Abstract
Background
Chronic myeloid leukemia (CML) is a myeloproliferative disorder of blood stem cells. The tyrosine kinase inhibitor (TKI) imatinib was the first targeted therapy licensed for patients with chronic-phase CML, and its introduction was associated with substantial improvements in response and survival compared with previous therapies. Clinical trial data are now available for the second-generation TKIs (nilotinib, dasatinib, and bosutinib) in the first-, second-, and third-line settings. A qualitative systematic review was conducted to qualitatively compare the clinical effectiveness, safety, and effect on quality of life of TKIs for the management of chronic-, accelerated-, or blast-phase CML patients.
Methods
Included studies were identified through a search of electronic databases in September 2011, relevant conference proceedings and the grey literature.
Results
In the first-line setting, the long-term efficacy (up to 8 years) of imatinib has been confirmed in a single randomized controlled trial (International Randomized Study of Interferon [IRIS]). All second-generation TKIs reported lower rates of transformation, and comparable or superior complete cytogenetic response (CCyR), major molecular response (MMR), and complete molecular response rates compared with imatinib by 2-year follow-up. Each of the second-generation TKIs was associated with a distinct adverse-event profile. Bosutinib was the only second-generation TKI to report quality-of-life data (no significant difference compared with imatinib treatment). Data in the second- and third-line setting confirmed the efficacy of the second-generation TKIs in either imatinib-resistant or -intolerant patients, as measured by CCyR and MMR rates.
Conclusion
Data from first-line randomized controlled trials reporting up to 2-year follow-up indicate superior response rates of the second-generation TKIs compared with imatinib. Current evidence from single-arm studies in the second-line setting confirm that nilotinib, dasatinib, and bosutinib are valuable treatment options for the significant subgroup of patients who are intolerant or resistant to imatinib treatment.
Introduction/background
Chronic myeloid leukemia or chronic myelogenous leukemia (CML) is a myeloproliferative disorder of blood stem cells. It is primarily due to a single genetic anomaly: a reciprocal chromosomal translocation between the C-ABL (Abelson leukemia virus) oncogene on chromosome 9 and the BCR (breakpoint cluster region) on chromosome 22.Citation1,Citation2 The resulting BCR-ABL gene encodes a fusion tyrosine kinase, which causes cell-cycle deregulation and apoptosis, as well as affecting differentiation and DNA repair.Citation3
The incidence of CML ranges from 0.6 to 2.0 cases per 100,000 per year.Citation4 Since the introduction of tyrosine kinase inhibitors (TKIs), prevalence rates have increased (due to their efficacy in controlling CML).Citation4 The median age of onset of CML was reported to be between 45 and 55 years in 2001,Citation5 but has more recently been reported to be 66 years.Citation6
Early treatments for CML included chemotherapeutic agents such as hydroxyurea and busulfan, which were able to control the symptoms of the disease but did not slow disease progression. The introduction of interferon-α (IFN-α) and stem cell transplantation enabled patients to achieve cytogenetic responses and durable remission.Citation7 However, increasing understanding of the abnormal activity of the BCR-ABL protein and its role in CML led to the development of targeted therapies such as TKIs, eg, imatinib, dasatinib, nilotinib, and bosutinib. Imatinib was the first targeted therapy licensed for patients with chronic-phase (CP) CML, although dasatinib and nilotinib have also received approval in this setting. Dasatinib and nilotinib are extensively used in the second-line setting for patients with intolerance and/or resistance to imatinib. They have recently also received regulatory approval in the US, the EU, and Japan in the first-line setting. Bosutinib has been shown to be efficacious with an acceptable safety profile in an open-label phase 2 trial in the second and third line,Citation8–Citation10 as well as in an ongoing phase 3 trial in patients with newly diagnosed CP CML.Citation11,Citation12
Objective
To provide a qualitative overview of the clinical effectiveness, safety, and quality of life of TKI treatments in CP, accelerated- and blast-phase (AP/BP) CML patients.
Methods
Study inclusion criteria
Inclusion criteria are detailed in . Only randomized controlled trials (RCTs) evaluating a TKI, as well as registrational studies of TKIs, published before September 2011, were included. Participants had to be adults (≥18 years) with chronic, AP, and/or BP CML. First to third-line treatment with bosutinib, imatinib, dasatinib or nilotinib was considered. Studies on IFN-α and older agents as well as studies on stem cell transplantation were excluded. There were no restrictions placed on comparators used in the studies.
Table 1 Summary of inclusion and exclusion criteria for the systematic review
Efficacy outcomes were included, but were not restricted to duration and time to response, response rates (cytogenetic, molecular, and hematological), overall survival (OS), event-free survival (EFS), time to treatment failure (TTF), time to and rate of transformation to AP or BP, and health-related quality of life (HRQoL). Reported safety outcomes included adverse events (AE) (all grades) and the incidence of serious AEs.
Search strategy
The following electronic databases were searched: the Cochrane Library (incorporating the Central Register of Controlled Trials, Central), OVID Medline, and OVID Embase. No restrictions on date of publication or language were applied.
Search terms included both free text and Medical Subject Headings terms (eg, leukemia, myelogenous, myeloid, chronic, imatinib, dasatinib, nilotinib, bosutinib). The following conference proceedings were also searched (2007–2011): American Society of Hematology, American Society of Clinical Oncology, and the European Hematology Association. Pfizer provided copies of two conference posters, the abstracts of which had been identified in the database searches.Citation11,Citation12
Quality assessment
The methodological quality of RCTs was assessed independently by two reviewers, according to methods recommended in section six of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0.Citation13 The likelihood of bias was assessed according to three criteria: adequacy of randomisation and allocation concealment procedures, adequacy of blinding procedures, and completeness of follow-up.
Results
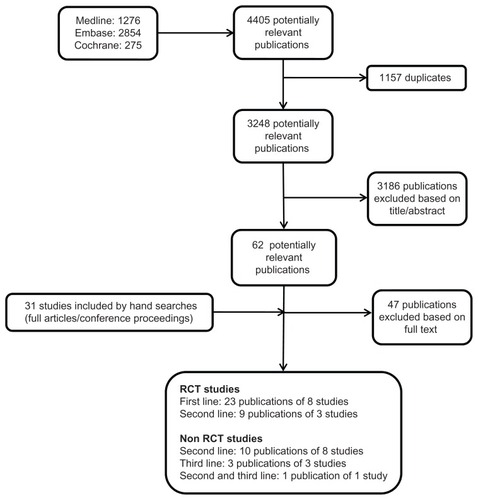
Electronic and manual searches identified 3248 potentially relevant publications, of which 3186 were excluded on the basis of title and abstract. Upon examination of the full texts, a further 47 were excluded. Thirty-one additional publications were identified via hand searching. In total, 46 publications, describing eleven RCTs and twelve single-arm studies, were included for detailed analysis (). Of the 11 RCTs identified,Citation11,Citation14–Citation23 eight investigated first-lineCitation11,Citation14–Citation20 and three second-line treatments.Citation21–Citation23 Only CP CML patients were included in the RCTs. No RCTs on third-line treatments were identified for inclusion in this systematic review, although one second-line trial included extensively pretreated patients.Citation21,Citation24 Of the single-arm studies, eight investigated second-line treatments,Citation9,Citation25–Citation31 three third-line treatments,Citation10,Citation32,Citation33 and one study enrolled both second- and third-line patients.Citation34 CP patients were enrolled in six trials,Citation9,Citation10,Citation25,Citation30,Citation31,Citation33 AP patients in one trial,Citation26 BP patients in one trial,Citation27 acute lymphoblastic leukemia (ALL) patients in a subgroup of one trial,Citation29 and mixed-patient populations were enrolled in three trials.Citation28,Citation32,Citation34
First-line treatments
One study (International Randomized Study of Interferon and STI571 [IRIS]) (eight publications) compared imatinib with IFN-α plus cytarabine.Citation14,Citation35–Citation41 Two trials (Dasatinib vs Imatinib Study In Treatment-Naïve CML [DASISION]Citation15,Citation42,Citation43 and S0325Citation16), compared imatinib with dasatinib. Imatinib was also compared with nilotinib in one trial (Evaluating Nilotinib Efficacy and Safety in Clinical Trials – Newly Diagnosed Patients [ENESTnd]).Citation17,Citation44–Citation46 A single RCT (Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia [BELA]) compared bosutinib with imatinib.Citation11,Citation12,Citation47 Different doses/administration schedules of imatinib were compared in two trials.Citation18,Citation19,Citation48 Different dose regimens of dasatinib were compared in one trial.Citation20 An overview of the included publications, including patient baseline characteristics and main efficacy outcomes, is provided in and .
Table 2 Overview of included publications: study details and baseline patient characteristics
Table 3 Overview of included publications – efficacy results
Imatinib versus interferon-α plus cytarabine (IRIS)
A total of eight publications reported results from a prospective, multicenter, open-label, phase 3 RCT comparing imatinib 400 mg/day with IFN-α (target dose of 5 MU/m2/ day) plus cytarabine (20 mg/m2 for 10 days per month, once maximum IFN-α dose was reached). Overall, 1106 patients were randomized in the IRIS trial.
In 2003, O’Brien et alCitation14 published the first data (after a median follow-up of 19 months): estimated complete cytogenetic response (CCyR) rates at 18 months were 76.2% with imatinib versus 14.5% with IFN-α plus cytarabine (P < 0.001). At 18 months, the estimated rate of freedom from progression to AP or BP was 96.7% with imatinib and 91.5% with the combination-therapy (P < 0.001). The most common AEs reported with imatinib were superficial edema, nausea, muscle cramps, and rashes. Rates of discontinuation (for any reason) and crossover to the alternative treatment were higher in the IFN-α group than in the imatinib group (discontinuations 12.3% with imatinib vs 31.6% with IFN-α; crossover 2.0% with imatinib vs 57.5% with IFN-α). Further results from this study were published in 2006,Citation36 when (after a median follow-up of 60 months) cumulative CCyR rates were estimated at 69% by 12 months and 87% by 60 months. Newly occurring or worsening grade 3/4 AEs were infrequent after 4 years of therapy, and there had been no change in the AE profile. Hochhaus et alCitation37 published 6-year follow-up data (focusing on patients treated with imatinib), and they reported no further cases of disease progression and an unchanged AE profile. Seven-year data reported a best CCyR of 82%, and a total of 317 (57%) of all randomized patients remained on imatinib and were in CCyR (38). At 8 years,Citation39 55% of patients initially randomized to imatinib were still on study treatment. The estimated OS rate was 85% (or 93% for CML-related deaths only). The authors also concluded that most progression events occurred within 3 years of imatinib treatment, with a very low risk of progression thereafter. A retrospective analysis of the trial dataCitation35 favored imatinib dose escalation for the initial treatment of CML patients with suboptimal CCyR or cytogenetic resistance.
Guilhot et alCitation41 investigated the relationship between time to CCyR and long-term outcomes in patients treated with imatinib. Results were reported in an abstract and indicated that the durability of major cytogenetic response did not differ significantly, regardless of when CCyR was achieved (P = 0.76) in patients who were treated for at least 1 year and achieved CCyR during therapy. Patients who did not achieve CCyR had significantly worse outcomes than those who did achieve CCyR (P < 0.001). However, there was a nonstatistically significant difference observed when categorized according to time to response.
In 2010, Hughes et alCitation40 published an analysis of the longterm prognostic significance of an early molecular response (in imatinib-treated patients taking part in the IRIS trial). The authors found that EFS was shorter and rates of progression higher in patients with BCR-ABL transcripts > 10% at 6 months and >1% at 12 months. Also, only 3% of patients who had achieved a major molecular response (MMR) by 18 months lost CCyR by 7 years, compared with 26% of patients without MMR (but with CCyR) at 18 months (P < 0.001). Of patients with MMR (at 12 or 18 months), 99% did not progress to AP or BP, compared with approximately 90% of patients without MMR (at 12 or 18 months). The authors concluded that molecular response status early during treatment may serve as a predictor of optimal response to therapy.
Nilotinib versus imatinib (ENESTnd)
A single phase 3, open-label RCT (ENESTnd) compared nilotinib (300 mg or 400 mg twice daily [BID]) with imatinib 400 mg once daily (OD) in 846 patients.Citation17 Rates of MMR at 12 months were significantly higher in the nilotinib treatment groups (44% with 300 mg, and 43% with 400 mg) compared with imatinib (22%, P < 0.001 for both comparisons). The difference in cumulative CCyR rates by 12 months was also statistically significant: 80% and 78% for nilotinib 300 mg and 400 mg, respectively, compared with 65% for imatinib (P < 0.001 for both comparisons). Transformation to AP or BP occurred in eleven patients (4%) receiving imatinib, two patients (<1%) receiving nilotinib 300 mg, and one patient (<1%) on nilotinib 400 mg. There were some differences in the AE profile between nilotinib and imatinib. With nilotinib, the incidence of rash, headache, and pruritus increased, as did levels of bilirubin, lipase, alanine aminotransferase (ALT) and aspartate aminotransferase (AST). There were, however, fewer cases of nausea, vomiting, diarrhea, muscle spasms, edema, neutropenia, and creatinine increase. Discontinuation rates were comparable between the treatment arms. At 18-month follow-up,Citation44 MMR was reported in 66% of patients treated with nilotinib 300 mg and 62% of those receiving nilotinib 400 mg, compared with 40% of imatinib-treated patients (P < 0.0001 vs imatinib for both nilotinib doses). A complete molecular response (CMR) was reported in 21% of patients treated with nilotinib 300 mg and 17% of those receiving nilotinib 400 mg, compared with 6% of imatinibtreated patients (P < 0.0001 vs imatinib for both nilotinib doses). Similarly, CCyR rates of 85% (P < 0.001) and 82% (P = 0.017) were reported for nilotinib 300 mg and 400 mg treated patients, respectively, versus 74% of imatinib-treated patients. The 24-month follow-up data confirmed the previous results favouring nilotinib.Citation45,Citation46 The MMR rates at 24 months were 37% for imatinib and 62% (P < 0.001) and 59% (P < 0.001) for nilotinib 300 mg and 400 mg, respectively.Citation45 Rates of CCyR were also significantly better with nilotinib (87%, P = 0.0018 with 300 mg, and 85%, P = 0.016 with 400 mg) than with imatinib (77%).Citation45,Citation46 In addition, CMR (4.5-log reduction) at any time was achieved by 26% and 21% of nilotinib 300 mg and 400 mg-treated patients, respectively, versus 10% of imatinib-treated patients (P < 0.0001 vs imatinib for nilotinib 300 mg, and P = 0.0004 for nilotinib 400 mg).Citation46 Progression rates were 4.2% with imatinib compared with 0.7% (P = 0.006) and 1.1% (P = 0.020) with nilotinib 300 mg and 400 mg, respectively. There was, however, no significant difference in OS: 96.3% for imatinib, and 97.4% (P = 0.65) and 97.8% (P = 0.21) for nilotinib 300 mg and 400 mg, respectively. There were no notable changes in the AE profile of nilotinib.
Dasatinib versus imatinib (DASISION, SO325)
The open-label, phase 3 DASISION RCT (519 patients) compared imatinib 400 mg OD with dasatinib 100 mg OD.Citation15 Confirmed cumulative rates of CCyR by 12 months and MMR by 12 months were significantly higher with dasatinib compared with imatinib (77% v 66%, P = 0.007, and 46% vs 28%, P < 0.0001). This difference in CCyR and MMR was observed across all Hasford risk (HR) categories. In addition, the times to CCyR and MMR were significantly shorter with dasatinib compared with imatinib (HR 1.5, P < 0.0001, and HR 2.0, P < 0.0001, respectively). In the dasatinib and imatinib treatment arms, 1.9% and 3.5% of patients, respectively, progressed to AP/BP. Rates of progression-free survival (PFS) at 12 months were similar between the treatment arms. Notable differences in the incidence of AEs were grade 3/4 thrombocytopenia (19% with dasatinib vs 10% with imatinib), fluid retention (19% vs 42%), nausea (8% vs 20%), myalgia (6% vs 12%), and muscle inflammation (4% vs 17%). Discontinuation rates were similar between the treatment groups. Efficacy and safety results at 18-month follow-upCitation42 were consistent with those published at 12 months. The rates of cumulative confirmed CCyR and MMR rates at any time for dasatinib vs imatinib were 78% vs 70% (P = 0.0366), and 57% vs 41% (P = 0.0002), respectively. Transformation to AP or BP occurred in six (2.3%) patients on dasatinib and nine (3.5%) patients on imatinib. After 24 months of minimum follow-up, cumulative rates of confirmed CCyR and MMR were 80% and 64% for dasatinib and 74% and 46% for imatinib, respectively.Citation43 The cumulative rate of CMR (4.5-log reduction) by 24 months was 17% for dasatinib compared with 8% for imatinib (P = 0.002). The transformation rates for dasatinib and imatinib were 2.3% (n = 6) and 5% (n = 13), respectively (during treatment). With regards to the AE profile, most cytopenias occurred in the first 12 months.
Radich et alCitation16 compared imatinib 400 mg OD with dasatinib 100 mg OD in an open-label phase 2 trial, randomising 253 patients. The rates of CCyR were not significantly different between the treatment arms at 12 months (69% with imatinib, 82% with dasatinib, P = 0.097), although data were only available for 51% of patients. Progression data were not reported. In the dasatinib and imatinib arms, 15% and 11% of patients, respectively, discontinued due to toxicity. Hematologic AEs were the most common grade 3/4 AEs (eg, thrombocytopenia reported in 18% and 8% of patients in the dasatinib and imatinib treatment groups, respectively, P = 0.024). Several nonhematologic grade 4 AEs (not defined in the publication) were reported for 6% of dasatinib-treated patients and no imatinib patients. Pleural effusion (any grade) was more common with dasatinib compared with imatinib (11% vs 2%, P = 0.0017).
Bosutinib versus imatinib (BELA)
A single ongoing, open-label, phase 3 RCT (BELA) (502 patients randomized) of bosutinib (500 mg OD) compared with imatinib (400 mg OD) in the first-line setting has been reported with patients followed for up to 24 months.Citation11,Citation12,Citation47 Numerically higher CCyR at 1 year (70% versus 68%) and cumulative CCyR rates by 1 year (79% versus 75%) were reported for bosutinib-treated versus imatinib-treated patients, although these differences were not statistically significant.Citation12 Bosutinib-treated patients reported both significantly higher MMR at 1 year (41% vs 27%, P = 0.002) and a 1-year cumulative MMR rate (47% vs 32%, P < 0.001) compared with imatinib-treated patients.Citation12 Adverse events that were more frequent with bosutinib compared with imatinib at 12 months were mainly gastrointestinal (GI), and included diarrhea (69% vs 22%) and vomiting (32% vs 14%).Citation12 With imatinib, the incidence of edema (peripheral, 11% vs 4%; periorbital, 14% vs 1%), muscle cramps (20% vs 4%), and bone pain (10% vs 4%) were higher compared with bosutinib. Citation12 Imatinib was also associated with a higher incidence of hematological AEs, including neutropenia (21% vs 3%). With regard to laboratory abnormalities, hypophosphatemia was reported more frequently with imatinib compared with bosutinib (17% vs 4%), while more bosutinib-treated patients experienced elevated ALT (23% vs 3%) or AST (11% vs 3%) compared with imatinib. The rates of discontinuation due to AEs were 22% for bosutinib and 5% for imatinib at 18 months (12-month discontinuation data: 19% vs 6%); none of these discontinuations were due to diarrhea.Citation12 By 18-month follow-up,Citation12 the rates of cumulative CCyR were identical for bosutinib and imatinib (both 79%). However, cumulative CMR (18% vs 10%) and MMR (55 vs 45%) remained significantly in favor of bosutinib. By 24 months,Citation47 the reported CCyR was similar for bosutinib (79%) and imatinib (80%), although the cumulative MMR remained significantly in favor of bosutinib (61% vs 50%, P < 0.05). At 24 months, the times to CCyR and MMR were also significantly in favor of bosutinib (P < 0.001). The cumulative rate of CMR (4.5-log reduction) by 24 months was 23% for bosutinib compared with 16% for imatinib (P = 0.002). Transformation to AP or BP occurred in a numerically higher percentage of patients treated with imatinib at both 12 (4% vs 2% with bosutinib, P = 0.053) and 18 and 24 months (5% vs 2% at both time points). Treatment failure was less common with bosutinib compared with imatinib (4% vs 13%). At the time of reporting, median OS had not been reached at 24-month follow-up (survival estimates for bosutinib and imatinib were 97% and 95%, respectively).Citation47 Patient- reported outcome measures of functioning and health status showed that the different AEs associated with bosutinib and imatinib had minimal overall impact.Citation49
Dose-finding studies (imatinib, n = 2; dasatinib, n = 1)
The two imatinib studies (Baccarani et alCitation48 and the Tyrosine Kinase Inhibitor Optimization and Selectivity [TOPS] studyCitation18) randomized patients (n = 200Citation48 and n = 460Citation18) to treatment with imatinib 400 mg/day or 800 mg/day. A numerically higher, nonsignificant response was reported for patients in the 800 mg/day group compared with 400 mg/day for both CCyRCitation18,Citation48 and MMRCitation18 at 1 year, which was confirmed at 2-year follow-up in the TOPS study.Citation19 With imatinib 800 mg/day, a higher incidence of edema, GI AEs, and rash, as well as grade 3/4 hematological toxicities, was reported with imatinib 800 mg/day.
Cortes et alCitation20 randomized 62 patients to dasatinib 100 mg OD or 50 mg BID. No significant difference between treatment arms was reported with regard to response (CCyR and MMR rates) or the incidence of AEs at 1-year follow-up.
Second-line treatments
Of the nine included single-arm studies, six were on dasatinib,Citation25–Citation30 one reported on nilotinib,Citation31,Citation50 one on bosutinib,Citation9,Citation51 and a further study enrolled second-line patients to treatment with either nilotinib or dasatinib.Citation34 Three RCTs investigated second-line treatments, all with dasatinib. One trial (START-R) compared high-dose imatinib with dasatinib.Citation21,Citation24 Different dose regimens of dasatinib were compared in two trials.Citation22,Citation23,Citation52–Citation56 An overview of the included publications, including patient baseline characteristics and main efficacy outcomes, is provided in and .
Dasatinib single-arm (registrational) studies
In an international, open-label, phase 2 study,Citation25 387 imatinib-resistant or -intolerant CP CML patients were treated with dasatinib. Results were only available for the first 186 patients. The best confirmed CCyR rate at 8-month follow-up was 39% (n = 73). Rates of MMR and OS were not reported, whereas the PFS rate was 92.4%. Baseline BCR-ABL mutational status was analysed in 180 of 186 patients. With the exception of a single mutation (T315I, identified in 2% of patients, none of whom attained a major CyR [MCyR] or a complete hematological response), there was no notable influence on the response rate. Imatinib-resistance mutations were only identified in 41% of patients analyzed. After 8 months, 9% (n = 6) of patients had discontinued due to AEs. The most frequent all-grade AEs were AST and ALT elevation (60% and 52%, respectively), followed by headache (34%), diarrhoea (30%), fatigue (28%), and dyspnea (27%). Cytopenias were the most common grade 3/4 AEs (ranging from 22% for anemia to 49% for neutropenia).
Talpaz et alCitation28 enrolled 40 CP CML patients, as well as 44 patients with AP CML, BP CML, or ALL, in a phase 1, open-label dose-escalation study. All patients were resistant or intolerant to imatinib. The overall CCyR rate was 30% (n = 25). Whereas the responses were maintained after 2–19 months in CP or AP CML patients, the responses of BP CML and ALL patients were of short duration. Mutational testing was performed in all patients, although mutations were only detected in 71% of patients at baseline. Responses were observed across all BCR-ABL genotypes, with the exception of T315I (associated with resistance to both imatinib and dasatinib). Diarrhea (23%), peripheral edema (19%), and headache (10%) were commonly reported AEs. Neutropenia and thrombocytopenia (grade 3/4) were reported in 45% and 35% of CP CML patients and 89% and 80% of BP CML and ALL patients.
Cortes et alCitation27 reported data from 74 myeloid BP (MBP) CML and 42 lymphoid BP (LBP) CML patients, who took part in two phase 2, open-label, single-arm, international studies on dasatinib (START-B and START-L), respectively. The CCyR rates at 8 months were 27% (n = 20) and 43% (n = 43) for MBP and LBP patients, respectively. After 8 months, the discontinuation rates (due to AEs) were 11% and 2% for MBP and LBP patients, respectively. Disease progression was reported for three imatinib-resistant and no imatinib-intolerant patients. Baseline BCR-ABL mutation data were available from about 95% of patients. Mutations associated with very high imatinib resistance (M244V, G250E, Y253H, E255K, E255V, T315I, F359V, H396R) were associated with the lowest response rates to dasatinib. Among MBP CML patients, the most frequently reported AEs (any grade) were diarrhea (36%), pleural effusion (28%), peripheral edema (19%), and dyspnea (18%). The most common grade 3/4 AEs in MBP patients were pleural effusion (14%), diarrhea (8%), GI hemorrhage (8%), and dyspnea (7%). Diarrhea (31%), fatigue (29%), and nausea and vomiting (24%) were the most common AEs (any grade) reported by LBP CML patients. The most frequent grade 3/4 AE in LBP CML patients was febrile neutropenia (12%).
Ottmann et alCitation29 reported results from the START-L trial, focusing on ALL patients (n = 36). The rate of best CCyR at 8-month follow-up was 58% (n = 21). Of the 67% (n = 15) of patients who had achieved a major hematologic response (MHR), five had experienced disease progression by the 8-month follow-up. In this study, the T315I mutation was found in six patients (17%), and was, as expected, associated with a lack of response. However, overall response rates for patients with resistance mutations were comparable to those for the total population (eg, MCyR was achieved by 56% of patients with any mutation, compared with 58% of the total patient population). The most frequently reported AEs of any grade were diarrhea (31%), pyrexia (25%), and nausea (22%), whereas the most common grade 3/4 events were febrile neutropenia (11%), diarrhea (8%), and asthenia (8%).
In the international phase 2 START-C study, 387 CP CML patients who were resistant (n = 288) or intolerant (n = 99) to imatinib were enrolled. Rates of CCyR and MMR after a minimum follow-up of 24 months were 53% and 47%, respectively. Rates of PFS and OS at 24 months were 80% and 94%, respectively. With the exception of the T315I mutation, responses were observed across all mutations. Thrombocytopenia (49%), neutropenia (50%), pleural effusion (9%), dyspnea (6%), bleeding (4%), diarrhea (3%), and fatigue (3%) were among the most common grade 3/4 AEs.
Guilhot et alCitation26 recruited 107 AP CML patients (resistant or intolerant to imatinib) to an international, open-label phase 2 study. At 8-month follow-up, the CCyR rate was 24%. After a minimum of 8 months of follow-up, 76% of patients were progression-free. Imatinib-resistance mutations were identified in 60% of patients tested at baseline. With the exception of T315I, the identified imatinib-resistance mutations were generally not associated with low response rates to dasatinib. Grade 3/4 hematological AEs occurred in 61% (leukopenia) to 82% (thrombocytopenia) of patients. The most frequent nonhematological AEs of any grade were diarrhea (50%), headache (28%), pyrexia, fatigue, and pleural effusion (23%), and of grade 3/4 were GI bleeding (7%) and diarrhea (6%).
Nilotinib single-arm (registrational) study
In a phase 2, open-label, international study, 318 CP CML patients intolerant or resistant to imatinib received nilotinib 400 mg BID.Citation31 Rates of CCyR and MCyR at 6 months were 31% and 48%, respectively, and 12-month OS was estimated at 95%. Baseline BCR-ABL mutation status data were available for 56% of patients. Rates of MCyR and CCyR were lower in patients with mutations than in those without (42% and 23% vs 51% and 35%, respectively). T315I was the only mutation associated with MCyR and complete hematologic response (CHR) rates of 0%. Rash, nausea, pruritus, fatigue, and headache were the most common AEs (all grades, 28%–19%), with rash, headache, and diarrhea as the most frequent grade 3/4 AEs (3%, 2%, and 2%, respectively). In 2011, Kantarjian et alCitation50 published 24-month follow-up data: 44% of patients achieved a cumulative CCyR (41% of imatinib-resistant and 51% of imatinib-intolerant patients). The median time to CCyR was approximately 3.2 months. MMR was reported in 28% of patients (294 of 321 were evaluated). At 24 months, the estimated PFS was 64%. Baseline CHR was found to be a predictive factor for achieving MCyR, CHR, MMR, and PFS. No changes in the overall AE profile were observed after 24 months.
Bosutinib single-arm study
Bosutinib was evaluated in 288 CP CML patients resistant or intolerant to imatinib in a phase 1/2 open-label, multicenter study by Cortes et al.Citation51 After a median follow-up period of 24.2 months, the cumulative CCyR rate was 41% (n = 110), and 64% (n = 50) of these patients achieved an MMR. Rates of OS were 97% and 92% at 1 and 2 years, respectively. Baseline mutation status was available for 40% of patients (42% of imatinib-resistant and 36% of imatinib-intolerant patients). Rates of CHR and MCyR were similar for patients with and without mutations. Diarrhea (84%), nausea (44%), rash (44%), and vomiting (35%) were the most frequent nonhematological AEs. Reported grade 3/4 hematological abnormalities were thrombocytopenia (24%), neutropenia (18%), and anemia (13%). On-treatment grade 3/4 elevations of ALT and AST were reported by 10% and 5% of patients, respectively. Gambacorti-Passerini et alCitation9 reported 31.8-month (median) follow-up data. The best cumulative CCyR observed was 43% (n = 114). MMR was also observed in 43% (n = 85) of evaluable patients. The authors estimated the OS to be 97% and 91% at 1 and 2 years, respectively. At 1 and 2 years, the estimated PFS rates were 91% and 81%. Rates of CHR between 33% and 100% were reported for the different mutations identified, including one of three patients with the T315I mutation. Rates of MCyR ranged from 0% (T315I) to 75%. Diarrhea, nausea, and vomiting were the most common AEs, and the AE profile was broadly similar to that previously reported for bosutinib. The study also collected HRQoL data.Citation8 Significant improvements in five subscales were reported by imatinib-resistant patients at 12, 24, and 48 weeks, exceeding the minimally important difference (MID) at 48 weeks. At 96 weeks, changes were significant for all but two subscales (imatinib-resistant patients), all but one exceeding the MID. Imatinib-intolerant patients first reported significant changes in four subscales at 24 weeks, six subscales at 48 weeks (of which the MID was exceeded for five), and similar to the imatinib-resistant patients, experienced improvement in all but two subscales, of which all but one exceeded the MID at 96 weeks (see ).
Table 4 QoL data
Nilotinib/dasatinib after imatinib
Garg et alCitation34 reported both second- and third-line results. Of the 34 patients treated with second-line nilotinib, 17 were in CP, ten in AP, and seven in BP. The best observed CCyR and MMR rates were 9% (n = 3) and 15% (n = 5), respectively. In the second-line dasatinib arm, eight CP, three AP and three BP patients were treated. Best responses included 14% CCyR (n = 2) and no MMR. The median time on the second-line treatment was 8.3 months. Data on AEs are reported in the third-line section.
Dasatinib dosing studies
Two dasatinib dosing studies were identified. Dasatinib 140 mg OD was compared with 70 mg BID in an open-label phase 3 trial, with results for two separate populations reported.Citation22,Citation52 Enrolling 317 AP patients resistant or intolerant to imatinib, Kantarjian et alCitation22 reported results after a minimum of 0.16 months and a median of 15 months of follow-up. The CCyR rates were 32% with the OD and 33% with the BID administration schedule, respectively (MMR rates were not reported). At 24 months, estimated PFS rates were 51% and 55% with the OD and BID administration schedules, respectively. The administration schedule did not appear to affect the response rate by mutation status. The most common AEs were diarrhea, fluid retention, nausea, headache, and fatigue. The incidence of GI bleeding and fluid-retention events was lower with the OD administration schedule. Saglio et alCitation52 reported results for 149 patients in myeloid and 61 patients in LBP. Both groups were randomized 1:1 to dasatinib 140 mg OD or 70 mg BID. In MBP patients, the CCyR rates were 14% with the OD and 21% with the BID administration schedule. In LBP patients, the CCyR rates were higher, with 38% and 36% for OD and BID administration schedules, respectively (no MMR rates reported). The 24-month PFS rate was 11% for the OD and 18% for the BID administration schedule. Rates of MHR were similar for patients with or without baseline mutations for both dosing schedules, except for patients with the T315I mutation, none of whom achieved an MHR. Safety results were generally consistent with those reported by Kantarjian et al;Citation22 only pleural effusion was less frequent with the OD regimen versus BID administration in LBP but not MBP patients.
Shah et alCitation23 randomized 670 patients to dasatinib 100 mg OD, 50 mg BID, 140 mg OD, or 70 mg BID in an open-label phase 3 trial. The minimum and median follow-ups at the time of the analysis were 6 and 8 months, respectively. No major differences in response rates were observed: CCyR rates were 41% with 100 mg OD, 42% with 50 mg BID, 44% with 140 mg OD, and 45% with 70 mg BID. Rates of MMR were not reported. Rates of disease progression or death were 8% with 100 mg OD, 50 mg BID, and 140 mg OD, and 11% with 70 mg BID. The dose/administration schedule did not appear to affect the response rate by mutation status. Patients in the 100-mg OD treatment arm experienced fewest treatment-related AEs (eg, pleural effusion, thrombocytopenia, or nausea). Two-year follow-up results were reported in subsequent publications.Citation53,Citation54 The observed CCyR rates were 54% with 50 mg BID, and 50% with each of the other three administration schedules; MMR rates were 37% with 100 mg OD, and 38% with each of the other three administration schedules. Response rates for patients with any or no mutations were comparable between the treatment groups (except for T315I, which was associated with no CCyR). The differences between the AE profiles of the different dose/administration schedules were consistent with those reported earlier. In 2010, Shah et alCitation55 presented 4-year follow-up results, reporting that response rates were similar in all treatment arms. Results were presented for the 100-mg OD arm only,Citation56 as the percentage remaining on treatment was highest in this arm (35%, compared with 31% on 50 mg BID or on 70 mg BID, and 27% on 140 mg OD). The best overall response within 24 months was 50% CCyR. Within 5 years, the cumulative MMR rate was 44%, and 5% (n = 8) of patients had experienced transformation. It was reported that nonhematological and hematological AEs first occurred generally within 24 and 12 months of treatment.
High-dose imatinib versus dasatinib in extensively pretreated patients
In a study by Kantarjian et alCitation21 150 patients who were resistant to imatinib were randomized (2:1) to either dasatinib 140 mg or imatinib 800 mg. Patients had undergone previous treatments for between 6 and 166 months. Crossover was permitted upon progression, lack of response, or intolerance. After 12 weeks of randomized treatment, CCyR rates with dasatinib were significantly higher than those with high-dose imatinib (22% vs 8%, P = 0.041). After a median follow-up of 15 months, the superiority of dasatinib was maintained, with CCyR rates of 40% (dasatinib) and 16% (imatinib, P = 0.004); MMR rates also favored dasatinib (16%) over imatinib (4%, P = 0.038). In total, 15% of patients crossed from the dasatinib group to the imatinib group, and 80% of patients randomized to imatinib crossed over to dasatinib. At baseline, 52 patients (38%) had an imatinib-resistant BCR-ABL mutation. Of these, 19 of 41 patients (46%) in the dasatinib group and three of 11 patients (27%) on imatinib achieved an MCyR (P = 0.282). The observed AEs corresponded to the known safety profile of the treatments, and there was no major difference between treatments. Two-year follow-up data indicated that favorable response rates were maintained with dasatinib. Citation24 The CCyR was 44% with dasatinib versus 18% with imatinib (P = 0.0025), and the MMR rates were 29% with dasatinib versus 12% with imatinib (P = 0.028). Compared with imatinib, dasatinib-treated patients reported a higher incidence of grade 3/4 neutropenia, thrombocytopenia, and leukopenia.
Third-line treatments
BosutinibCitation10 and nilotinibCitation32 have been investigated as third-line treatment options in single-arm studies, whereas two studies allowed a choice of either nilotinib or dasatinib as treatment.Citation33,Citation34 An overview of the included publications, including patient baseline characteristics and main efficacy outcomes, is provided in and .
Nilotinib
Patients with CML in CP (n = 37) or AP (n = 17) were enrolled in an international phase 2 study by Giles et al.Citation32 After a median follow-up of 12 months, no patients in AP and 24% (n = 9) of patients in CP achieved CCyR. At 18 months, the PFS was estimated to be 59%, with a survival rate of 86%; median OS was not reached at that point. Disease progression (n = 19, 35%), AEs (n = 10, 19%), and death (n = 2, 4%) were the most common reasons for discontinuation. The most common grade 3/4 AEs were neutropenia (23% CP, 33% AP), thrombocytopenia (28% CP, 19% AP), hyperphosphatemia (13% CP, 24% AP), and elevated lipase levels (25% CP, 10% AP).
Nilotinib/dasatinib
Ibrahim et alCitation33 and Garg et alCitation34 evaluated 26 CP CML patients and 48 CML patients (25 in CP, 10 in AP, 13 in BP) who had failed on imatinib therapy as well as on dasatinib or nilotinib. The third-line study treatment was nilotinib or dasatinib in both studies. In the study by Ibrahim et al,Citation33 cumulative rates of CCyR and MMR after a median of 21.5 months’ follow-up were 34.6% (n = 9) and 19.2% (n = 5), respectively. Probabilities of EFS and OS at 30 months were 45.7% and 46.7%, respectively. AEs were not reported. The authors found that previous achievement of a cytogenetic response was a predictor for third-line treatment success as well as OS.
During third-line treatment in the study by Garg et al,Citation34 CCyR was achieved by five patients (31%) in CP, two (25%) in AP, and two (20%) in BP in patients receiving dasatinib third-line. In the nilotinib group, one (11%) patient in CP, no patients in AP, and one (33%) in BP achieved CCyR. The corresponding numbers of patients reaching MMR were two (13%) in CP, one (13%) in AP, and one (10%) in BP in patients receiving dasatinib, and three (33%) in CP, one (50%) in AP, and no patients in BP in nilotinib-treated patients. AEs were not reported. The median EFS was 13 months overall, ranging from 20 months for CP patients, over 5 months for AP patients, and only 3 months for BP patients.
Bosutinib
In a phase 1/2, open-label, multicenter study,Citation57 bosutinib 500 mg/day was evaluated in CP CML patients in the third-line setting. Of the 118 enrolled patients, 64 were resistant to prior imatinib and either dasatinib (n = 37) or nilotinib (n = 27), 50 were intolerant to prior imatinib and dasatinib, and four received fourth-line treatment (having received prior imatinib, nilotinib, and dasatinib). After a median follow-up time of 28.5 months (ranging from 20.0 months in the dasatinib- resistant group to 34.5 months in the dasatinib-intolerant group), the best cumulative CCyR rate in the overall study population was 24% (n = 26). Within the separate cohorts, the reported CCyR rates were 14% (n = 5) and 27% (n = 7) in the dasatinib- and nilotinib-resistant groups, respectively, 28% (n = 12) in the dasatinib-intolerant group, and 50% (n = 2) in the fourth-line patients. Estimated OS was 91% at 1 year and 83% at 2 years. Within the separate cohorts, the estimated 2-year OS was 75% and 92% in the dasatinib- and nilotinib-resistant groups, respectively, 85% in the dasatinib-intolerant group and 75% in the fourth-line patients. Five patients (three dasatinib-resistant, one nilotinib-resistant, and one fourth-line) progressed to AP CML. The most common AEs were GI-related. The most common grade 3/4 AEs were thrombocytopenia (25%, n = 30), neutropenia (19%, n = 23), hypermagnesemia (12%, n = 14), diarrhea (8%, n = 10), and elevated ALT (7%, n = 8).
Discussion
This qualitative review is limited by the small number of trials investigating any given drug or combination treatment. Initially, the second-generation TKIs were investigated in the second- and third-line setting, as there were no active comparators available to be used in clinical trials and a license in this setting could therefore be obtained on the basis of a single-arm trial.
The structured literature search, including conference abstracts, and the assessment of the methodological quality of the included articles by two individuals separately, contribute to the strength of evidence provided by this systematic review.
First-line treatments
First-generation TKIs represent the first targeted therapy for CML, superseding chemotherapeutic agents and IFN-α. The comparison of imatinib with IFN-α (plus cytarabine) in a key trial (IRIS), follow-up data which is reported up to 8 years, demonstrated the clear superiority of imatinib over IFN-α.Citation14,Citation35–Citation39 Imatinib was the first TKI to be extensively used for the treatment of CML. The two included dose-finding studiesCitation18,Citation19,Citation48 did not report a difference between 400 mg/day and 800 mg/day in CCyR rates at 1 year, although results up to 24 months favored the higher dose.Citation18,Citation19
When second-generation TKIs became available, they were naturally compared with imatinib. Dasatinib was found to result in significantly higher cumulative response rates by 12 and 18 months than imatinib in the single trial comparing the treatments.Citation15,Citation42 At 24-month follow-up, CCyR, MMR, and CMR were higher in the dasatinib treatment group compared with imatinib,Citation45 although the difference was no longer statistically significant for CCyR. Nilotinib was also associated with higher response rates reported at 12, 18, and 24 months,Citation17,Citation44,Citation46 compared with imatinib in a single trial. At 12 and 24 months, CCyR, MMR, and CMR rates were significantly higher in both nilotinib treatment groups than in the imatinib group (P < 0.001). With bosutinib,Citation11,Citation12 CCyR rates at 1 year were numerically higher than those in the imatinib group, whereas MMR rates at and by 1 year were significantly higher with bosutinib than with imatinib (P = 0.002 and P < 0.001, respectively). With bosutinib, responses were also achieved significantly faster (P < 0.001). Superior rates of MMR, CMR, and similar rates of CCyR were reported for bosutinib compared with imatinib at 24 months of follow-up.Citation47 QoL data from the bosutinib study were published,Citation49 indicating that after a minimum follow-up period of 12 months (median 16.6 months), no significant between-group difference was reported between the bosutinib and imatinib treatment arms.
Therefore, although relatively few RCTs have been published to date, available results indicate that treatment with the second-generation TKIs is associated with higher response rates for most outcomes at 2-year follow-up compared with imatinib.Citation43,Citation46,Citation47 There are also indications that a faster response can be achieved with second-generation TKIs,Citation12 and there is emerging evidence that a quicker response may be associated with a more favorable outcome for imatinib.Citation58 All second-generation TKIs reported higher rates of CMR (≤4.5-log reduction) by 24 months compared with imatinib. Achievement of a “deeper” response appears to be clinically relevant, as indicated by results from the Stop Imatinib (STIM) study,Citation59 in which imatinib treatment was stopped in 100 patients who had been on the drug for at least 2 years and who had achieved CMR during treatment. After stopping imatinib treatment, 41% of patients maintained CMR at 1-year follow up, suggestive of a TKI-induced “cure” in a subset of patients.
In the absence of head-to-head RCTs comparing the second-generation TKIs, it is challenging to make robust conclusions on their relative efficacy. A recent indirect comparison reported on the relative efficacy of nilotinib and dasatinib and concluded that patients treated with nilotinib 300 mg BID experienced significantly higher MMR by 12 months compared with dasatinib-treated patients.Citation60
Second-line treatments
The second-line single-arm studies on dasatinib, nilotinib, and bosutinib showed that these agents can elicit responses (CyRs, HRs, and MRs) in patients who are resistant or intolerant to imatinib. Indeed, results from the studies conducted for nilotinib and dasatinib resulted in these agents being granted a license for this indication. Recently published data for bosutinib are also encouraging, and it is currently undergoing regulatory review in several countries. In contrast to the included RCTs (regardless of first- or second-line), which enrolled only CP CML patients, four of the five registrational single-arm studies on dasatinib focused on patients in BP, AP, or mixed-patient populations. In the course of the bosutinib single-arm study,Citation9,Citation51 QoL data were collectedCitation8 with a statistically significant and clinically meaningful increase reported at weeks 36, 48, and 96 follow-up.
Third-line treatments
No RCTs on third-line treatments were eligible for inclusion in this systematic review. This is primarily a result of the small numbers of eligible patients who are resistant/intolerant to multiple TKIs and therefore available for enrollment into a study and the paucity of active comparators in this setting. However, four single-arm studies were identified. Garg et al,Citation34 who followed 48 patients treated successively with three TKIs (starting with imatinib, followed by dasatinib and nilotinib second- or third-line), found that while a response was induced in some patients, it was not durable. Nilotinib was also found to be efficacious as a third-line TKI treatment.Citation32 Another similar study on third-line TKIs concluded that while therapy was only efficacious in a small proportion of patients, prior CCyR on first- or second-line TKI treatment could serve as a predictor for CCyR to third-line TKI treatment.Citation33 The largest study to date has been conducted with bosutinib,Citation57 and demonstrated clinical activity (comparable with other second-generation TKIs) with an acceptable AE profile.
Toxicity profiles
Each of the TKIs is associated with a characteristic AE profile (). Treatment with imatinib is predominantly accompanied by superficial edema, nausea, muscle cramps, and elevated rates of some hematological AEs (neutropenia and hypophosphatemia). Considering the second-generation TKIs, treatment with nilotinib is associated with increased incidence of rash, dasatinib with certain hematological AEs, and fluid retention (including pleural effusion), and bosutinib-treated patients report increased GI AEs.
Table 5 Incidence (percentage) of adverse events reported in included studies (all grade and grade 3/4)
Conclusion
There are a number of findings from the present systematic review. Firstly, there is now a wealth of data available over a long follow-up period (up to 8 years) to indicate that imatinib is clinically superior to IFN plus cytarabine,Citation14 and that the efficacy of imatinib is not improved by the addition of IFN-α, cytarabine, or granulocyte-macrophage colony-stimulating factor. Secondly, despite relatively short follow-up (2 years), there is increasing evidence to indicate that treatment with the second-generation TKIs dasatinib, nilotinib, and bosutinib offers improved, “deeper” responses that are achieved more rapidly compared with standard-dose imatinib in CP CML patients in both the first- and second-line setting. Although each of these therapies is associated with a distinct AE profile, the majority of AEs are low-grade and manageable. However, longer follow-up is required to confirm that the improved efficacy of the second-generation TKIs is maintained and to allow robust conclusions with regard to the effect of these improved response rates on OS. Although outside the scope of the current review, there are several therapies currently under investigation. In particular, ponatinib, a TKI inhibitor active against the BCR-ABL gene, is a promising agent. The single-arm, phase 2 Ponatinib Ph+ ALL and CML Evaluation (PACE) trialCitation61 was conducted in 397 patients with refractory CML in CP, AP, or BP, or Ph+ ALL, resistant or intolerant to dasatinib or nilotinib, or with the resistant T315I mutation. Initial data at a median follow-up of 57 days suggest that ponatinib has activity in heavily pretreated patients and in patients with the T315I mutation.
The current availability of several second-generation TKIs should allow selection of the most relevant treatment on an individualized basis, taking into account any comorbid conditions and mutation status if known. Currently, there is a paucity of data reporting on the effects of treatment on QoL outcomes (of importance in the management of CML, which requires long-term therapy) and in the third-line setting, where patients currently have limited treatment options. Further studies are required to address both these issues.
Acknowledgment
The authors would like to thank Dr David Marin for his work on reviewing our manuscript.
Disclosure
Stephen Mitchell and Sarah Batson are employees of Abacus International who were paid consultants to Pfizer in connection with development of this manuscript. All other authors have no conflicts of interest to disclose.
References
- HeisterkampNStamKGroffenJde KleinAGrosveldGStructural organization of the bcr gene and its role in the Ph’ translocationNature198531560227587612989703
- RowleyJDA new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa stainingNature197324354052902934126434
- PensergaETSkorskiTFusion tyrosine kinases: a result and cause of genomic instabilityOncogene2007261112016785987
- RohrbacherMHasfordJEpidemiology of chronic myeloid leukaemia (CML)Best Pract Res Clin Haematol200922329530219959081
- KantarjianHFaderlSTalpazMChronic myelogenous leukemiaDeVitaHellmanRosenbergCancer: Principles and Practice of OncologyPhiladelphiaLippincott Williams & Wilkins2001
- DeVitaVLawrenceTRosenbergSDeVita, Hellman, and Rosenberg’s Cancer: Cancer: Principles and Practice of Oncology8th edPhiladelphiaLippincott Williams & Wilkins2008
- PavlovskyCKantarjianHCortesJEFirst-line therapy for chronic myeloid leukemia: past, present, and futureAm J Hematol200984528729319306355
- TraskPCCellaDBessonNKellyVMassziTKimDWHealth- related quality of life of bosutinib (SKI-606) in imatinib-resistant or imatinib-intolerant chronic phase chronic myeloid leukemiaLeuk Res201236443844222036634
- Gambacorti-PasseriniCKimDWTurkinaABosutinib (SKI-606) as second-line therapy for chronic phase chronic myeloid leukemia following imatinib failure: analyses of cross-intolerance and response by prior response to imatinib16th Congress of the European Hematology AssociationJune 9–12, 2011London, UK
- Gambacorti-PasseriniCBrümmendorfTCortesJBosutinib as third-line therapy for chronic phase chronic myeloid leukemia following failure with imatinib and dasatinib or nilotinib16th Congress of the European Hematology AssociationJune 9–12, 2011London, UK
- Gambacorti-PasseriniCKimDWKantarjianHAn ongoing phase 3 study of bosutinib (SKI-606) versus imatinib in patients with newly diagnosed chronic phase chronic myeloid leukemia. Abstract 20852nd American Society of Hematology Annual MeetingDecember 10–13, 2010Orlando, FL
- Gambacorti-PasseriniCCortesJKhouryHJBosutinib (BOS) versus imatinib (IM) in patients with chronic phase chronic myeloid leukemia (CP CML) in the BELA trial: 18-month follow-up. Abstract 6509American Society of Clinical Oncology Annual MeetingJune 3–7, 2011Chicago, IL.
- The Cochrane CollaborationCochrane Handbook for Systematic Reviews of Interventions Version 5.1.02011 Available from: http://www.cochrane-handbook.orgAccessed June 26, 2012.
- O’BrienSGGuilhotFLarsonRAImatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemiaN Engl J Med200334811994100412637609
- KantarjianHShahNPHochhausADasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemiaN Engl J Med2010362242260227020525995
- RadichJPKopeckyKJKamel-ReidSA randomized phase II trial of dasatinib 100 mg vs imatinib 400 mg in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): the S0325 Intergroup Trial52nd American Society of Hematology Annual Meeting and ExpositionDecember 4–7, 2010Orlando, FL
- SaglioGKimDWIssaragrisilSNilotinib versus imatinib for newly diagnosed chronic myeloid leukemiaN Engl J Med2010362242251225920525993
- CortesJEBaccaraniMGuilhotFPhase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: tyrosine kinase inhibitor optimization and selectivity studyJ Clin Oncol201028342443020008622
- BaccaraniMDrukerBCortesJ24 months update of the TOPS study: a phase iii, randomized open label study of 400 mg/d (SD-IM) versus 800 mg/d (HD-IM) of imatinib mesylate (IM) in patients (Pts) with newly diagnosed, previously treated chronic myeloid leukemia in chronic phase (CML-CP). Abstract 33751st American Society of Hematology Annual Meeting and ExpositionDecember 5–8, 2009New Orleans, LA
- CortesJEJonesDO’BrienSResults of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemiaJ Clin Oncol201028339840420008620
- KantarjianHPasquiniRHamerschlakNDasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trialBlood2007109125143515017317857
- KantarjianHCortesJKimDWPhase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-upBlood2009113256322632919369231
- ShahNPKantarjianHMKimDWIntermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemiaJ Clin Oncol200826193204321218541900
- KantarjianHPasquiniRLevyVDasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R)Cancer2009115184136414719536906
- HochhausAKantarjianHMBaccaraniMDasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapyBlood200710962303230917138817
- GuilhotFApperleyJKimDWDasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phaseBlood2007109104143415017264298
- CortesJRousselotPKimDWDasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisisBlood200710983207321317185463
- TalpazMShahNPKantarjianHDasatinib in imatinib-resistant Philadelphia chromosome-positive leukemiasN Engl J Med2006354242531254116775234
- OttmannODombretHMartinelliGDasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 studyBlood200711072309231517496201
- MauroMJBaccaraniMCervantesFDasatinib 2-year efficacy in patients with chronic-phase chronic myelogenous leukemia (CML-CP) with resistance or intolerance to imatinib (START-C)J Clin Oncol200826May 20 SupplAbstr 7009
- KantarjianHMGilesFGattermannNNilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intoleranceBlood2007110103540354617715389
- GilesFJAbruzzeseERostiGNilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapyLeukemia20102471299130120520639
- IbrahimARPaliompeisCBuaMEfficacy of tyrosine kinase inhibitors (TKIs) as third-line therapy in patients with chronic myeloid leukemia in chronic phase who have failed 2 prior lines of TKI therapyBlood2010116255497550020833982
- GargRJKantarjianHO’BrienSThe use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow-upBlood2009114204361436819729517
- KantarjianHMLarsonRAGuilhotFEfficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phaseCancer2009115355156019117345
- DrukerBJGuilhotFO’BrienSGFive-year follow-up of patients receiving imatinib for chronic myeloid leukemiaN Engl J Med2006355232408241717151364
- HochhausAO’BrienSGGuilhotFSix-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemiaLeukemia20092361054106119282833
- O’BrienSGuilhotFGoldmanJInternational randomized study of interferon versus ST1571 (IRIS) 7-year follow-up: sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (PTS) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib (IM). Abstract 18650th American Society of Hematology Annual Meeting and ExpositionDecemeber 6–9, 2008San Francisco, CA.
- DeiningerMO’BrienSGuilhotFInternational randomized study of interferon vs ST1571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Abstract 112651st American Society of Hematology Annual Meeting and ExpositionDecemeber 5–8, 2009New Orleans, LA
- HughesTPHochhausABranfordSLong-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS)Blood2010116193758376520679528
- GuilhotFLarsonJO’BrienSGTime to complete cytogenetic response (CCyR) does not affect long-term outcomes for patients on imatinib therapy49th American Society of Hematology Annual Meeting and ExpositionDecemeber 8–11, 2007Atlanta, GA
- ShahNKantarjianHHochhausACortesJBradley-GarelikMBZhuCDasatinib versus imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) in the DASISION trial: 18 month follow-up. Abstract 20652nd American Society of Hematology Annual MeetingDecemeber 4–7, 2010Orlando, FL
- KantarjianHMShahNPCortesJEDasatinib or imatinib in newly diagnosed chronic phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION)Blood201211951123112922160483
- HughesTHochhausASaglioGENESTnd update: continued superiority of nilotinib versus imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP)52nd American Society of Hematology Annual MeetingDecember 4–7, 2010Orlando, FL
- HughesPHochhausASaglioGENESTnd 24-month update: continued superiority of nilotinib versus imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP)Blood2010116Abstr 207
- KantarjianHMHochhausASaglioGNilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trialLancet Oncol201112984185121856226
- CortesJEMaruAAntonio de SouzaCBosutinib versus imatinib in newly diagnosed chronic phase chronic myeloid leukemia– BELA trial: 24-month follow-up53rd American Society of Hematology Annual Meeting and ExpositionDecember 10–13, 2011San Diego, CA
- BaccaraniMRostiGCastagnettiFComparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: a European LeukemiaNet StudyBlood2009113194497450419264678
- LiptonJHTraskPCCellaDHealth-related quality of life in newly diagnosed chronic phase chronic myelogenous leukemia patients treated with bosutinib or imatinibAmerican Society of Clinical Oncology Annual MeetingJune 3–7, 2011Chicago, IL
- KantarjianHMGilesFJBhallaKNNilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up resultsBlood201111741141114521098399
- CortesJEKantarjianHMBrummendorfTHSafety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive CML patients with resistance or intolerance to imatinibBlood201111874567457621865346
- SaglioGHochhausAGohYTDasatinib in imatinib-resistant or imatinib-intolerant chronic myeloid leukemia in blast phase after 2 years of follow-up in a phase 3 study: efficacy and tolerability of 140 milligrams once daily and 70 milligrams twice dailyCancer2010116163852386120564086
- ShahNPKimDWKantarjianHPotent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinibHaematologica201095223224020139391
- ShahNKimDWKantarjianHDasatinib dose-optimization in chronic phase chronic myeloid leukemia (CML-CP): two-year data from CA180-034 show equivalent long-term efficacy and improved safety with 100 mg once daily dose. Abstract 322552nd American Society of Hematology Annual MeetingDecember 4–7, 2010Orlando, FL
- ShahNCortesJSchifferCAFour-year follow-up of patients with chronic-phase chronic myeloid leukemia (CP-CML) receiving 100 mg of dasatinib once daily. Abstract 6512American Society of Clinical Oncology Annual MeetingJune 4–8, 2010Chicago, IL
- ShahNCortesJSchifferCFive-year follow-up of patients with imatinib-resistant or -intolerant chronic-phase chronic myeloid leukemia (CML-CP) receiving dasatinibAmerican Society of Clinical Oncology Annual MeetingJune 3–7, 2011Chicago, IL
- KhouryHJCortesJEKantarjianHMBosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failureBlood2012119153403341222371878
- JabbourEKantarjianHO’BrienSThe achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitorsBlood2011118174541454621803854
- MahonFXReaDGuilhotJDiscontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trialLancet Oncol201011111029103520965785
- SignorovitchJEWuEQBettsKAComparative efficacy of nilotinib and dasatinib in newly diagnosed chronic myeloid leukemia: a matching-adjusted indirect comparison of randomized trialsCurr Med Res Opin20112761263127121524239
- CortesJEKimDWPinilla-IbarzJInitial findings from the PACE trial: a pivotal phase 2 study of ponatinib in patients with CML and Ph+ ALL resistant or intolerant to dasatinib or nilotinib, or with the T315I mutationPresented at 53rd American Society Hematology Annual MeetingDecember 10–13, 2011Atlanta, GA