Abstract
Introduction
The optimal treatment for young patients with high-risk newly diagnosed multiple myeloma (NDMM) remains a challenge.
Methods
We retrospectively evaluated 58 NDMM patients younger than 55 years treated in our center from 2010 to 2021 with the current recommended protocols.
Results
After a median follow-up of 48 months, median overall survival (OS) was not reached; however, approximately 25% of them died within 4 years after diagnosis. Advanced disease stage, presence of extramedullary disease, elevated LDH, and less than very good remission before autologous hematopoietic stem-cell transplantation adversely affected patient survival. Based on these factors, we created a risk-assessment scoring system that sufficiently discriminated young NDMM patients at risk of poor outcome. The 4-year OS was superior for patients with zero to two factors to those with three to five factors (86% vs 44%, p<0.001).
Conclusion
The proposed scoring system could be reliably used at diagnosis and at interim disease evaluation in aiming for personalized treatment for young NDMM patients.
Introduction
Multiple myeloma (MM) is the second–most common hematological malignancy, accounting for approximately 13% of all malignant hematological disorders.Citation1 The current therapeutic protocols using combinations of novel agents (proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies) along with dexamethasone plus or without autologous stem-cell transplantation (AHSCT) indisputably result in prolonged survival rates and better quality of life; however, the disease remains incurable.Citation2–4 The truth is that clinical trials with the innovative agents have been designed with a focus on the median age of newly diagnosed MM (NDMM) patients, which is approximately 65–70 years.Citation5–9 However, 40% of NDMM patients are aged <65 years and around 15%–17% <55 years, which raises the question of whether young NDMM (Y-NDMM) patients, especially those with high-risk disease, should be treated with the same rationale as older patients or those with standard-risk MM. Also, how can Y-NDMM patients, who may need more intensive treatment approaches, be easily and early identified?Citation10–19
In this retrospective study, we reviewed outcomes of NDMM patients who had been treated in our center with the current recommended treatment protocols.Citation19–21 Focusing on the clinical course and ultimate outcome in younger patients (≤55 years), we created a risk-assessment scoring system (MM-RASS) based on just five easily assessable parameters (sex, serum lactate dehydrogenase [LDH] levels, stage as per the International Staging System [ISS], presence of extramedullary disease [EMD], and response to initial treatment), which were found to have a strong impact on survival rates. This novel MM-RASS was validated in Y-NDMM patients (≤55 years) and proved to be efficient and reliable in discriminating patients with poor outcomes who might have benefited from more intensive treatment approaches.
Methods
Study Design
Following the Declaration of Helsinki for ethical principles and after King Fahad Specialist Hospital’s Institutional Review Board approval, we retrospectively evaluated the records of 116 NDMM patients who had been treated in our department during the last decade and had had a minimum of 6 months follow-up. All had signed informed-consent forms regarding the diagnostic procedures, treatment approaches, and use of their clinical data for research purposes. Patients with asymptomatic/smoldering MM, plasma-cell leukemia, or inadequate data regarding disease characteristics or disease response after the initial treatment were excluded from the study. Patients considered too frail for treatment as per primary physician assessment (based on age, comorbidities, and inability to tolerate duplet or triplet therapies) were also excluded from the study. Because a considerable number of patients had missing cytogenetic/molecular data, for statistical analysis purposes, we used the International Staging System (ISS) instead of the revised ISS (R-ISS).Citation10,Citation11 All patients received initial treatment with combinations of a proteasome inhibitor (bortezomib) plus immunomodulatory drugs (lenalidomide or thalidomide) or cyclophosphamide with dexamethasone with or without AHSCT. Disease response was evaluated after three or four cycles of treatment, and remission status was defined as per International Myeloma Working Group (IMWG) criteria.Citation22
Statistical Analysis
SPSS version 22 was used for the statistical analysis. The Kaplan–Meier method with log-rank test and Cox regression analysis, crosstabs, and Student’s independent t-test were used to estimate survival rates and to identify potential risk factors for patient outcomes. Overall survival (OS) was defined as the duration from the day of diagnosis until death or the date of last follow-up for surviving patients. Relapse/progression was defined as the period from treatment initiation till the first documentation of disease recurrence, while progression-free survival was defined as the period from treatment initiation till the first documentation of disease recurrence or the last follow-up for non-relapsed/progressed patients. For the purposes of this study, disease reappearance within 18 months post–treatment initiation was defined as early relapse/progression. Non–disease-related mortality (NDRM) was defined as any cause of death other than related to refractory MM. Patients’ sex, age, LDH levels, ISS stage, cytogenetic profile, presence of EMD at diagnosis, response to initial treatment, and early relapse were analyzed as risk factors affecting the survival rates. Factors with missing data for >20% of patients were not incorporated in the statistical analysis.
Patient Characteristics
In all, 67 male and 49 female NDMM patients who fulfilled the aforementioned criteria were included in the analysis. Median age at diagnosis was 55 (30–85) years. The male:female ratio was 1.3:1. A total of 25 patients had elevated LDH levels, eleven poor cytogenetic characteristics at diagnosis (del[17p] four, del[13q] five, t[4;14] two), whereas for 23 (20%) karyotypic analysis either failed or was not available. According to the ISS, 51 were assessed as stage III, 47 as stage II, and 17 as stage I. Among stage III patients, 13 had LDH in upper to normal ranges (reference lab values 125–220 IU/L), two had poor cytogenetic characteristics (17p[del]), while for five, karyotypic analysis had failed. Based on the R-ISS, eleven patients had stage I, 78 stage II and 16 stage III, while for eleven patients, staging was not able to be calculated as per the R-ISS due to unavailable data. Twelve (10%) patients presented with EMD at diagnosis, which was confirmed by imaging tests (CT, MRI, or PET/CT) and from tissue biopsy if feasible. IgG isotype (83/116) and k light chain (78/116) were the dominant MM subtypes. We did not find any statistical difference between younger and older (than 55 years) NDMM patients regarding sex, disease stage, EMD presence, high LDH levels, high β2 microglobulin (B2M) levels or high-risk cytogenetics. However, in the group of patients >55 years old, a significantly higher number had albumin levels <3.5 g/dL at disease diagnosis ().
Table 1 Patient and disease characteristics, treatment approaches, and response to treatment for all patients (n=116) patients and those aged ≤55 years and >55 years
Treatment Approaches
The majority of patients received a median four (three to 12) cycles of combined therapy with bortezomib, lenalidomide, and dexamethasone (VRD; n=53), bortezomib, cyclophosphamide, and dexamethasone (VCD, n=31), bortezomib, thalidomide, and dexamethasone (VDT, n=7), or bortezomib or lenalidomide plus dexamethasone (n=21). As per the treatment plan, early AHSCT was offered to all eligible and fit-enough individuals (shown in ).
Results
At data-collection cutoff, median follow-up for the surviving patients was 48 (6.5–132) months. The overall response rate (ORR) after frontline treatment was 83%: 43 (37%) achieved stringent complete remission (sCR) or CR, 28 (24%) very good partial remission (VGPR), 26 (22%) PR, and 20 (17%) were assessed as having stable or progressive disease. Eventually, 74 of 116 (65%) patients underwent early ASCHT. The reasons for not performing ASCHT were age >70 years (15%, n=17), disease refractoriness (9%, n=10), severe comorbidities, or patient refusal (13%, n=15; ).
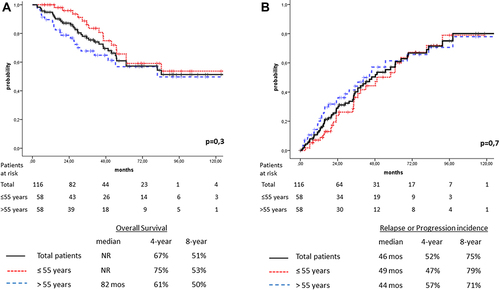
Median OS was not reached, and projected 4- and 8-year OS was 67% and 51%, respectively. Median time to disease relapse/progression was 46 months, while the 4- and 8-year probability of disease recurrence was 52% and 75%, respectively (). For the whole cohort (n=116), on univariate analysis, advanced stage (ISS stage III), elevated LDH levels, response of less than VGPR pre-AHSCT, presence of EMD at diagnosis, and early disease relapse/progression had an adverse impact on survival rates, while disease recurrence was negatively affected only by less than VGPR achievement pre-AHSCT (shown in ). On multivariate analysis, only LDH level upper to normal was found to be an independent factor for inferior long-term OS, whereas age >55 years and achievement of less than VGPR pre-AHSCT adversely influenced disease relapse/progression. Finally, 38 patients died. A majority of them (31 of 38, 82%) succumbed to uncontrolled disease and seven died from NDR causes. The 10-year NDRM rate was estimated to be 6%.
Table 2 Univariate and multivariate analysis of factors affecting survival and disease/progression incidence in multiple myeloma patients
Figure 1 (A) Overall survival for the total cohort of patients (n=116, solid line) and those aged ≤55 years (n=58, dotted line), and >55 years (n=58, dashed line). (B) Relapse/progression of disease for the total cohort of patients (n=116, solid line), and those aged ≤55 years (n=58, dotted line) and >55 years (n=58, dashed line).
Patients ≤55 Years Old
A total of 58 patients were aged ≤55 years at the time of disease diagnosis: 48 (83%) received VRD (n=26), VCD (n=17), or VTD (n=5), and ten (17%) received only the VD combination (). The ORR was superior for patients aged ≤55 years (88% vs 78%); however, this difference was not statistically significant (p=0.15), while sCR and CR rates were similar (38% vs 36%; ). Given their fitness and the lower incidence of comorbidities, it was expected that the vast majority of patients ≤55 years (n=53, 91%) underwent AHSCT.
All in all, 29 patients found to have disease progression/recurrence (either biochemically only or along with clinical symptoms) after a median of 49 months. The 4- and 8-year probability of disease relapse/progression was 47% and 79%, respectively (). Patients with clinical symptoms or who had only biochemical relapse/progression but experienced fast disease turnover were salvaged with combinations of carfilzomib plus pomalidomide, while lenalidomide was used for those patients who did not receive lenalidomide as frontline treatment. Only a minority of relapsed/progressed patients received daratumumab-based regimens, since this agent has been available for only the last 3 years in our center.
Median OS for the subgroup of patients aged ≤55 years was not reached, while 4- and 8-year OS was only marginally superior thano those >55 years (75% vs 61% and 53% vs 50%, respectively, p=0.3; ). However, based on the OS analysis, it was also conspicuous that a considerable number of the younger patients (eleven of 58) died within 4 years after diagnosis. The 4-year probability of death was estimated to be 25%, and in nine of eleven, death was attributed to uncontrolled MM. Median time from disease recurrence to death was 18.5 (3–31) months, despite the fact that they were salvaged with worldwide recommended therapeutic protocols. Two patients died from NDR causes (SARS-CoV-2 and car accident).
Contributing Factors in Survival of NDMM Patients ≤55 Years Old and Risk-Assessment System
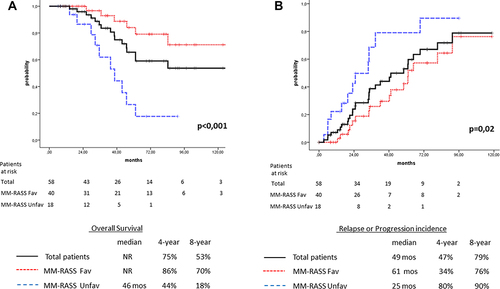
Similarly to previously published studies, in our group of Y-NDMM patients, advanced disease stage (ISS III), LDH levels higher than the upper normal limit, and EMD at diagnosis were identified as negatively contributing factors to prolonged survival.Citation10,Citation11,Citation22–28 Although the male preponderance in MM patients is a well-known phenomenon, in patient aged ≤55 years, the male:female ratio was similar (30:28).Citation17 However, an interesting finding was that young female patients demonstrated significantly inferior survival rates to male patients, with projected 4- and 8-year OS of 65% vs 76% and 35% vs 76%, respectively (p=0.05). Karyotypic analysis was available for almost 90% of patients ≤55 years old. Only seven (12%) had high-risk karyotypes, and presumably for this reason high-risk cytogenetics unexpectedly showed no impact on OS in this group of patients. Nevertheless, five of seven with high-risk cytogenetics relapsed in a median of 20 (3–33) months. Based on these clinical findings, we created a novel risk-assessment system for Y-NDMM patients (MM-RASS), using five risk factors: female sex, LDH levels higher than the normal limit, EMD at presentation, ISS stage III, and less than VGPR achievement prior to AHSCT. Although in patients aged <55 years, achievement of VGPR prior to AHSCT showed no statistically significant impact on survival rates, we chose to incorporate the response rates after frontline treatment in the proposed MM-RASS as an indicator of disease kinetics.Citation30–33 With regard to OS, no significant differences were found for patients with zero, one, or two factors; however, for those with more than two factors, the outcome was extremely dismal. Therefore, we separated patients in two groups: those with zero to two factors (n=40, favorable group) and those with three or more factors (n=18, unfavorable group). The favorable group achieved significant better 4- and 8-year OS of 86% and 70%, respectively, and median OS was not reached, while for the unfavorable group 4- and 8-year OS was 44% and 18%, respectively, and median OS estimated to be 46 months (p<0.001, ). More importantly, in the Cox proportional hazard model, the MM-RASS emerged as an independent predictive factor for prolonged survival (p=0.004, ). The MM-RASS was also studied as a predictive factor for disease recurrence. On univariate analysis, median time to relapse/progression was 2.5-fold shorter for the unfavorable group than the favorable group (25 vs 60 months, p=0.02), while on multivariate analysis the MM-RASS proved also to be an independent prognostic factor for disease relapse/progression (p=0.031, and ).
Figure 2 (A) Overall survival for patients aged ≤55 years according to the MM-RASS: total cohort (n=58, solid line), favorable score (n=40, dotted line), and unfavorable score (n=18, dashed line). (B) Relapse/progression of disease for patients aged ≤55 years according to the MM-RASS: total cohort of patients (n=58, solid line), favorable score (n=40, dotted line), and unfavorable score (n=18, dashed line).
Comparison Between ISS and MM-RASS
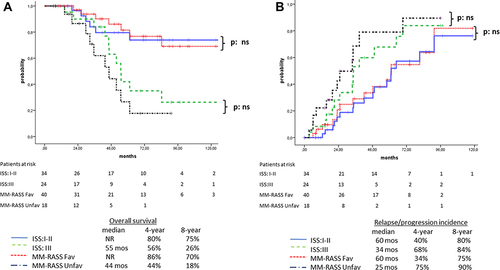
Five patients who had been assessed with non-advanced disease (ISS stage I or II) were escalated to the unfavorable group as per the proposed MM-RASS: three of five (60%) died early due to disease relapse/progression at 20, 30, and 32 months postdiagnosis. On the other hand, eleven patients with advanced disease (ISS III) were de-escalated to the favorable group as per the MM-RASS, and interestingly only three of eleven (27%) succumbed to relapse/progression at 46, 55, and 84 months after disease diagnosis. Survival rates were similar for the non–high-risk patients (ISS I–II or favorable MM-RASS). Median OS was not reached for either system, 4-year OS was 80% vs 86% and 8-year OS was 75% vs 70% for the ISS and MM-RASS, respectively. For high-risk patients (ISS III or MM-RASS unfavorable), median OS was 55 vs 44 months, 4-year OS 56% vs 44%, and 8-year OS 26% vs 18% for the ISS and MM-RASS, respectively (p=0.25, ). Although the MM-RASS discriminated patients at high-risk for relapse/progression better than the ISS (4- and 8-year relapse/progression incidence 68% vs 80% and 84% vs 90% and median time for disease relapse/progression 34 months vs 25 months for ISS III and MM-RASS unfavorable, respectively), this superiority was not statistically significant (p=0.3, ).
Figure 3 (A) Overall survival for patients with ISS I–II (n =34, solid line) and ISS III (n=24, dashed line) versus patients with favorable MM-RASS score (n=40, dotted line) and unfavorable MM-RASS score (n=18, dot/dashed line). The differences were not statistically significant. (B) Relapse/progression of disease for patients with ISS I–II (n =34, solid line) and ISS III (n=24, dashed line) versus patients with favorable MM-RASS score (n=40, dotted line) and unfavorable MM-RASS score (n=18, dotted/dashed line). The differences were not statistically significant.
Discussion
The advent of novel agents has completely altered the clinical course of MM, improving patients’ quality of life and survival rates; however, they have failed to put an end to the typical course of repeated relapses, which inevitably lead to death due to uncontrolled disease. Since MM is typically a disease of elderly people, scant data exist regarding the long-term efficacy of the currently used treatment protocols for Y-NDMM patients, especially for those with high-risk characteristics. In our study group, the median age of 55 years was lower than the reported median age in other published studies from the US and Europe, apparently because <5% of Saudis are aged >65 years, while the vast majority of patients had been treated with the current recommended protocols.Citation19–21,Citation34
It is ambiguous whether Y-NDMM patients have different disease characteristics from older patients. In the largest published study, which evaluated more than 10,000 NDMM patients, female sex, advanced ISS stage, elevated B2M, and low albumin were more frequent in patients aged >50 years, while LDH levels did not differ significantly between the two groups.Citation35 In our study, though with a smaller patient series, we did not observe any significant differences between patients younger or older than 55 years in terms of sex, advanced stage, B2M, levels of LDH, or presence of EMD at diagnosis, and we only observed low albumin levels more often in patients aged >55 years.
In disagreement with other studies, we observed only slightly better OS for patients aged ≤55 years than those aged >55 years.Citation35,Citation36 Possible explanations for this marginal OS superiority could be that the treatment protocols used were effective enough to get over the adverse impact of older age and that only 25% of our patients were older than 65 years. In the few studies designed for young patients, the only available data regarding the impact of novel agents on the outcome of younger patients come from studies with AHSCT-eligible patients.Citation19 Undoubtedly, over the last few years OS has been prolonged to 7–10 years for transplant-eligible individuals. However, looking at the opposite edge of the survival curves, the indisputable fact is that even for this favorable age-group, the probability of death within 4–5 years post-MM diagnosis is approximately 20%–30%.Citation35–40 In Ludwig et al study, autografted patients with aged <50 years demonstrated 4-year probability of death of approximately 30%. Nevertheless, it should be noted that patients were treated between 1981 and 2002 and thus novel triplet or quadruplet combinations were not in use.Citation35 Similarly, in a more recent multicenter study on NDMM up to 30 years, the 5-year risk of death was approximately 23%, but the evaluation period was 1989–2016, so the currently used treatment protocols were not available for a considerable number of patients.Citation40 In another study from Israel on a small series of Y-NDMM patients who received bortezomib-based therapy, eventually all patients relapsed shortly after treatment in a median 12 months. No deaths were reported, but it should be kept in mind that follow-up of the surviving patients was not mentioned in the study.Citation41
In our study with 58 patients aged ≤55 years treated with widely accepted triplet therapies and salvaged with novel agents and with quite prolonged follow-up, we found that the probability of death within 4 years post-diagnosis was 25%. This result confirms that the heterogeneity of disease outcome is not only a characteristic of older but also of younger patients. On uni- and multivariate analyses, we found that female sex, elevated LDH, presence of EMD at diagnosis, and ISS stage III after frontline treatment were strongly associated with poor outcomes. Disease response of less than VGPR after initial treatment was also associated with inferior survival rates, though with no statistical significance, probably due to the number of patients and/or because of therapies given to control relapsed disease post-AHSCT.
Data on the impact of patient sex on outcomes in MM are conflicting. A survey from the UK and an analysis from the International Myeloma Working Group reported similar survival rates for male and female MM patients.Citation18,Citation35 However, in the MRC Myeloma IX phase III clinical trial that evaluated 1,960 patients, significantly better median OS for male patients was observed (49.9 vs 44.8 months, p=0.02). The inferior survival of female patients was correlated with higher prevalence of poor prognosis and cytogenetic abnormalities.Citation42 In our whole cohort (n=116), patient sex had no significant impact on survival or relapse/progression (), but in Y-NDMM patients, similarly to the MRC Myeloma IX trial, female patients had poorer outcomes ().
Elevated LDH levels suggest aggressive disease and usually indicate extramedullary and/or extraosseous MM, which is associated with shorter OS.Citation11,Citation23,Citation24 In our Y-NDMM group, high LDH was correlated with a strong trend for inferior outcomes (). There is increasing evidence that EMD represents a poor prognostic factor in MM. Although satisfying responses can be achieved with novel agent combinations, they are not sustainable and relapses are common, even after consolidation with AHSCT.Citation25–27 In our patient series, in agreement with previous reports, the presence of EMD at diagnosis was associated with poor prognosis. It is generally accepted that achievement of CR or VGPR before AHSCT is not a mandatory objective for prolonged OS, but it has also been well documented that the deeper the response prior AHSCT, the better the outcome.Citation30–33,Citation43 We found that the achievement of at least VGPR was a favorable predictor of prolonged survival in both the whole group and <55-year-old patients, though it was not statistically significant ().
An advantage of our proposed MM-RASS is that all the factors are easily assessable in any hematology center, not requiring special or laborious processing. A limitation could be the nonincorporation of cytogenetics or molecular characteristics in the proposed system. The high number of patients (20% of the whole cohort) lacking cytogenetic profiles did not allow us to incorporate cytogenetics in the proposed MM-RASS.Citation16 This fact, at least partially, reflects the difficulty of analyzing cytogenetically NDMM patients because of the low proliferation rate of the malignant clone.Citation44 The sensitivity of interphase fluorescence in situ hybridization analysis does not require cell proliferation, but is sometimes affected by the low percentage of malignant plasma cells in whole-bone marrow samples. Therefore, for accurate cytogenetic/molecular results, more specific, laborious, and costly methods are required, which are eventually infeasible for several centers lacking specialized laboratory and experienced staff.Citation45,Citation46
The MM-RASS showed better predictability of OS and relapse/progression incidence than the ISS, though the differences were not statistically significant. A plausible explanation for the better discrimination with the MM-RASS could be that two more factors with well-documented impact on MM outcome are additionally incorporated in the MM-RASS: EMD presence at diagnosis and response after frontline treatment, which reflects disease kinetics.Citation25–33,Citation43 Moreover, since the proposed MM-RASS takes into account disease response prior to AHCT (three to four cycles following initial treatment), it could be used as a reliable interim risk-assessment system during the treatment course.
Conclusion
This retrospective analysis of 58 patients aged ≤55 years represents one of the few studies of Y-NDMM patients who have been treated with the currently recommended triplet or doublet therapies. Our results confirm that even in the contemporary era of MM treatment, the appropriate therapy for a considerable number of Y-NDMM patients remains a major challenge. The MM-RASS we propose herein, which is exclusively targeted at Y-NDMM patients, shows remarkable strength in discriminating younger patients at higher risk of dismal outcomes. The MM-RASS merits further investigation and validation in larger series of patients and could potentially serve as an additional tool not only for initial but also for interim risk assessment, thus increasing the capability of early identification of high-risk Y-NDMM patients who are candidates for more intensive treatment approaches.
Data Sharing
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether is in conception, study design, execution, acquisition of data, analysis and interpretation, or all these areas, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no competing interests.
Acknowledgments
The authors wish to thank Sudair Pharmaceutical Company for assistance with manuscript publication.
Additional information
Funding
References
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC. Multiple myeloma. Lancet. 2009;374(9686):324–339. doi:10.1016/S0140-6736(09)60221-X
- Palumbo A, Anderson K. Multiple Myeloma. N Engl J Med. 2011;364(11):1046–1060. doi:10.1056/NEJMra1011442
- Touzeau C, Moreau P, Dumontet C. Monoclonal antibody therapy in multiple myeloma. Leukemia. 2017;31(5):1039–1047. doi:10.1038/leu.2017.60
- Richardson PG, Laubach JP, Munshi NC, Anderson KC. Early or delayed transplantation for multiple myeloma in the era of novel therapy: does one size fit all? Hematology Am Soc Hematol Educ Program. 2014;2014(1):255–261. doi:10.1182/asheducation.V2014.1.255.3885263
- Anderson KC, Alsina M, Bensinger W, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. J Natl Compr Canc Netw. 2009;7(9):908–942. doi:10.6004/jnccn.2009.0061
- Reeder CB, Reece DE, Kukreti V, et al. Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a Phase II clinical trial. Leukemia. 2009;23(7):1337–1341. doi:10.1038/leu.2009.26
- Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116(5):679–686. doi:10.1182/blood-2010-02-268862
- Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem‐cell transplantation in newly diagnosed multiple myeloma: a randomized Phase 3 study. Lancet. 2010;376(9758):2075‐2085.
- Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A Phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120(9):1801–1809. doi:10.1182/blood-2012-04-422683
- Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi:10.1200/JCO.2005.04.242
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from international myeloma working group. J Clin Oncol. 2015;33(26):2863–2869. doi:10.1200/JCO.2015.61.2267
- Moreau P, Touzeau C, Vij R, Goldsmith SR, Rosko AE. Newly diagnosed Myeloma in 2020. Am Soc Clin Oncol Educ Book. 2020;40:1–15. doi:10.1200/EDBK_280221
- Goldman-Mazur S, Kumar SK. Current approaches to management of high-risk multiple myeloma. Am J Hematol. 2021;96(7):854–871. doi:10.1002/ajh.26161
- Moreau P, Kumar SK, San Miguel J, et al. Treatment of relapsed and refractory multiple myeloma: recommendations from the International Myeloma Working Group. Lancet Oncol. 2021;22(3):e105–e118. doi:10.1016/S1470-2045(20)30756-7
- Kaufman JL. Roundtable: how I treat a newly diagnosed patient with high-risk myeloma. Hematology Am Soc Hematol Educ Program. 2019;2019(1):120–124. doi:10.1182/hematology.2019000015
- Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. doi:10.1182/blood-2016-01-631200
- Howlader N, Noone AM, Krapcho M, et al. Eds. SEER Cancer Statistics Review, 1975–2016. Bethesda, MD, USA: National Cancer Institute; 2018. Available from: https://seer.cancer.gov/csr/1975_2016.
- Cancer Research UK Myeloma survival statistics; 2014. Available from: www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma/survival#heading-Two. Accessed October 4, 2022.
- Gandolfi S, Prada CP, Richardson PG. How I treat the young patient with multiple myeloma. Blood. 2018;132(11):1114–1124. doi:10.1182/blood-2017-05-693606
- Kumar SK, Callander NS, Alsina M, et al. Multiple Myeloma, Version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(2):230–269. doi:10.6004/jnccn.2017.0023
- Mikhael JR, Dingli D, Roy V, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc. 2013;88(4):360–376. doi:10.1016/j.mayocp.2013.01.019
- Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi:10.1016/S1470-2045(16)30206-6
- Gkotzamanidou M, Kastritis E, Gavriatopoulou MR, et al. Increased serum lactate dehydrogenase should be included among the variables that define very-high-risk multiple myeloma. Clin Lymphoma Myeloma Leuk. 2011;11(5):409–413. doi:10.1016/j.clml.2011.07.001
- Chim CS, Sim J, Tam S, Tse E, Lie AK, Kwong YL. LDH is an adverse prognostic factor independent of ISS in transplant-eligible myeloma patients receiving bortezomib-based induction regimens. Eur J Haematol. 2015;94(4):330–335. doi:10.1111/ejh.12434
- Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica. 2012;97(11):1761–1767. doi:10.3324/haematol.2012.065698
- Gagelmann N, Eikema DJ, Iacobelli S, et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: a study from the chronic malignancies working party of the EBMT. Haematologica. 2018;103(5):890–897. doi:10.3324/haematol.2017.178434
- Jagosky MH, Usmani SZ. Extramedullary Disease in Multiple Myeloma. Curr Hematol Malig Rep. 2020;15(2):62–71. doi:10.1007/s11899-020-00568-3
- Majithia N, Rajkumar SV, Lacy MQ, et al. Early relapse following initial therapy for multiple myeloma predicts poor outcomes in the era of novel agents. Leukemia. 2016;30(11):2208–2213. doi:10.1038/leu.2016.147
- Giralt S, Garderet L, Durie B, et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transplant. 2015;21(12):2039–2051. doi:10.1016/j.bbmt.2015.09.016
- Harousseau JL, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood. 2009;114(15):3139–3146. doi:10.1182/blood-2009-03-201053
- Kim JS, Kim K, Cheong JW, et al. Complete remission status before autologous stem cell transplantation is an important prognostic factor in patients with multiple myeloma undergoing upfront single autologous transplantation. Biol Blood Marrow Transplant. 2009;15(4):463–470. doi:10.1016/j.bbmt.2008.12.512
- Tandon N, Sidana S, Rajkumar SV, et al. Outcomes with early response to first-line treatment in patients with newly diagnosed multiple myeloma. Blood Adv. 2019;3(5):744–750. doi:10.1182/bloodadvances.2018022806
- Aggarwal M, Agrawal N, Yadav N, et al. Autologous stem cell transplantation in first remission is associated with better progression-free survival in multiple myeloma. Ann Hematol. 2018;97(10):1869–1877. doi:10.1007/s00277-018-3370-1
- Saudi Arabia: age structure from 2008 to 2018 (published by Plecher H, Feb 5, 2020). Available from: https://www.statista.com/statistics/262478/age-structure-in-saudi-arabia. Accessed July 4, 2020.
- Ludwig H, Durie BG, Bolejack V, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group Blood. Blood. 2008;111(8):4039–4047. doi:10.1182/blood-2007-03-081018
- Bove V, Garrido D, Riva E. Young age and autologous stem cell transplantation are associated with improved survival in newly diagnosed multiple myeloma. Hematol Transfus Cell Ther. 2020;4:30113–30119.
- Nishimura KK, Barlogie B, van Rhee F, et al. Long-term outcomes after autologous stem cell transplantation for multiple myeloma. Blood Adv. 2020;4(2):422–431. doi:10.1182/bloodadvances.2019000524
- Cavo M, Gay F, Beksac M, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020;7(6):e456–e468. doi:10.1016/S2352-3026(20)30099-5
- Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376(14):1311–1320. doi:10.1056/NEJMoa1611750
- Jurczyszyn A, Davila J, Kortüm KM, et al. Multiple myeloma in patients up to 30 years of age: a multicenter retrospective study of 52 cases. Leuk Lymphoma. 2019;60(2):471–476. doi:10.1080/10428194.2018.1480766
- Duek A, Trakhtenbrot L, Avigdor A, Nagler A, Leiba M. Multiple myeloma presenting in patients younger than 50 years of age: a single institution experience. Acta Haematol. 2021;144(1):58–65. doi:10.1159/000507414
- Boyd KD, Ross FM, Chiecchio L, et al. Gender disparities in the tumor genetics and clinical outcome of multiple myeloma. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1703–1707. doi:10.1158/1055-9965.EPI-11-0157
- Mainou M, Madenidou AV, Liakos A, et al. Association between response rates and survival outcomes in patients with newly diagnosed multiple myeloma: a systematic review and meta-regression analysis. Eur J Haematol. 2017;98(6):563–568.
- Fonseca R, Barlogie B, Bataille R, et al. Genetics and cytogenetics of multiple myeloma: a workshop report. Cancer Res. 2004;64(4):1546–1558. doi:10.1158/0008-5472.CAN-03-2876
- Fonseca R, Bergsagel PL, Drach J, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23(12):2210–2221. doi:10.1038/leu.2009.174
- Ross FM, Avet-Loiseau H, Ameye G, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders Haematologica. Haematologica. 2012;97(8):1272–1277. doi:10.3324/haematol.2011.056176
