2105/AJPH.2004.051318
- Lin TF, Carhill P, Huang JN, Baker JR. Capacity building for rare bleeding disorders in the remote commonwealth of the Northern Mariana Islands. Am J Public Health. 2016;106(4):658–661. doi:10.2105/AJPH.2016.303093
- Recht M, Konkle BA, Jackson S, et al. Recognizing the need for personalization of haemophilia patient-reported outcomes in the prophylaxis era. Haemophilia. 2016;22(6):825–832. doi:10.1111/hae.13066
- Roberts JC, Lattimore S, Recht M, et al. Goal Attainment Scaling for haemophilia (GAS-Hem): testing the feasibility of a new patient-centric outcome measure in people with haemophilia. Haemophilia. 2018;24(4):e199–e206. doi:10.1111/hae.13454
- Escobar MA, Brewer A, Caviglia H, et al. Recommendations on multidisciplinary management of elective surgery in people with haemophilia. Haemophilia. 2018;24(5):693–702. doi:10.1111/hae.13549
- Foundation for Women & Girls With Blood Disorders. Blood disorders resources. Available from: https://www.fwgbd.org/resources/blood-disorders. Accessed August 3, 2021.
- Chaudhury A, Sidonio R Jr., Jain N, et al. Women and girls with haemophilia and bleeding tendencies: outcomes related to menstruation, pregnancy, surgery and other bleeding episodes from a retrospective chart review. Haemophilia. 2021;27(2):293–304. doi:10.1111/hae.14232
- American College of Obestricians and Gynecologists. Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891–896. doi:10.1097/01.AOG.0000428646.67925.9a
- Kirtava A, Crudder S, Dilley A, Lally C, Evatt B. Trends in clinical management of women with von Willebrand disease: a survey of 75 women enrolled in haemophilia treatment centres in the United States. Haemophilia. 2004;10(2):158–161. doi:10.1046/j.1351-8216.2003.00832.x
- Angelini D, Konkle BA, Sood SL. Aging among persons with hemophilia: contemporary concerns. Semin Hematol. 2016;53(1):35–39. doi:10.1053/j.seminhematol.2015.10.004
- Mannucci PM. Aging with hemophilia: the challenge of appropriate drug prescription. Mediterr J Hematol Infect Dis. 2019;11(1):e2019056. doi:10.4084/mjhid.2019.056
- Schieve L, Byams V, Dupervil B, et al. Evaluation of CDC’s Hemophilia Surveillance Program — universal data collection (1998–2011) and community counts (2011–2019), United States. MMWR Surveill Summ. 2020;69(5):1–18. doi:10.15585/mmwr.ss6905a1
- Byams VR, Kouides PA, Kulkarni R, et al. Surveillance of female patients with inherited bleeding disorders in United States haemophilia treatment centres. Haemophilia. 2011;17(Suppl 1):6–13. doi:10.1111/j.1365-2516.2011.02558.x
- Kulkarni R, Presley RJ, Lusher JM, et al. Complications of haemophilia in babies (first two years of life): a report from the Centers for Disease Control and Prevention Universal Data Collection System. Haemophilia. 2017;23(2):207–214. doi:10.1111/hae.13081
- Pepper L. National databases improve bleeding disorders care. Available from: https://hemaware.org/bleeding-disorders-z/national-databases-improve-bleeding-disorders-care. Accessed October 25, 2020.
- American Thrombosis and Hemostasis Network. My life, our future research repository. Available from: https://athn.org/what-we-do/national-projects/mlof-research-repository.html. Accessed August 3, 2021.
- Soucie JM, Nuss R, Evatt B, et al. Mortality among males with hemophilia: relations with source of medical care. The Hemophilia Surveillance System Project Investigators. Blood. 2000;96(2):437–442.
- Soucie JM, Symons J 4th, Evatt B, et al. Home-based factor infusion therapy and hospitalization for bleeding complications among males with haemophilia. Haemophilia. 2001;7(2):198–206. doi:10.1046/j.1365-2516.2001.00484.x
- Okolo AI, Soucie JM, Grosse SD, et al. Population-based surveillance of haemophilia and patient outcomes in Indiana using multiple data sources. Haemophilia. 2019;25(3):456–462. doi:10.1111/hae.13734
- American Thrombosis and Hemostasis Network. American thrombosis and hemostasis network: overview. Available from: https://athn.org/who-we-are/about/overview.html. Accessed November 21, 2020.
- National HTC Patient Satisfaction Survey. Third patient satisfaction survey of the U.S. Hemophilia Treatment Centers. Available from: http://www.htcsurvey.com/examplesurvey. Accessed April 17, 2021.
- Riske B, Shearer R, Baker JR. Patient satisfaction with US hemophilia treatment center care, teams and services: the first national survey. Haemophilia. 2020;26(6):991–998. doi:10.1111/hae.14176
- Lattimore S, Shearer R, Ashton M, Riske B, Baker J. Patient satisfaction with US hemophilia treatment centers: national trends 2014 and 2017. Presented at: Hempstasis and Thrombosis Research Society; May 2019; New Orleans, LA.
- Butler RB, Cheadle A, Aschman DJ, et al. National needs assessment of patients treated at the United States federally-funded hemophilia treatment centers. Haemophilia. 2016;22(1):e11–e17. doi:10.1111/hae.12810
- Shapiro A, Pipe SW, Geraghty S, et al. Hemophilia and managed care: partnering to achieve cost-effective care. Pharm Times. 2011;3(5):248–255.
- Tarantino MD, Pindolia VK. Hemophilia management via data collection and reporting: initial findings from the Comprehensive Care Sustainability Collaborative. J Manag Care Spec Pharm. 2017;23(1):51–56.
- United States Congress. The Public Health Service Act establishing the hemophilia diagnostic and treatment center program. In: Congress US, editor. Number 1131 of Public Law 9463. Washington, D.C.: Government Printing Office; 1975.
- National Hemophilia Foundation. What we do. Available from: https://www.hemophilia.org/who-we-are/our-story/what-we-do. Accessed August 5, 2021.
- Health Resources and Services Administration. Comprehensive hemophilia treatment centers. Available from: https://www.hrsa.gov/opa/eligibility-and-registration/specialty-clinics/hemophilia/index.html. Accessed August 5, 2021.
- Qian W, Lam TT, Lam HHW, Li CK, Cheung YT. Telehealth interventions for improving self-management in patients with hemophilia: scoping review of clinical studies. J Med Internet Res. 2019;21(7):e12340. doi:10.2196/12340
- Valentino LA, Skinner MW, Pipe SW. The role of telemedicine in the delivery of health care in the COVID-19 pandemic. Haemophilia. 2020;26(5):e230–e231. doi:10.1111/hae.14044
- Boccalandro EA, Dallari G, Mannucci PM. Telemedicine and telerehabilitation: current and forthcoming applications in haemophilia. Blood Transfus. 2019;17(5):385–390.
