Abstract
While platelet activation is essential to maintain blood vessel patency and minimize loss of blood upon injury, untimely or excessive activity can lead to unwanted platelet activation and aggregation. Resultant thrombosis has the potential to block blood vessels, causing myocardial infarction or stroke. To tackle this major cause of mortality, clinical therapies that target platelet responsiveness (antiplatelet therapy) can successfully reduce cardiovascular events, especially in people at higher risk; however, all current antiplatelet therapies carry an increased probability of bleeding. This review will evaluate new and emerging targets for antithrombotics, focusing particularly on platelet glycoprotein VI, as blockade or depletion of this platelet-specific receptor conveys benefits in experimental models of thrombosis and thromboinflammation without causing major bleeding complications.
Introduction
The platelet response to vessel injury or infection is essential for normal hemostasis. Platelets also have multifaceted roles in inflammation and immunity.Citation1 In response to vascular injury, circulating platelets rapidly adhere to exposed subendothelial matrix proteins, such as von Willebrand factor (VWF) and collagen.Citation2,Citation3 Adherent platelets become activated, spread, and release the contents of storage organelles. These are dense bodies that contain prothrombotic substances, including serotonin and adenosine diphosphate (ADP), membrane glycoproteins P-selectin and CD40 ligand, coagulation proteins, fibrinolytic proteins, growth factors, cytokines, and chemokines.Citation4 The platelet response is enhanced by signaling pathways initiated through the thromboxane A2 receptor and P2Y1 and P2Y12 receptors for ADP, ultimately leading to the activation of the platelet-specific integrin αIIbβ3 and platelet aggregation, thereby maintaining blood vessel patency and minimizing the loss of blood upon injury.
Myocardial infarction or stroke can result from untimely or excessive platelet activity leading to unwanted platelet activation and aggregation, causing thrombosis. Antiplatelet therapies target the ADP receptor P2Y12 (thienopyridine-based inhibitors clopidogrel and prasugrel) or thromboxane generation (cyclooxygenase inhibitor, acetylsalicylic acid) and aim to modulate platelet responsiveness in people at risk of thrombosis.Citation5 When given together (dual antiplatelet therapy), these treatments significantly reduce the risk of vascular events in high-risk patients.Citation6,Citation7 Critically, however, the risk of bleeding in patients also increases with the use of antiplatelet therapies.Citation8,Citation9
There is a clear need to develop new antiplatelet reagents ideally with high antithrombotic properties but negligible effects on normal hemostasis. Platelet glycoprotein (GP)VI represents an attractive new target as an antiplatelet reagent, because it is only expressed on platelets and platelet precursor cells in bone marrow (megakaryocytes), and GPVI blockade has demonstrated efficient antithrombotic potential in the experimental models of thrombosis without enhancing pathological bleeding. Targeting GPVI in the setting of myocardial infarction makes good sense as platelet levels of GPVI are elevated in people who have coronary syndromes.Citation10–Citation12 More recent data indicate that the blockade of GPVI may also be advantageous for therapies that target inflammatory processes involving platelet function. As several excellent reviews on this topic have been published elsewhere recently,Citation13–Citation16 we will briefly cover the background rationale for targeting GPVI before highlighting the existing and novel approaches to modulate GPVI function.
GPVI structural and functional features
GPVI is found exclusively on platelets and megakaryocytes and is the predominant platelet receptor for collagen.Citation17 GPVI has also been shown to bind laminin, an additional extracellular matrix protein.Citation18 Human GPVI is an approximately 64 kDa type I transmembrane glycoprotein of the immunoreceptor family, with two extracellular immunoglobulin (Ig)-like domains, an extracellular mucin-like domain, followed by a 19-amino acid transmembrane domain, and a cytoplasmic tail of approximately 50 residues that is important for the transmission of ligand-regulated signals.Citation19 Platelets from healthy donors contain approximately 10,000 copies per platelet of GPVI.Citation20 Critical residues within the transmembrane domain of GPVI enable this receptor to link with the fragment crystallizable (Fc) γ chain and form a cooperative signaling complex, which utilizes two immunoreceptor tyrosine-based activation motifs (ITAMs) within the cytoplasmic tail portions of dimerized Fcγ chain.Citation21,Citation22 The cytoplasmic tail of GPVI also contains a proline-rich region that can recruit sarcoma (Src) family kinase membersCitation23 and a calmodulin-binding sequence in the juxtamembrane region.Citation24 The Ig domains of GPVICitation25 are able to bind specific glycine–proline–hydroxyproline motifs within collagenCitation26 and, in doing so, cluster GPVI on the platelet membrane to enhance the signaling response triggered by binding collagen.Citation17
Like many other Ig-like receptor family members,Citation27–Citation29 GPVI can exist as a dimer on the platelet surface.Citation30,Citation31 Dimerization is enhanced by ligand binding and by platelet activation.Citation31,Citation32 In the case of GPVI, dimers can be stabilized via the formation of a disulfide bond between cysteinyl thiol groups on the penultimate residues within the cytoplasmic tails of the adjacent GPVI molecules.Citation27 Information gleaned from the crystal structure of the ligand-binding Ig-like domains of GPVI also hints that an interaction between adjacent GPVI ectodomains is likely.Citation33 Such a receptor dimerization step is likely to enhance ligand-induced signaling and to strengthen collagen-binding, as the affinity of dimerized GPVI ectodomains for collagen is significantly greater than for the monomer.Citation30,Citation34
An additional feature of GPVI is that the receptor can be downregulated at the platelet membrane.Citation35 This modulation of receptor levels can be achieved by the cleavage of the ectodomain upon the ligand engagement of GPVICitation36 or FcγRIIaCitation37 by activation of the coagulation cascadeCitation38 or exposure to elevated levels of shear stress.Citation39 Under certain experimental conditions, GPVI may also be internalized and degraded.Citation40,Citation41
Throughout biology, the shedding of the receptor ligand-binding ectodomains is a consistent recurring mechanism that is used to regulate the function of adhesion and signaling receptors.Citation42–Citation44 This irreversible process occurs within seconds to minutes of the exposure of the platelets to collagen or to pathophysiological levels of shear and may be a mechanism by which ligand- or shear-exposed platelets can reduce levels of functional GPVI at the membrane surface and downregulate activation signals. This process is mediated by members of the a disintegrin and metalloproteinase (ADAM) family of membrane-bound metalloproteinases, predominantly ADAM10 in the human system.Citation45 In nucleated cells, ADAMs are synthesized and stored as proenzymes within the cytoplasmic vesicles. Upon appropriate stimulation, they are then enzymatically processed to remove the prodomain and brought to the surface of the cell as an active metalloproteinase.Citation46 This activation process can take 4–16 hours. In platelets, the ADAM proteolytic processing of substrates can be detected within seconds to minutes of platelet activation. There is no evidence that platelets store zymogen forms of ADAMs, implying that the ADAMs proteins are present on the surface of nonactivated platelets (Qiao, Andrews, Arthur, Gardiner; unpublished data, 2012). A peptide with sequence matching residues 228–248 of the extracellular juxtamembrane sequence within GPVI could be proteolyzed by recombinant ADAM10 at position R242–Q243.Citation45 This site is also presumably present and accessible for enzymatic cleavage on the circulating platelet surface. Cleavage within this region of GPVI results in the liberation of an approximately 55 kDa ectodomain fragment of GPVI and production of an approximately 10 kDa remnant portion that remains associated with the platelet surface. However, unlike other platelet receptors that are constitutively shed from the platelet surface, the majority of GPVI remains intact on platelets under resting conditions. The proteolytic cleavage of GPVI only occurs upon specific activation of platelets.
Platelet processes mediating thrombosis
Platelet adhesion and activation are mediated by one or more adhesion-signaling receptors on the platelet membrane (). Principal players in these adhesive events are the receptors GPIb-IX-V that binds VWF as well as other important vascular proteins, (P-selectin, leukocyte integrin αMβ2, thrombin, high molecular weight kininogen, thrombospondin-1, factor XI, factor XII) and GPVI which binds collagen and also laminin,Citation18 both of which are exposed within damaged vascular walls.
Figure 1 Platelet adhesion and aggregation.
Abbreviations: ADP, adenosine diphosphate; VWF, von Willebrand factor; GP, glycoprotein.
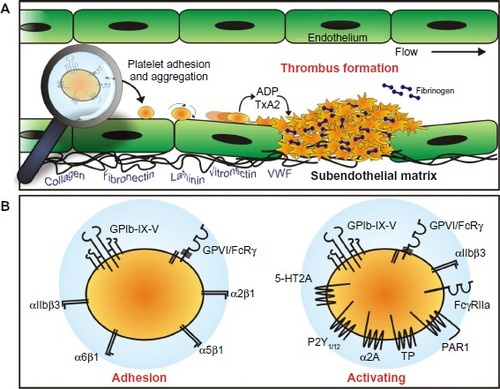
Importantly, GPIb-IX-V and GPVI engage their respective ligands differentially, according to local blood rheological conditions, with GPVI binding collagen exposed within the blood vessel walls at relatively low physiological shear ratesCitation17 and GPIb-IX-V binding VWF after exposure to high shear rates as found in arterioles and stenotic arteries.Citation48
Engagement of either of these receptors leads to the activation of intracellular signaling pathways leading to the upregulation of platelet-specific αIIbβ3 integrin and resulting in enhanced ability of αIIbβ3 to bind the plasma protein fibrinogen.Citation49,Citation50 Triggering of these signaling pathways also initiates or enhances metalloproteolytic shedding of the extracellular ligand-binding portions of GPVI,Citation36 and GPIbα and GPV.Citation45,Citation51
Interestingly, GPIbα and GPVI are directly and functionally linked on the platelet surfaceCitation52 and activate common signaling pathways,Citation53 further underscoring the extent of cooperation between these adhesion molecules. This differential involvement of GPIb-IX-V and GPVI in the initiation of platelet responses implies a coordinated response by platelets determined at least in part by specific vascular conditions and may provide an opportunity to selectively target a single prothrombotic process from one adhesion receptor while maintaining an adequate response from the other adhesion receptor.
Intracellular molecular signals that result from the engagement of either GPIb-IX-V or GPVI by their ligands may also represent attractive targets for antiplatelet therapeutics (). The signaling cascades involve similar events where receptor binding results in the activation of the Src family kinases followed sequentially by spleen tyrosine kinase (Syk), lymphocyte cytosolic protein 2 (also know as SLP-76), phosphatidylinositide (PI) 3-kinase, Bruton’s tyrosine kinase (Btk), phospholipase (PL) Cγ2, and protein kinase (PK) C, ultimately resulting in the elevation of the cytosolic Ca2+ and αIIbβ3 activation.Citation54,Citation55 Small differences exist regarding the order of activation of the signaling molecules and some signaling/adaptor molecules. For example, Src and Lyn appear to be recruited to the GPIb-IX-V complex upon platelet activation, whereas Fyn and Lyn are constitutively associated with GPVI.
Figure 2 Signaling pathways orchestrate platelet activation and aggregation.
Abbreviations: GPCR, G protein-coupled receptor; GP, glycoprotein; ADAM, a disintegrin and metalloproteinase; ROS, reactive oxygen species; Syk, spleen tyrosine kinase; Btk, Bruton’s tyrosine kinase; CLEC-2, C-type lectin receptor-2; PLC, phospholipase C; PI3K, phosphatidylinositide 3-kinase; CaM, calmodulin; ITAM, immunoreceptor tyrosine-based activation motif.
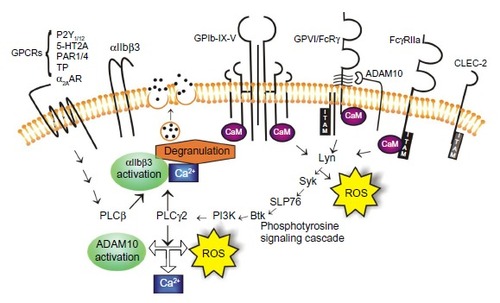
Additionally, it is reported that GPVI activation induces significantly more inositol phosphate production and PLCγ2 activity relative to GPIb-IX-V activation.Citation55 Differences regarding the tyrosine phosphorylation patterns downstream of GPIbα and GPVI, where the GPVI agonists induce more rapid tyrosine phosphorylation of platelet proteins relative to GPIbα engagement, are also evident.Citation56 The engagement of GPVI also produces significant amounts of intracellular reactive oxygen species (ROS), possibly as part of the signaling processCitation57 and the blockade of the ROS-generating nicotinamide adenine dinucleotide phosphate (NADP+)-oxidase downstream of GPVI engagement, reduces platelet activation and aggregation, and results in the formation of smaller thrombi,Citation58,Citation59 possibly via the ablation of thromboxane production.Citation59 ROS-generating machinery is also associated with the cytoplasmic tails of the GPIb-IX-V complex;Citation60 however, evidence that the engagement of GPIb-IX-V also leads to the generation of ROS has not yet emerged. These small differences in signaling responses between GPVI and GPIb-IX-V, once engaged by their respective ligands, may provide some biological leads for the design of agents that would selectively target one receptor over the other and so provide tools to mediate platelet activation under the differential shear conditions found in specific vascular beds.
GPVI involvement in thrombosis
In vascular lesions, where the endothelium has been denuded/disrupted or there exists plaques, collagen as well as VWF are exposed and form a prothrombotic surface, a potent trigger for the platelet adhesion and aggregation.Citation2 Platelet engagement with the blood vessel wall is predominantly mediated by GPVI and GPIb-IX-V, which cooperate under a large range of blood shear conditions. Additional involvement of other adhesion proteins – including integrins α2β1Citation61,Citation62 and α6β1Citation63 – helps to stabilize the initial attachment and facilitate platelet recruitment and thrombus growth.
However, the engagement of GPIb-IX-V and GPVI generates a cascade of signaling events that lead to Ca2+ mobilization, the rapid release of a battery of soluble agonists that includes ADP and thromboxane, inorganic polyphosphates,Citation64 microparticles, and thiol oxidoreductase ERp57,Citation65 and produces a negatively charged phosphatidylserine-expressing platelet surfaceCitation66,Citation67 to aid and enhance the generation of active tissue factor.
Consequences of reduction of GPVI function
provides a summary of the known data collected from both mouse and human systems evaluating the contribution of GPVI to normal hemostasis and to thrombosis and other responses involving platelets. Platelets with reduced/absent GPVI, as found in people with platelet-targeting autoantibodiesCitation68,Citation69 or genetic mutation,Citation70–Citation72 display a mild-to-more-severe bleeding diathesis that can include ecchymosis, epistaxis, easy bruising, and prolonged bleeding from mucosal membranes and gums. Experimentally, these platelets display reduced response to collagen and other GPVI agonists by aggregation or flow cytometry-based assaysCitation69,Citation73 and a reduction in thrombus size when exposed to collagen in ex vivo blood flow-based assays.Citation74
Table 1 Extent of involvement of GPVI in onset of thrombotic and inflammatory disorders
Similarly, platelets from mice genetically deficient for the GPVI/Fcγ chainCitation75,Citation76 or treated with anti-GPVI antibodies that cause the removal of GPVI from plateletsCitation76,Citation77 do not respond efficiently or effectively to collagen in assays in vitro. Significantly, however, mice that are genetically deficient in GPVI do not display a prolonged bleeding time, but they do demonstrate a moderate-to-strong protection from thrombosis, depending on the injury model employed.Citation75,Citation78–Citation81 Clearly, in animal models of thrombosis, the initiating event and the vascular bed being studied in part determine the extent of the contribution and the relative importance of GPVI to platelet activation and thrombus formation ().
Under normal or healthy blood rheological conditions, the contribution of GPVI is only minor, probably because other adhesion receptors (GPIb-IX-V and collagen-binding integrin α2β1, for example) deliver necessary platelet adhesion and thrombus stability properties, and the vascular conditions permit an accentuated contribution to platelet activation from thrombin and other soluble agonists.Citation79,Citation82,Citation83 Keeping this in mind, the findings imply overall that GPVI may be a reasonable target for antithrombotic therapies as its nonessential role in hemostasis but important role in thrombosis means that the therapeutic targeting is unlikely to lead to unacceptable bleeding.
Existing clinical strategies to target platelet adhesion/activation
Numerous clinical therapeutics exist that target platelet activation and platelet adhesiveness ().Citation84,Citation85 The majority of the therapeutics aims to block the receptors involved in the initial and amplification stages of platelet activation; several reagents are often used in combination to achieve a potent antiplatelet effect. Dual and triple antiplatelet therapies have been shown to prevent ischemic events in high-risk patients with coronary artery disease or during percutaneous coronary interventions, but they can cause bleeding complications.Citation8 Further, the incidence of recurrence of adverse vascular events remains of concern. The range of currently approved reagents includes:
Table 2 Examples of existing and novel therapeutics targeting platelet receptors
Anticoagulants, such as lepirudin, warfarin, unfractionated heparin, low molecular weight heparin, bivalirudin, edoxaban, rivaroxaban, argatroban, and dabigatranCitation86,Citation87 that are direct or indirect inhibitors of thrombin and so block coagulation and interfere with the thrombin engagement of protease-activated receptors (PARs) on platelets
Clopidogrel, prasugrel, and ticagrelor,Citation88 which serve to block platelet ADP receptor P2Y12
Aspirin, which blocks thromboxane generation (a strong platelet agonist) via inhibition of cyclooxygenases
Abciximab, eptifibatide, and tirofiban,Citation89 which target fibrinogen interactions with the platelet-specific fibrinogen receptor αIIbβ3.
The main issue with these reagents is the elevated risk of adverse bleeding, due to the therapeutics targeting molecular pathways that are important for both thrombotic processes and normal hemostasis.Citation90 This issue is compounded because the assessment of bleeding risk in patients receiving one or more of these therapies is complicated,Citation91,Citation92 generally requiring specialized platelet function analysis where the relationship between platelet function testing and bleeding in different patient groups on combinational therapy is not clear, due to limited data.
Targeting GPVI therapeutically
GPVI has emerged as a potential target for antithrombotic therapy for a number of reasons. First, GPVI is only expressed on platelets and megakaryocyte (platelet precursor cells in bone marrow) populationsCitation17 in relatively low abundance,Citation93 thus permitting high specificity while minimizing potential side effects of a therapeutic agent. Second, GPVI appears to play only a supporting role in normal hemostasis,Citation72,Citation94 implying that targeting GPVI would not increase bleeding risk to unacceptable levels. Third, in animal models of thrombosis as well as in studies of inflammationCitation95 and reperfusion injury following ischemia,Citation96,Citation97 a significant contribution of GPVI to tissue injury – together with experiments demonstrating the benefits of blockade of GPVI to reduce the extent of injuryCitation16 – have been well-documented.
Several options exist to modulate or inhibit GPVI-mediated platelet activation. GPVI–collagen interaction can be disturbed by collagen-binding molecules (GPVI mimics), by GPVI-function blocking reagents (aptamers, small molecules, and antibodies), or by GPVI depletion.
GPVI mimetics
Taking advantage of the stronger collagen-binding affinity of dimeric GPVI, a recombinant fusion protein was formed between the extracellular collagen-binding domain of GPVI and the C-terminal of human immunoglobulin Fc domain to form a soluble dimeric GPVI (GPVI-Fc).Citation98 This reagent specifically bound to collagen with high affinity and attenuated platelet adhesion to immobilized collagen in vitro and to sites of vascular injury in vivo.Citation98
Importantly, at doses sufficient to reduce platelet adhesion, the soluble form of GPVI only moderately prolonged tail bleeding times.Citation99,Citation100 Such a reagent holds several advantages over other types of GPVI inhibitors. The GPVI-Fc predominantly targets the exposed subendothelium at a site of vascular injury, suggesting that collagen exposed within a damaged vascular site is the primary site of binding. By directly targeting the site of interaction, there is no requirement for prolonged systemic inhibition of platelet function.Citation99 Further, there have been no reports of aberrant platelet activation, loss of GPVI on circulating platelets, or thrombocytopenia associated with the use of this reagent in animal models. By the addition of an appropriate molecular tag to GPVI-Fc, it is possible that this reagent may also be developed as a bioimaging tool as it selectively binds to presumably prothrombotic regions of a vascular bed that is enriched for collagen.Citation16,Citation98,Citation101
Injection of GPVI-Fc (Revacept; Janssen-Cilag GmbH, Neuss, Germany) has improved endothelial dysfunction and vascular morphology in atherosclerotic rabbitsCitation100 and reduced the cerebral infarct size and edema after ischemic stroke, with improved functional and prognostic outcome without intracranial bleeding.Citation102 In a Phase I study, Revacept efficiently inhibited collagen-induced platelet aggregation ex vivo, with no alteration of primary hemostasis in 30 healthy donors;Citation103 however, GPVI-Fc had only limited antithrombotic effects in animal models where the direct blockade of GPVI function was effective in preventing occlusive thrombus formation.Citation104
While the experimental and early phase trial results with this fusion protein are encouraging, the precise clinical setting and the appropriate dosing and treatment regimens where this reagent may be useful still remain to be defined. Also, similar to other immunoglobulin fusion proteins and antibody therapies, the possibility of immunogenicity with repeated injections of Revacept remains a potential hazard.
GPVI-function blocking reagents
Anti-GPVI antibodies are of great interest as candidate antithrombotic reagents as they may be able to accomplish the dual purpose of interfering with collagen-GPVI interactions as well as triggering GPVI shedding and/or internalization. Numerous antibodies raised against human GPVI now exist,Citation10,Citation35 most of which activate platelets either directly through GPVI engagement or indirectly via the interaction with FcγRIIa when the antibody is intact. The fragment antigen-binding (Fab) fragments of most of these antibodies, including 9O12, 5C4, 1G5, and OM2 have strong-to-very-strong affinity for GPVI and inhibit GPVI-induced platelet activation. Single domain antibody clones,Citation105 consisting of single heavy and light chain variable domains (11–13 kDa) and single chain antibodies,Citation106 are highly stable and protease-resistant reagents that can be humanized and readily expressed in phage display libraries. As monomers, these reagents also have the advantage of not clustering or crosslinking GPVI, causing unwanted platelet activation.Citation105,Citation107
The signaling pathways utilized by GPVI to transmit activation signals () may also be targeted therapeutically. Existing reagents targeting SykCitation108 or BtkCitation109 – which are already approved as antitumor reagents for use in patients with lymphoma – have shown strong efficacy in blocking platelet-collagen responses via GPVI.
Interestingly, the anticancer histone deacetylase inhibitors have additional effects on platelet GPVI. They reduce the surface and total expression of GPVI and impair GPVI signaling, which results in the inhibition of the final common pathways of platelet activation.Citation110 New approaches that target GPVI-mediated reactive oxygen speciesCitation58 or limit GPVI signaling via the activation of nuclear receptors (liver X receptors) on platelets that dampen platelet-collagen responsesCitation111 may represent novel, more subtle approaches to diminish but not block GPVI-signaling function. The fact that many of these agents have been used therapeutically in patients and do not seem to cause clinically significant bleeding provides encouragement and possibly underscores the relatively minor role for GPVI in normal hemostasis.
Final remarks
For a number of reasons, GPVI presents as an attractive and feasible target to modulate platelet function. Peripherally, it is found exclusively on platelets, meaning that its modulation is less likely to lead to off-target effects. GPVI is accessible on the surface of platelets and tools and reagents exist to: 1) rapidly block the function or downregulate surface levels of the molecule; and 2) quantify changes in the surface levels of GPVI (by fluorescence-activated cell sorting [FACS]) and/or shed soluble GPVI in plasma (by enzyme-linked immunosorbent assay [ELISA] or bead-based immunoassay).
While the loss of GPVI does not appear to seriously disrupt normal hemostasis, pathological thrombus formation is significantly attenuated by targeting GPVI. Despite these positive milestones, it is valuable to remember that many proteins and gene products contribute to a given platelet phenotype in the complex human system. In the case of mouse GPVI, at least one or more unknown modifier genes in a modifier of hemostasis locus were shown to control the extent to which platelet thrombus formation in vivo was disrupted by the absence of platelet GPVI. Conceivably this could occur by altering the composition and thrombotic nature of the extracellular matrix through the regulation of gene expression in endothelial cells, smooth muscle cells, and/or fibroblasts.Citation112
Further, GPVI-based inhibitors need to be carefully evaluated for safety, efficacy, and potency in the different patient groups and – as a monotherapy – suitability for acute conditions, or in combination with the existing antiplatelet and anticoagulant therapies as part of an approach to chronic treatment. For these reasons, data from human trials are eagerly awaited. However, together with new tools to specifically examine the antithrombotic (and other) effects of new and existing anti-GPVI reagents, such as a mouse expressing human GPVI,Citation113 a reagent that controls GPVI expression and function is practical and a reasonable proposition.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia.
Disclosure
The authors report no conflicts of interest in this work.
References
- RondinaMTWeyrichASZimmermanGAPlatelets as cellular effectors of inflammation in vascular diseasesCirc Res2013112111506151923704217
- FurieBFurieBCMechanisms of thrombus formationNew Engl J Med2008359993894918753650
- MackmanNTriggers, targets and treatments for thrombosisNature2008451718191491818288180
- JacksonSPArterial thrombosis – insidious, unpredictable and deadlyNat Med201117111423143622064432
- UngererMMünchGNovel antiplatelet drugs in clinical developmentThromb Haemost2013110586887524108565
- Antithrombotic Trialists’ CollaborationCollaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patientsBMJ20023247329718611786451
- HankeyGJAntiplatelet therapy for the prevention of recurrent stroke and other serious vascular events: a review of the clinical trial data and guidelinesCurr Med Res Opin20072361453146217559741
- ChengJWUpdates in antiplatelet agents used in cardiovascular diseasesJ Cardiovas Pharmacol Ther2013186514524
- BergerPBBhattDLFusterVCHARISMA InvestigatorsBleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: Results from the clopidogrel for high atherothrombotic risk and ischemic stabilization, management, and avoidance (CHARISMA) trialCirculation2010121232575258320516378
- BigalkeBStellosKWeigHJRegulation of platelet glycoprotein VI (GPVI) surface expression and of soluble GPVI in patients with atrial fibrillation (AF) and acute coronary syndrome (ACS)Basic Res Cardiol2009104335235719190951
- BigalkeBStellosKGeislerTGlycoprotein VI for diagnosis of acute coronary syndrome when ECG is ambiguousInt J Cardiol2011149216416820071043
- BigalkeBStellosKGeislerTLindemannSMayAEGawazMGlycoprotein VI as a prognostic biomarker for cardiovascular death in patients with symptomatic coronary artery diseaseClin Res Cardiol201099422723320049463
- DüttingSBenderMNieswandtBPlatelet GPVI: a target for antithrombotic therapyTrends Pharmacol Sci2012331158359022901552
- ZahidMManginPLoyauSThe future of glycoprotein VI as an antithrombotic targetJ Thromb Haemost201210122418242723020554
- BigalkeBKrämerBFSeizerPFateh-MoghadamSGawazMLindemannSDiagnostic and therapeutic potentials of platelet glycoprotein VISemin Thromb Hemost201036220321120414836
- GawazMVogelSPfannenbergCPichlerBLangerHBigalkeBImplications of glycoprotein VI for theranosticsThromb Haemost2014112116
- NieswandtBWatsonSPPlatelet-collagen interaction: is GPVI the central receptorBlood2003102244946112649139
- InoueOSuzuki-InoueKMcCartyOJLaminin stimulates spreading of platelets through integrin α6β1-dependent activation of GPVIBlood200610741405141216219796
- ClemetsonJMPolgarJMagnenatEWellsTNClemetsonKJThe platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcαR and the natural killer receptorsJ Biol Chem199927441290192902410506151
- BurkhartJMVaudelMGambaryanSThe first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathwaysBlood201212015e73e8222869793
- TsujiMEzumiYAraiMTakayamaHA novel association of Fc receptor γ-chain with glycoprotein VI and their co-expression as a collagen receptor in human plateletsJ Biol Chem19972723823528235319295288
- BergmeierWStefaniniLPlatelet ITAM signalingCurr Opin Hematol201320544545023921514
- GibbinsJMOkumaMFarndaleRBarnesMWatsonSPGlycoprotein VI is the collagen receptor in platelets which underlies tyrosine phosphorylation of the Fc receptor γ-chainFEBS Lett199741322552599280292
- AndrewsRKSuzuki-InoueKShenYTulasneDWatsonSPBerndtMCInteraction of calmodulin with the cytoplasmic domain of platelet glycoprotein VIBlood200299114219422112010829
- LecutCArocasVUlrichtsHIdentification of residues within human glycoprotein VI involved in the binding to collagen: evidence for the existence of distinct binding sitesJ Biol Chem200427950522935229915466473
- KnightCGMortonLFOnleyDJCollagen-platelet interaction: Gly-Pro-Hyp is uniquely specific for platelet Gp VI and mediates platelet activation by collagenCardiovasc Res199941245045710341844
- CasasnovasJMStehleTLiuJHWangJHSpringerTAA dimeric crystal structure for the N-terminal two domains of intercellular adhesion molecule-1Proc Natl Acad Sci USA1998958413441399539702
- CornishALFreemanSForbesGCharacterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33Blood1998926212321329731071
- RamslandPAFarrugiaWBradfordTMStructural basis for Fc γRIIa recognition of human IgG and formation of inflammatory signaling complexesJ Immunol201118763208321721856937
- JungSMMoroiMSoejimaKConstitutive dimerization of glycoprotein VI (GPVI) in resting platelets is essential for binding to collagen and activation in flowing bloodJ Biol Chem201228735300003001322773837
- ArthurJFShenYKahnMLBerndtMCAndrewsRKGardinerEELigand binding rapidly induces disulfide-dependent dimerization of glycoprotein VI on the platelet plasma membraneJ Biol Chem200728242304343044117690106
- LoyauSDumontBOllivierVPlatelet glycoprotein VI dimerization, an active process inducing receptor competence, is an indicator of platelet reactivityArterioscler Thromb Vasc Biol201232377878522155453
- HoriiKKahnMLHerrABStructural basis for platelet collagen responses by the immune-type receptor glycoprotein VIBlood2006108393694216861347
- MiuraYTakahashiTJungSMMoroiMAnalysis of the interaction of platelet collagen receptor glycoprotein VI (GPVI) with collagen. A dimeric form of GPVI, but not the monomeric form, shows affinity to fibrous collagenJ Biol Chem200227748461974620412356768
- Al-TamimiMArthurJFGardinerEEAndrewsRKFocusing on plasma glycoprotein VIThromb Haemost2012107464865522274761
- GardinerEEArthurJFKahnMLBerndtMCAndrewsRKRegulation of platelet membrane levels of glycoprotein VI by a platelet-derived metalloproteinaseBlood2004104123611361715308568
- GardinerEEKarunakaranDArthurJFDual ITAM-mediated proteolytic pathways for irreversible inactivation of platelet receptors: de-ITAM-izing FcγRIIaBlood2008111116517417848620
- Al-TamimiMGrigoriadisGTranHCoagulation-induced shedding of platelet glycoprotein VI mediated by factor XaBlood2011117143912392021252089
- Al-TamimiMTanCWQiaoJPathological shear triggers shedding of vascular receptors: a novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vesselsBlood2012119184311432022431567
- RabieTVarga-SzaboDBenderMDiverging signaling events control the pathway of GPVI down-regulation in vivoBlood2007110252953517374738
- TakayamaHHosakaYNakayamaKA novel antiplatelet antibody therapy that induces cAMP-dependent endocytosis of the GPVI/Fc receptor γ-chain complexJ Clin Invest200811851785179518382762
- MurphyGRegulation of the proteolytic disintegrin metalloproteinases, the ‘Sheddases’Semin Cell Dev Biol200920213814518840536
- van der VorstEPKeijbeckAAde WintherMPDonnersMMA disintegrin and metalloproteases: molecular scissors in angiogenesis, inflammation and atherosclerosisAtherosclerosis2012224230230822698791
- HartmannMHerrlichAHerrlichPWho decides when to cleave an ectodomainTrends Biochem Sci201338311112023298902
- GardinerEEKarunakaranDShenYArthurJFAndrewsRKBerndtMCControlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinasesJ Thromb Haemost2007571530153717445093
- LudwigAHundhausenCLambertMHMetalloproteinase inhibitors for the disintegrin-like metalloproteinases ADAM10 and ADAM17 that differentially block constitutive and phorbol ester-inducible shedding of cell surface moleculesComb Chem High Throughput Screen20058216117115777180
- AndrewsRKBerndtMCBernard–Soulier syndrome: an updateSemin Thromb Hemost201339665666223929303
- KrollMHHellumsJDMcIntireLVSchaferAIMoakeJLPlatelets and shear stressBlood1996885152515418781407
- AndrewsRKGardinerEEShenYBerndtMCPlatelet interactions in thrombosisIUBMB Life2004561131814992375
- McFadyenJDJacksonSPDifferentiating haemostasis from thrombosis for therapeutic benefitThromb Haemost2013110585986723945664
- RabieTStrehlALudwigANieswandtBEvidence for a role of ADAM17 (TACE) in the regulation of platelet glycoprotein VJ Biol Chem200528015144621446815691827
- ArthurJFGardinerEEMatzarisMGlycoprotein VI is associated with GPIb-IX-V on the membrane of resting and activated plateletsThromb Haemost200593471672315841318
- OzakiYAsazumaNSuzuki-InoueKBerndtMCPlatelet GPIb-IX-V-dependent signalingJ Thromb Haemost2005381745175116102041
- WatsonSPHerbertJMPollittAYGPVI and CLEC-2 in hemostasis and vascular integrityJ Thromb Haemost2010871456146720345705
- Suzuki-InoueKWildeJIAndrewsRKGlycoproteins VI and Ib-IX-V stimulate tyrosine phosphorylation of tyrosine kinase Syk and phospholipase Cγ2 at distinct sitesBiochem J2004378Pt 31023102914656219
- GardinerEEArthurJFShenYGPIbα-selective activation of platelets induces platelet signaling events comparable to GPVI activation eventsPlatelets201021424425220367574
- ArthurJFQiaoJShenYITAM receptor-mediated generation of reactive oxygen species in human platelets occurs via Syk-dependent and Syk-independent pathwaysJ Thromb Haemost20121061133114122489915
- VaraDCampanellaMPulaGThe novel NOX inhibitor 2- acetylphenothiazine impairs collagen-dependent thrombus formation in a GPVI-dependent mannerBr J Pharmacol2013168121222422881838
- WalshTGBerndtMCCarrimNCowmanJKennyDMetharomPThe role of Nox1 and Nox2 in GPVI-dependent platelet activation and thrombus formationRedox Biol2014217818624494191
- ArthurJFShenYGardinerEETNF receptor-associated factor 4 (TRAF4) is a novel binding partner of glycoprotein Ib and glycoprotein VI in human plateletsJ Thromb Haemost20119116317220946164
- MillerMWBasraSKulpDWSmall-molecule inhibitors of integrin α2β1 that prevent pathological thrombus formation via an allosteric mechanismProc Natl Acad Sci USA2009106371972419141632
- NissinenLPentikäinenOTJouppilaAA small-molecule inhibitor of integrin α2 β1 introduces a new strategy for antithrombotic therapyThromb Haemost2010103238739720126829
- SchaffMTangCMaurerEIntegrin α6β1 is the main receptor for vascular laminins and plays a role in platelet adhesion, activation, and arterial thrombosisCirculation2013128554155223797810
- MüllerFMutchNJSchenkWAPlatelet polyphosphates are proinflammatory and procoagulant mediators in vivoCell200913961143115620005807
- SchulzCLeuschenNVFröhlichTIdentification of novel downstream targets of platelet glycoprotein VI activation by differential proteome analysis: implications for thrombus formationBlood2010115204102411020107233
- ArachicheAKerbiriou-NabiasDGarcinILetellierTDachary-PrigentJRapid procoagulant phosphatidylserine exposure relies on high cytosolic calcium rather than on mitochondrial depolarizationArterioscler Thromb Vasc Biol200929111883188919696403
- ChooHJSaafirTBMkumbaLWagnerMBJobeSMMitochondrial calcium and reactive oxygen species regulate agonist-initiated platelet phosphatidylserine exposureArterioscler Thromb Vas Biol2012321229462955
- MoroiMJungSMOkumaMShinmyozuKA patient with platelets deficient in glycoprotein VI that lack both collagen-induced aggregation and adhesionJ Clin Invest1989845144014452808700
- GardinerEEAl-TamimiMMuFTCompromised ITAM-based platelet receptor function in a patient with immune thrombocytopenic purpuraJ Thromb Haemost2008671175118218485087
- DumontBLasneDRothschildCAbsence of collagen-induced platelet activation caused by compound heterozygous GPVI mutationsBlood200911491900190319549989
- HermansCWittevrongelCThysCSmethurstPAVan GeetCFresonKA compound heterozygous mutation in glycoprotein VI in a patient with a bleeding disorderJ Thromb Haemost2009781356136319552682
- ArthurJFDunkleySAndrewsRKPlatelet glycoprotein VI-related clinical defectsBrit J Haematol2007139336337217910626
- QiaoJArthurJFCollecuttMAn acquired defect associated with abnormal signaling of the platelet collagen receptor glycoprotein VIActa Haematol2012128423324122922528
- BellucciSHuisseMGBovalBDefective collagen-induced platelet activation in two patients with malignant haemopathies is related to a defect in the GPVI-coupled signaling pathwayThromb Haemost200593113013815630503
- LockyerSOkuyamaKBegumSGPVI-deficient mice lack collagen responses and are protected against experimentally induced pulmonary thromboembolismThromb Res2006118337138016139873
- NieswandtBBergmeierWSchulteVRackebrandtKGessnerJEZirngiblHExpression and function of the mouse collagen receptor glycoprotein VI is strictly dependent on its association with the FcRγ chainJ Biol Chem200027531239982400210825177
- MassbergSGawazMGrünerSA crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivoJ Exp Med20031971414912515812
- DuboisCPanicot-DuboisLGainorJFFurieBCFurieBThrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury modelJ Clin Invest2007117495396017380206
- ManginPYapCLNonneCThrombin overcomes the thrombosis defect associated with platelet GPVI/FcRγ deficiencyBlood2006107114346435316391010
- KatoKKanajiTRussellSThe contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletionBlood200310251701170712738669
- BenderMHagedornINieswandtBGenetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl(3)-induced thrombosisJ Thromb Haemost2011971423142621535392
- GrünerSProstrednaMAktasBAnti-glycoprotein VI treatment severely compromises hemostasis in mice with reduced α2β1 levels or concomitant aspirin therapyCirculation2004110182946295115505105
- DuboisCPanicot-DuboisLMerrill-SkoloffGFurieBFurieBCGlycoprotein VI-dependent and -independent pathways of thrombus formation in vivoBlood2006107103902390616455953
- YousufOBhattDLThe evolution of antiplatelet therapy in cardiovascular diseaseNat Rev Cardiol201181054755921750497
- MoserMOlivierCBBodeCTriple antithrombotic therapy in cardiac patients: more questions than answersEur Heart J201435421622324302275
- van der HulleTKooimanJden ExterPLDekkersOMKlokFAHuismanMVEffectiveness and safety of novel oral anticoagulants compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysisJ Thromb Haemost201412332032824330006
- AhrensILipGYPeterKNew oral anticoagulant drugs in cardiovascular diseaseThromb Haemost20101041496020539909
- AradiDKomócsiAVorobcsukASerebruanyVLImpact of clopidogrel and potent P2Y 12 -inhibitors on mortality and stroke in patients with acute coronary syndrome or undergoing percutaneous coronary intervention: a systematic review and meta-analysisThromb Haemost201310919310123197191
- Muñiz-LozanoARolliniFFranchiFAngiolilloDJUpdate on platelet glycoprotein IIb/IIIa inhibitors: recommendations for clinical practiceTher Adv Cardiovasc Dis20137419721323818658
- SwieringaFKuijpersMJHeemskerkJWvan der MeijdenPETargeting platelet receptor function in thrombus formation: the risk of bleedingBlood Rev201428192124411640
- DahlenJRPriceMJPariseHGurbelPAEvaluating the clinical usefulness of platelet function testing: considerations for the proper application and interpretation of performance measuresThromb Haemost2013109580881623254993
- HarrisonPLordkipanidzéMTesting platelet functionHematol Oncol Clin North Am201327341144123714306
- BerlangaOBobeRBeckerMExpression of the collagen receptor glycoprotein VI during megakaryocyte differentiationBlood20009682740274511023507
- NieswandtBSchulteVBergmeierWLong-term antithrombotic protection by in vivo depletion of platelet glycoprotein VI in miceJ Exp Med2001193445946911181698
- BoilardENigrovicPALarabeeKPlatelets amplify inflammation in arthritis via collagen-dependent microparticle productionScience2010327596558058320110505
- KleinschnitzCPozgajovaMPhamMBendszusMNieswandtBStollGTargeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleedingCirculation2007115172323233017438148
- DeviSKuligowskiMPKwanRYPlatelet recruitment to the inflamed glomerulus occurs via an αIIbβ3/GPVI-dependent pathwayAm J Pathol201017731131114220651232
- MassbergSKonradIBültmannASoluble glycoprotein VI dimer inhibits platelet adhesion and aggregation to the injured vessel wall in vivoFASEB J200418239739914656994
- BültmannAHerdegCLiZLocal delivery of soluble platelet collagen receptor glycoprotein VI inhibits thrombus formation in vivoThromb Haemost200695576376616676064
- UngererMLiZBaumgartnerCThe GPVI-Fc fusion protein Revacept reduces thrombus formation and improves vascular dysfunction in atherosclerosis without any impact on bleeding timesPLoS One201388e7119323951109
- BigalkeBPhinikaridouAAndiaMEPositron emission tomography/computed tomographic and magnetic resonance imaging in a murine model of progressive atherosclerosis using (64)Cu-labeled glycoprotein VI-FcCirc Cardiovasc Imaging20136695796424107491
- GoebelSLiZVogelmannJThe GPVI-Fc fusion protein Revacept improves cerebral infarct volume and functional outcome in strokePLoS One201387e6696023935828
- UngererMRosportKBültmannANovel antiplatelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humansCirculation2011123171891189921502572
- GrünerSProstrednaMKochMRelative antithrombotic effect of soluble GPVI dimer compared with anti-GPVI antibodies in miceBlood200510541492149915507524
- WalkerAPughNGarnerSFBloodomics ConsortiumSingle domain antibodies against the collagen signaling receptor glycoprotein VI are inhibitors of collagen induced thrombus formationPlatelets200920426827619459133
- MuzardJBouabdelliMZahidMDesign and humanization of a murine scFv that blocks human platelet glycoprotein VI in vitroFEBS J2009276154207422219558491
- ZahidMLoyauSBouabdelliMAubreyNJandrot-PerrusMBillialdPDesign and reshaping of an scFv directed against human platelet glycoprotein VI with diagnostic potentialAnal Biochem2011417227428221771576
- SpaltonJCMoriJPollittAYHughesCEEbleJAWatsonSPThe novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in plateletsJ Thromb Haemost2009771192119919422460
- UckunFMVassilevABartellSZhengYMahajanSTibblesHEThe anti-leukemic Bruton’s tyrosine kinase inhibitor alpha-cyano-beta-hydroxy-beta-methyl-N-(2,5-dibromophenyl) propenamide (LFM-A13) prevents fatal thromboembolismLeuk Lymphoma20034491569157714565661
- BishtonMJGardinerEEHarrisonSJPrinceHMJohnstoneRWHistone deacetylase inhibitors reduce glycoprotein VI expression and platelet responses to collagen related peptideThromb Res2013131651452023642854
- SpyridonMMoraesLAJonesCILXR as a novel antithrombotic targetBlood2011117215751576121411760
- CheliYJensenDMarchesePThe Modifier of hemostasis (Mh) locus on chromosome 4 controls in vivo hemostasis of Gp6−/− miceBlood200811131266127317991808
- ManginPHTangCBourdonCA humanized glycoprotein VI (GPVI) mouse model to assess the antithrombotic efficacies of anti-GPVI agentsJ Pharmacol Exp Ther2012341115616322238212
- CosemansJMKuijpersMJLecutCContribution of platelet glycoprotein VI to the thrombogenic effect of collagens in fibrous atherosclerotic lesionsAtherosclerosis20051811192715939050
- KuijpersMJGilioKReitsmaSComplementary roles of platelets and coagulation in thrombus formation on plaques acutely ruptured by targeted ultrasound treatment: a novel intravital modelJ Thromb Haemost20097115216118983512
- Al-TamimiMGardinerEEThomJYSoluble glycoprotein VI is raised in the plasma of patients with acute ischemic strokeStroke201142249850021193745
- BigalkeBStellosKGeislerTExpression of platelet glycoprotein VI is associated with transient ischemic attack and strokeEur J Neurol201017111111719686349
- BoulaftaliYHessPRGetzTMPlatelet ITAM signaling is critical for vascular integrity in inflammationJ Clin Invest2013123290891623348738
